Structure and reactivity of sulfonamide- and acetate-chelated ruthenium alkylidene complexes†‡
Venkata R.
Sabbasani
 ,
Sang Young
Yun
and
Daesung
Lee
*
,
Sang Young
Yun
and
Daesung
Lee
*
Department of Chemistry, University of Illinois at Chicago, 845 West Taylor Street, Chicago, Illinois 60607, USA. E-mail: dsunglee@uic.edu
First published on 23rd May 2016
Abstract
Novel ruthenium alkylidene complexes are prepared via enyne ring-closing metathesis, and the structural features of acetate- and sulfonamide-chelates are explored by single crystal X-ray diffraction analyses. All 5-membered sulfonamide chelates and 6-membered acetate chelates have the chelated oxygen and the NHC ligand in cis relationship, which force the two chloride ligands into cis orientation. Upon exposure to carbon monoxide, while acetate chelates decomposed to generate vinyl acetates, the sulfonamide chelate formed an unprecedented CO-bound octahedral ruthenium alkylidene complex. In this complex, the alkylidene carbene moiety and the NHC ligand have a trans relationship, which is the first example of Grubbs-type ruthenium alkylidene to have this unique trans geometry. This complex is devoid of metathesis activity because of the coordinatively saturated nature of the ruthenium center with a completely locked ligand environment, which does not allow either associative or dissociative ligand–substrate exchange with alkene substrates.
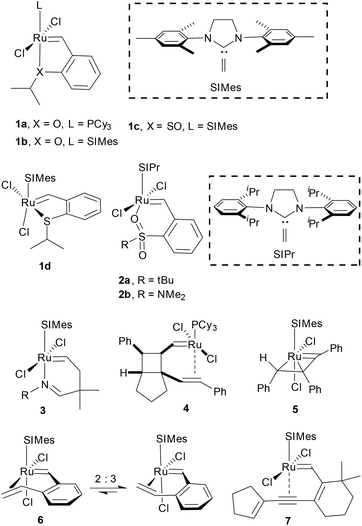
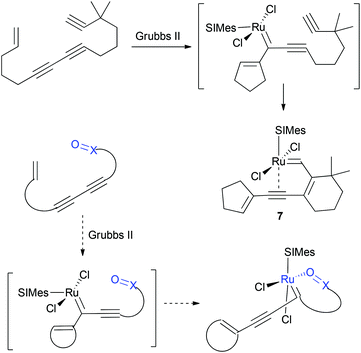
The emergence of the phosphine-free ruthenium alkylidene complex 1 containing an oxygen chelate developed by Hoveyda and coworkers has led to the expanded scope of the metathesis reaction in organic synthesis (Fig. 1).1–4 Therefore many research groups have continued to develop heteroatom-chelated ruthenium alkylidene complexes and studied their structure and reactivity. Sulfur-based complexes 1c and 1d were reported by Grela and Lemcoff, respectively.5 Six-membered chelates with sulfonyl oxygen6 (2a, 2b) and imino-nitrogen7 (3) were also reported. On the other hand, Snapper reported the alkene-chelated ruthenium complex 4 where an alkene is chelated to the metal trans to the phosphine ligand;8 the vinyl alkylidene complex95 and styryl-chelated10 complex 6 reported by Grubbs display a cis-relationship between the SIMes N-heterocyclic carbene (NHC) ligand and the chelated alkene. Previously, our group also reported the first well-defined alkyne-chelated ruthenium alkylidene complex generated by employing tandem ring-closing enyne metathesis and metallotropic [1,3]-shift. Interestingly complex 7 shows that the alkyne binds to the metal center trans to the SIMes NHC ligand.11
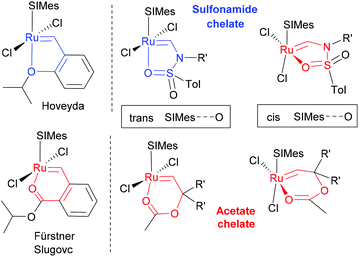
On the basis of the ruthenium alkylidene complexes derived from enyne metathesis and the metallotropic [1,3]-shift, we envisioned that the ring-closing metathesis (RCM) of enediynes would be the most effective method to deliver the ruthenium alkylidene moiety at an appropriate location such that the metal center and the installed chelating group would interact to form heteroatom chelates. For the RCM of enediynes, the metallotropic [1,3]-shift12 of the initially formed ruthenium alkylidene13 would be facilitated by the chelation event and vice versa the thermodynamic advantage of the metallotropic [1,3]-shift to generate a fully conjugated system would force the metal center to be positioned near the chelating group. Here we report the structure and reactivity of acetate- and sulfonamide-chelated ruthenium alkylidene complexes formed via a tandem reaction involving enyne ring-closing metathesis and metallotropic [1,3]-shift. To gain insight into the chelation behaviour of ruthenium alkylidenes, we investigated the factors that promote chelation by oxygen functionality (Scheme 1). The design of 5-membered sulfonamide chelates is modelled by replacing the sp2–sp2 carbon moiety of the phenyl group in the Hoveyda complex with a N–S(O) moiety of sulfonamides.
For less common 6-membered ester chelates, the sp2–sp2 carbon moiety of the phenyl group in the complexes reported by Fürstner and Slugovc was modeled by the (R′2)C–O moiety of acetate chelates (Fig. 2).14 We expected that the formation of acetate-based chelates might be less favourable than that of Fürstner's ester chelate because of the more flexible nature of the C–O linker than the phenyl linker, which will be partially compensated by the gem-dialkyl effect of the (R′2)C–O linker.
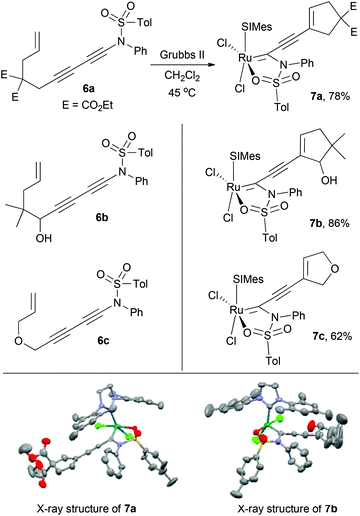
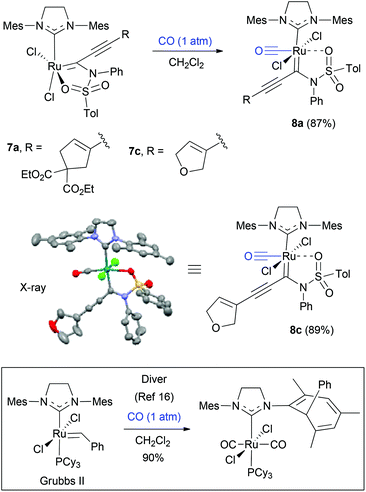
First, to prove the validity of the 5-membered sulfonamide chelates, terminal ynamide-containing enediyne 6a was treated with a stoichiometric amount of Grubbs second-generation complex (Grubbs-II) in CH2Cl2 at 45 °C (Scheme 2). Spectroscopic characterization of the newly formed material (78%) clearly indicates the characteristics of structure 7a, which was further confirmed by single crystal X-ray diffraction analysis.15 The newly formed sulfonamide-chelate has a SIMes-NHC ligand and a chelated oxygen in a cis relationship. Other enediynes 6b and 6c generated the corresponding ruthenium chelates 7b and 7c in 86% and 62% yield, respectively. The ligand orientation around the metal center in 7b was further confirmed by its X-ray structure.15 On the other hand, the crystallized material of 7c in a mixed solvent system of CH2Cl2/EtOAc/Et2O over 10 days, showed that the obtained material is not the expected sulfonamide-chelate 7c, but surprisingly, its carbon monoxide-coordinated form 8c (Scheme 3).15 The origin of CO during crystallization is not clear at this point, however, to further confirm this CO coordination event, 7a and 7c were treated with CO (balloon) in CH2Cl2. Thus, both complexes provided the carbon monoxide-coordinated complexes 8a and 8c within 10 minutes in 87% and 89% yield, respectively. During this time the characteristic green of 7a and 7c changed to pink. The formation of CO-coordinated complexes 8a and 8c are in stark contrast to Diver's observation that CO promotes a Buchner reaction in Grubbs-II ruthenium complexes.16 Chelates such as the Grubbs–Hoveyda complex did not cleanly give Buchner products but did give Buchner products when exposed to isocyanide ligands.17 The stability of the five-membered chelate in complex 7 may explain this unexpected difference in reactivity.
 | ||
| Scheme 3 Formation of octahedral complex with trans NHC–carbene geometry induced by CO coordination. | ||
These CO-bound octahedral ruthenium alkylidene complexes chelated with a sulfonamide moiety display a trans relationship between the SIMes NHC ligand and the carbenic carbon ligand. This is a rare example of Grubbs-type ruthenium alkylidenes to have this unique ligand geometry. This might be explained by the fact that the electronic effect of the initial coordinated CO ligand on the ruthenium center of complex 7, might force rearrangement of the ligand through pseudorotation at the metal center. As expected, complexes 8a and 8c are devoid of metathesis activity, because the ruthenium complex is inert under the tested reaction conditions (5 mol% 8a/8c with dimethyl-2,2-diallylmalonate in toluene-d8 at 100 °C) with a coordinatively saturated ligand environment, which would not allow either associative or dissociative ligand–substrate exchange with alkene substrates.18
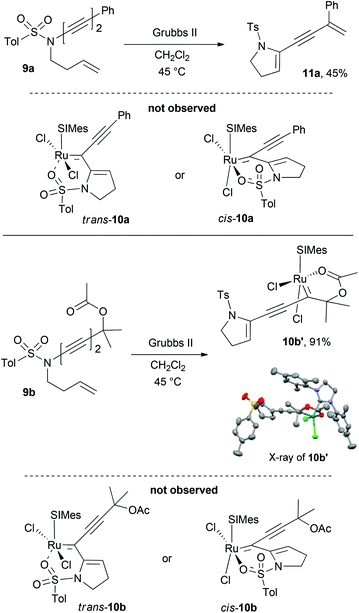
Next, we explore the formation of 6-membered sulfonamide chelates and their ligand disposition. Grela and coworkers have demonstrated that the chelated oxygen of the 6-membered sulfone and sulfonamide chelates maintain a trans relationship to the SIMes NHC ligand (Scheme 4).6 Based on these precedent examples, we expected that the RCM of ynamide-tethered 1,6-enyne 9a,b would undergo ring-closure to form either trans-10a,b or cis-10a,b. Although C–C–N–S of 10a,b connecting the metal center and chelating oxygen is different from C–C–C–S of 2a or 2b the overall ligand dispositions of these complexes are expected to be similar. To our surprise, however, ynamide 9a upon treating with Grubbs II provided only the metathesis product 11a instead of either sulfonamide chelate trans-10a or cis-10a. From the reaction of 9b, under otherwise identical conditions, only the acetate-chelated cis-complex 10b′ was obtained in 91% yield without forming the expected trans-10b or cis-10b.15 The result from the reaction of 9a and 9b suggests that, as opposed to the 5-membered sulfonamide chelates, the corresponding 6-membered chelates 10a and 10b should be relatively unfavourable, thus favourably generating 11a and 10b′, respectively.
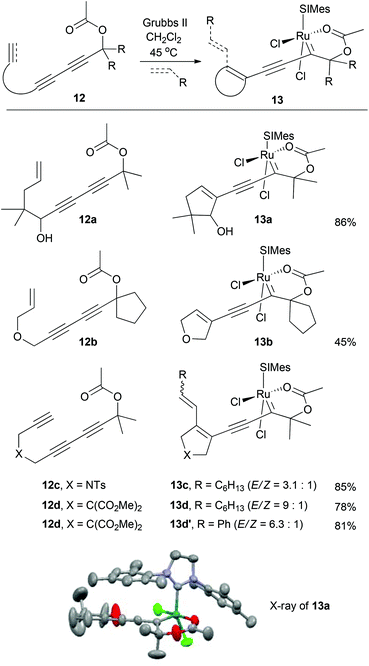
The unexpected formation of the 6-membered acetate chelate of ruthenium alkylidene 10b′ prompted us to further explore carbonyl-based sp2-oxygen chelates. To facilitate the delivery of the ruthenium alkylidene moiety near the chelating acetate functionality, we first employed a ring-closing metathesis strategy rather than cross metathesis with the substrates 1,3-diynes 12a–12d (Table 1). Upon treatment of 1,3-diynyl propargylic acetates 12a and 12b with a stoichiometric amount of Grubbs-II, the corresponding acetate-chelated complexes 13a and 13b were formed in 86% and 45% yield, respectively.19 The low yield of 13b is the consequence of its turn over to the final metathesis product (13%). The cis relationship between the NHC and the chelated oxygen of the acetate in complex 13a is evident in its X-ray structure.15 For triynyl propargylic acetates 12c and 12d, their cross metathesis with 1-octene and styrene followed by metallotropic [1.3]-shift provided chelates 13c, 13d, and 13d′ in 85%, 78% and 81% yield, respectively.
These results suggest that the subtle steric and electronic influence of the substituent on the carbenic carbon is critical for the formation of these chelates. The corresponding substrates devoid of the gem-dialkyl substituent at the propargylic carbon did not form chelates. The formation of 6-membered cis chelates is primarily due to the presence of an alkynyl group at the carbenic carbon compared to Fürstner's and Slugovc's trans chelates containing either a vinyl group or a proton. For 13a–13d to form trans chelates, 90° rotation of the Ru![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C axis is required, which will bring the linear alkynyl group to the same space where the mesityl group is.
C axis is required, which will bring the linear alkynyl group to the same space where the mesityl group is.
The complexation behaviour of these acetate-chelated ruthenium alkylidenes toward carbon monoxide was found to be different from that of sulfonamide chelates (Scheme 5).20 Upon exposure of a green coloured CH2Cl2 solution of 13a to carbon monoxide, the solution became colourless as opposed to pink with sulfonamide chelates. Isolation and characterization of the newly formed material shows that CO promoted the decomplexation of 13a to generate vinyl acetate 14a and the CO-bound C2-symmetrical dimeric ruthenium complex 15a in quantitative yield (Scheme 5).15,21 Regarding this transformation, we propose that the CO-coordination would decrease the electron density from the carbenic carbon because of the strong-acceptor capacity of CO, which promotes the acetate to rearrange with concurrent cleavage of the Ru![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond.
C bond.
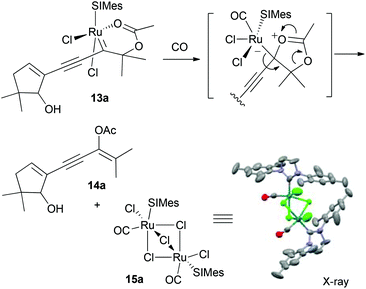 | ||
Scheme 5 CO-binding to a 6-membered acetate-chelate promotes acetate migration with concurrent Ru![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C cleavage. C cleavage. | ||
In conclusion, novel sulfonamide- and acetate-chelated ruthenium alkylidene complexes were synthesized from appropriately functionalized enynes and enediynes via ring-closing metathesis. In all the 5-membered sulfonamide- and 6-membered acetate-chelated complexes prepared in this study, the sulfonamide and acetate moieties are disposed in a cis relationship relative to the NHC ligands. Upon exposure to carbon monoxide, the sulfonamide chelates generated a CO-bound octahedral ruthenium alkylidene complex where the alkylidene carbene moiety and the NHC ligand maintain a trans relationship. This is the first example of Grubbs-type ruthenium alkylidenes to have this unique trans geometry. On the other hand, under the same conditions, the corresponding acetate chelate decomposed to generate vinyl acetates as pure organic products concurrently with a dimeric ruthenium CO-complex. The CO-bound octahedral ruthenium alkylidenes are metathesis inactive because the ligand environment of the ruthenium center is completely locked, which does not allow ligand–substrate exchange.
Financial support for this work from UIC (LAS Science Award) and TACOMA Technology is greatly acknowledged. We thank Prof. Neal Mankad (UIC), Mr Rodger F. Henry (AbbVie), Dr Huaqing Liu (AbbVie) and Prof. Donald Wink (UIC) for their help in obtaining X-ray structures. We thank the Mass Spectrometry Laboratory at the University of Illinois at Urbana–Champaign for acquisition of high-resolution mass spectrometry data.
Notes and references
- General reviews: (a) R. H. Grubbs and S. Chang, Tetrahedron, 1998, 54, 4413 CrossRef CAS; (b) A. Fürstner, Angew. Chem., Int. Ed., 2000, 39, 3012 CrossRef; (c) R. R. Schrock and A. H. Hoveyda, Angew. Chem., Int. Ed., 2003, 42, 4592 CrossRef CAS PubMed; (d) A. Deiters and S. F. Martin, Chem. Rev., 2004, 104, 2199 CrossRef CAS PubMed; (e) G. C. Vougioukalakis and R. H. Grubbs, Chem. Rev., 2010, 110, 1746 CrossRef CAS PubMed. For enyne metathesis: (f) A. J. Giessert and S. T. Diver, Chem. Rev., 2004, 104, 1317 CrossRef PubMed; (g) M. Mori, in Handbook of Metathesis, ed. R. H. Grubbs, Wiley-VCH, Weinheim, Germany, 2003, vol. 2, p. 176 Search PubMed.
- (a) A. Füstner and K. Langemann, J. Am. Chem. Soc., 1997, 119, 9130 CrossRef; (b) A. K. Ghosh, J. Cappiello and D. Shin, Tetrahedron Lett., 1998, 39, 4651 CrossRef CAS; (c) E. Vedrenne, H. Dupont, S. Oualef, L. Elkaim and L. Grimaud, Synlett, 2005, 670 CAS; (d) J. C. Conrad and D. E. Fogg, Curr. Org. Chem., 2006, 10, 185 CAS; (e) T. Wdowik, C. Samojłowicz, M. Jawiczuk, A. Zarecki and K. Grela, Synlett, 2010, 2931 CAS.
- J. S. Kingsbury, J. P. A. Harrity, P. J. Bonitatebus and A. H. Hoveyda, J. Am. Chem. Soc., 1999, 121, 791 CrossRef CAS.
- (a) S. B. Garber, J. S. Kingsbury, B. L. Gray and A. H. Hoveyda, J. Am. Chem. Soc., 2000, 122, 8168 CrossRef CAS; (b) H. Wakamatsu and S. Blechert, Angew. Chem., Int. Ed., 2002, 41, 2403 CrossRef CAS; (c) K. Grela, S. Harutyunyan and A. Michrowska, Angew. Chem., Int. Ed., 2002, 41, 4038 CrossRef CAS.
- (a) A. Ben-Asuly, E. Tzur, C. E. Diesendruck, M. Sigalov, I. Goldberg and N. G. Lemcoff, Organometallics, 2008, 27, 811 CrossRef CAS; (b) A. Szadkowska, A. Makal, K. Woźniak, R. Kadyrov and K. Grela, Organometallics, 2009, 28, 2693 CrossRef CAS.
- A. Szadkowska, K. Żukowska, A. E. Pazio, K. Woźniak, R. Kadyrov and K. Grela, Organometallics, 2011, 30, 1130 CrossRef CAS.
- A. Hejl, M. W. Day and R. H. Grubbs, Organometallics, 2006, 25, 6149 CrossRef CAS and references therein.
- J. A. Tallarico, P. J. Bonitatebus Jr. and M. L. Snapper, J. Am. Chem. Soc., 1997, 119, 7157 CrossRef CAS.
- T. M. Trnka, M. W. Day and R. H. Grubbs, Organometallics, 2001, 20, 3845 CrossRef CAS.
- (a) D. R. Anderson, D. D. Hickstein, D. J. O'Leary and R. H. Grubbs, J. Am. Chem. Soc., 2006, 128, 8386 CrossRef CAS PubMed; (b) D. R. Anderson, D. J. O'Leary and R. H. Grubbs, Chem. – Eur. J., 2008, 14, 7536 CrossRef CAS PubMed; (c) I. C. Stewart, D. Benitez, D. J. O'Leary, E. Tkatch-ouk, M. W. Day, W. A. Goddard III and R. H. Grubbs, J. Am. Chem. Soc., 2009, 131, 1931 CrossRef CAS PubMed.
- K.-P. Wang, S. Y. Yun, D. Lee and D. J. Wink, J. Am. Chem. Soc., 2009, 131, 15114 CrossRef CAS PubMed.
- Review of metallotropic shifts: (a) M. Kim and D. Lee, Org. Biomol. Chem., 2007, 5, 3418 RSC. Synthetic applications: (b) E. J. Cho and D. Lee, Org. Lett., 2008, 10, 257 CrossRef CAS PubMed; (c) J. Li, R. L. Miller and D. Lee, Org. Lett., 2009, 11, 571 CrossRef CAS PubMed; (d) S. Y. Yun, K.-P. Wang, M. Kim and D. Lee, J. Am. Chem. Soc., 2010, 132, 8840 CrossRef CAS PubMed; (e) K.-P. Wang, E. J. Cho, S. Y. Yun, J. Y. Rhee and D. Lee, Tetrahedron, 2013, 69, 9105 CrossRef CAS.
- S. Y. Yun, M. Kim, D. Lee and D. J. Wink, J. Am. Chem. Soc., 2009, 131, 24 CrossRef CAS PubMed.
- (a) A. Fürstner, O. R. Thiel and C. W. Lehmann, Organometallics, 2002, 21, 331 CrossRef; (b) M. Zirngast, E. Pump, A. Leitgeb, J. H. Albering and C. Slugovc, Chem. Commun., 2011, 47, 2261 RSC; (c) E. Pump, A. Poater, M. Zirngast, A. Torvisco, R. Fischer, L. Cavallo and C. Slugovc, Organometallics, 2014, 33, 2806 CrossRef CAS.
- CCDC 1471953 (7a), 1471954 (7b), 1471958 (8c), 1471955 (10b′), 1471956 (13a) and 1471957 (15a) contain the supplementary crystallographic data.
- B. R. Galan, M. Gembicky, P. M. Dominiak, J. B. Keister and S. T. Diver, J. Am. Chem. Soc., 2005, 127, 15702 CrossRef CAS PubMed.
- B. R. Galan, M. Pitak, M. Gembicky, J. B. Keister and S. T. Diver, J. Am. Chem. Soc., 2009, 131, 6822 CrossRef CAS PubMed.
- Although the CO-complexed chelates 8a and 8c are inert toward metathesis, their precursors 7a as well as 7c are metathesis-active. Upon treating dimethyl-2,2-diallylmalonate with 7a or 7c (5 mol%) in toluene-d8 at 80 °C, the corresponding RCM product was obtained in 78% yield (95% conversion) after 10 h, but at 45 °C in CDCl3 only around 10% conversion was observed after 24 h.
- Treating dimethyl-2,2-diallylmalonate with acetate-chelated complex 13a (2 mol%) in CDCl3 at 45 °C provided the RCM product in 89% yield within 2 h.
- Halide exchange of ruthenium ester chelates: E. Pump, R. C. Fischer and C. Slugovc, Organometallics, 2012, 31, 6972 CrossRef CAS.
- For trichloro-bridged ruthenium dimer-type complexes see: (a) X. Sauvage, Y. Borguet, A. F. Noels, L. Delaude and A. Demonceau, Adv. Synth. Catal., 2007, 349, 255 CrossRef CAS; (b) E. M. Leitao, S. R. Dubberley, W. E. Piers, Q. Wu and R. McDonald, Chem. – Eur. J., 2008, 14, 11565 CrossRef CAS PubMed.
Footnotes |
| † In celebration of Professor Barry Trost's 75th Birthday. |
| ‡ Electronic supplementary information (ESI) available. CCDC 1471953–1471958. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6qo00148c |
| This journal is © the Partner Organisations 2016 |