Base-induced synthesis of N-dialkylaminomethyl-2H-1,2,3-triazoles from N-sulfonyl-1,2,3-triazoles†
Yu
Jiang
a,
Qiang
Wang
b,
Run
Sun
b,
Xiang-Ying
Tang
a and
Min
Shi
*ab
aState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032, China
bKey Laboratory for Advanced Materials and Institute of Fine Chemicals, East China University of Science and Technology, Meilong Road No. 130, Shanghai, 200237, China. E-mail: mshi@mail.sioc.ac.cn
First published on 11th April 2016
Abstract
A facile synthetic method to access N-dialkylaminomethyl-2H-1,2,3-triazoles has been developed via a novel reaction mode from N-sulfonyl-1,2,3-triazoles by a base induced reaction. Moreover, 4-phenyl-1H-1,2,3-triazoles can also give the same products even in the absence of a base at high temperature.
Introduction
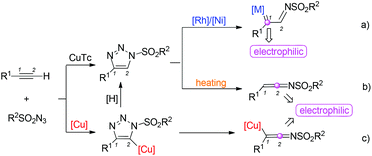
1,2,3-Triazoles are an important class of heterocycles in organic synthesis, medicinal chemistry and materials science.1 Among them, N-sulfonyl-1,2,3-triazoles, which can be easily prepared from copper(I)-catalyzed azide–alkyne cycloadditions,2 have attracted much attention of organic chemists because of their potential applications in accessing other heterocycles. Recently, investigation on the reaction types of these compounds has emerged at the forefront of current research. Several different reaction types have been found to date (Scheme 1).In the presence of Rh/Ni catalysts, N-sulfonyl-1,2,3-triazoles will undergo a ring-opening process to afford α-imino metal carbenes.3–8 The C1 position with a strong electrophilic ability can be attacked by other nucleophiles.4–6 Moreover, a nitrogen atom with an increased nucleophilic ability offers more synthetic flexibility. The α-imino metal carbenes can react with many other compounds to afford useful heterocycles (Scheme 1a).7 On the other hand, at high temperature, N-sulfonyl-1,2,3-triazoles can also undergo a ring-opening process to afford N-sulfonyl ketenimines, which can be attacked by nucleophiles at the C2 position or undergo pericyclic reactions (Scheme 1b).9 Indeed, in many reports, N-sulfonyl ketenimines were afforded by a one-pot strategy.10–12 Cycloaddition between terminal alkynes and sulfonyl azides catalyzed by copper(I) afforded the N-sulfonyl triazolyl copper species, which can undergo a ring-opening rearrangement leading to the ketenimine intermediate upon the release of a dinitrogen molecule (Scheme 1c).
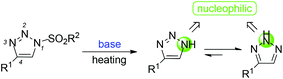
However, we recently also found that the sulfonyl group of N-sulfonyl-1,2,3-triazoles can undergo elimination to afford 4-phenyl-1H-1,2,3-triazoles as nucleophilic intermediates13 in the presence of a base at high temperature. Moreover, the intermediates could be converted into more stable 4-phenyl-2H-1,2,3-triazoles to trap other electrophiles in the N2-position (Scheme 2).14,15 Indeed, bis(dialkylamino)methane was chosen as a reaction partner, which could afford an electrophilic iminium ion.16 Herein, we would like to report these interesting new findings.
Results and discussion
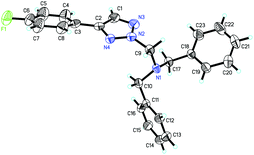
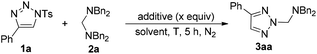
We initially utilized N-sulfonyl-1,2,3-triazole 1a (0.2 mmol) and tetrabenzylmethanediamine 2a (0.2 mmol) as substrates to examine the reaction outcomes. The results are shown in Table 1. To our delight, in the presence of 1.0 equiv. of K2CO3 as an additive, we found that 3aa was obtained in 39% yield determined by 1H NMR spectroscopy along with the formation of HNBn2 (Table 1, entry 2). Subsequently, solvents were examined (Table 1, entries 3–8). The yield of 3aa had no obvious improvement when THF or toluene was used (Table 1, entries 3 and 4). While the use of high polar solvents such as DMF and DMSO afforded 3aa in higher yields (Table 1, entries 5 and 6). Then we turned our attention to identify the best base for this reaction. Both inorganic and organic bases were used in the reaction, and 1,4-diazabicyclo[2,2,2]octane (DABCO) was identified as the best base for the reaction, affording 3aa in 90% isolated yield. Meanwhile, the corresponding TsNBn2 was also obtained at the same time (Table 1, entry 14). The structure of 3aa has been unambiguously determined by the X-ray crystal structure of its analogue 3ha (Fig. 1).17| Entrya | Additive | X | Solvent | T (°C) | Yieldb (%) |
|---|---|---|---|---|---|
| a Unless otherwise specified, all reactions were performed with 1a (0.20 mmol), 2a (0.20 mmol) and additive (0.20 mmol). b Yields are determined by 1H NMR using trimethoxybenzene as an internal standard. c HNBn2 was obtained in 25% yield. d TsNBn2 was obtained in 91% yield. e Isolated yield. | |||||
| 1 | — | — | DCE | 100 | 25 |
| 2 | K2CO3 | 1.0 | DCE | 100 | 39c |
| 3 | K2CO3 | 1.0 | THF | 100 | 40 |
| 4 | K2CO3 | 1.0 | Toluene | 100 | 51 |
| 5 | K2CO3 | 1.0 | DMF | 100 | 70 |
| 6 | K2CO3 | 1.0 | DMSO | 100 | 68 |
| 7 | K2CO3 | 1.0 | DMF | 100 | 92 |
| 8 | NaHCO3 | 1.0 | DMF | 100 | 92 |
| 9 | NaHCO3 | 1.0 | DMF | 100 | 86 |
| 10 | NaOH | 1.0 | DMF | 100 | 12 |
| 11 | Et3N | 1.0 | DMF | 100 | 11 |
| 12 | iPr2NH | 1.0 | DMF | 100 | 11 |
| 13 | DBU | 1.0 | DMF | 100 | 58 |
| 14 | DABCO | 1.0 | DMF | 100 | 96d (90e) |
| 15 | DMAP | 1.0 | DMF | 100 | 95 |
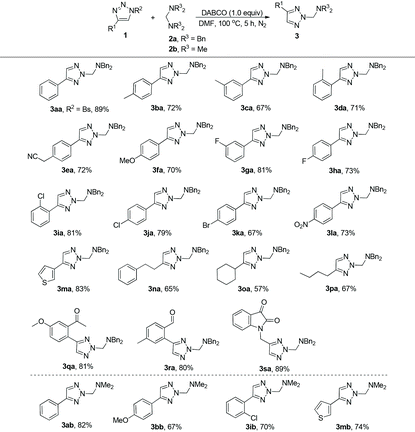
With the optimal conditions in hand, we next surveyed the substrate scope of this reaction and the results are shown in Table 2. At first, we synthesized a series of triazoles with different substituents on the aromatic ring and examined their reaction properties. As for substrates 2b–f, with electron-donating groups on the aromatic ring, the desired products 3 were afforded in 67–72% yields. While for substrates 2g–l, with electron-withdrawing groups, the corresponding products 3 were afforded in 67–81% yields. These results suggested that the substrates with electron-withdrawing groups on the aromatic ring may be more conducive to the formation of 3 compared to those of electron-donating ones. When the benzene ring was replaced by the thiophene ring, product 3ma could also be afforded in good yield. The reaction could also tolerate various triazoles with aliphatic substituents, indicating a broad substrate scope, and giving the desired products 3na, 3oa and 3pa in moderate yields. Furthermore, the substrates bearing functional groups such as a carbonyl group could also afford the corresponding products 3qa, 3ra and 3sa in good yields. When the triazole was protected by the Bs group (4-bromophenylsulfonyl), the reaction also proceeded efficiently, giving the corresponding product 3aa in 89% yield. Finally, tetramethylmethanediamine 2b was also used in this reaction, affording the desired products 3ab, 3bb, 3ib and 3mb in 67–82% yields.
| a Conditions: 1 (0.20 mmol), 2 (0.20 mmol) and DABCO (0.20 mmol) were heated in DMF (2.0 ml) at 100 °C for 5 h. |
|---|
|
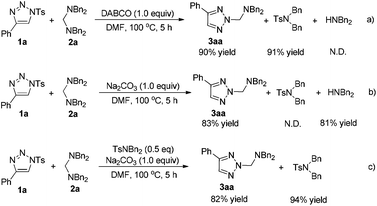
The next experiments mainly focused on the investigation of the mechanism of this reaction. The different by-products of the reaction with different additives caught our attention (Table 1, entries 2 and 14). As shown in Scheme 3, several control experiments were performed. If DABCO was used as the additive, the corresponding TsNBn2 was obtained in 91% yield along with the product 3aa in 90% yield (Scheme 3a). However, if Na2CO3 was used, HNBn2 was obtained in 81% yield instead of TsNBn2 (Scheme 3b). To eliminate the possibility that HNBn2 is derived from the decomposition of TsNBn2, another control experiment was also performed. 0.5 equiv. of TsNBn2 was added to the Na2CO3-induced reaction, and it could be recovered in 94% yield after 5 h, suggesting that HNBn2 is not derived from TsNBn2 (Scheme 3c).
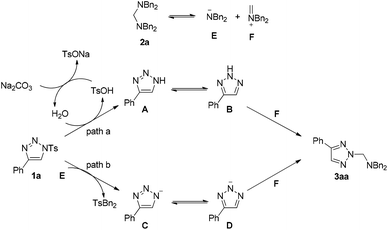
Based on the above results, two possible reaction pathways using substrates 1a and 2a as models are outlined in Scheme 4. As for substrate 2a, it will decompose to give intermediates E and F. When Na2CO3 is used as an additive, substrate 1a will decompose to form intermediate A in the presence of a trace amount of H2O in the solvent. TsOH is obtained at the same time, which can be neutralized by Na2CO3 to deliver H2O again. Intermediate A can be converted into its resonance B, which can react with intermediate F to give the product 3aa (path a). If DABCO is used as an additive, substrate 1a reacts with E to give intermediate C and TsNBn2. Intermediate D, derived from C, can also react with F to give the product 3aa (path b).
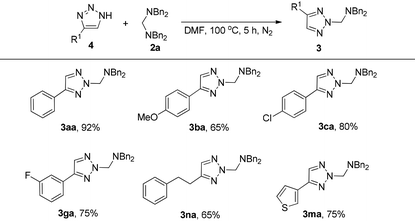
The investigations on the reaction mechanism suggested that the in situ generated 4-phenyl-1H-1,2,3-triazole could also react with 2a to give a similar product. As we expected, substrate 4a can react with 2a smoothly even in the absence of DABCO to give the product 3aa in 92% yield. This result also suggested that intermediate B is more stable than A. For substrates with electron-donating or -withdrawing groups on the aromatic ring, the reaction proceeded efficiently to give the desired products 3ba, 3ca and 3ga in 65%, 80% and 75% yields, respectively. Meanwhile, for the triazole with an aliphatic substituent, the corresponding product 3na was formed in 65% yield. In addition, in the case of thiophene-tethered triazole 4m, the reaction also proceeded smoothly to afford the desired product 3ma in 75% yield (Table 3).
| a Conditions: 4 (0.20 mmol) and 2 (0.20 mmol) were heated in DMF (2.0 ml) at 100 °C for 5 h. |
|---|
|
In summary, a new base-induced reaction mode of N-sulfonyl-1,2,3-triazoles has been established, thereby providing a simple procedure for preparation of triazoles bearing dialkylaminomethyl groups. In this reaction, a wide range of substrates can tolerate this reaction. Moreover, 4-phenyl-1H-1,2,3-triazoles can also give the same product even in the absence of a base. The potential applications and extension of the substrate scope of this novel synthetic methodology are currently underway in our laboratory.
Acknowledgements
We are grateful for the financial support from the National Basic Research Program of China (973)-2015CB856603 and the National Natural Science Foundation of China (20472096, 21372241, 21361140350, 20672127, 21102166, 21121062, 21302203, 20732008 and 21572052).Notes and references
- For selected reviews, see: (a) W. Q. Fan and A. R. Katritzky, in Comprehensive Heterocyclic Chemistry II, ed, A. R. Katritzky, Pergamon Press, Oxford, 1996, vol. 4, p. 1 Search PubMed; (b) T. Muller and S. Bräse, Angew. Chem., Int. Ed., 2011, 50, 11844 CrossRef CAS PubMed; (c) P. Thirumurugan, D. Matosiuk and K. Jozwiak, Chem. Rev., 2013, 113, 4905 CrossRef CAS PubMed.
- (a) Y. Liu, X. Wang, J. Xu, Q. Zhang, Y. Zhao and Y. Hu, Tetrahedron, 2011, 67, 6294 CrossRef CAS; (b) J. Raushel and V. V. Fokin, Org. Lett., 2010, 12, 4952 CrossRef CAS PubMed; (c) E. J. Yoo, M. Ahlquist, S. H. Kim, I. Bae, V. V. Fokin, K. B. Sharpless and S. Chang, Angew. Chem., Int. Ed., 2007, 46, 1730 CrossRef CAS PubMed.
- For examples on the previous reviews: (a) B. Chattopadhyay and V. Gevorgyan, Angew. Chem., Int. Ed., 2012, 51, 862 CrossRef CAS PubMed; (b) A. V. Gulevich and V. Gevorgyan, Angew. Chem., Int. Ed., 2013, 52, 1371 CrossRef CAS PubMed; (c) H. M. L. Davie and J. S. Alford, Chem. Soc. Rev., 2014, 43, 5151 RSC; (d) Y. Wang, X. Lei and Y. Tang, Synlett, 2015, 2051 Search PubMed.
- (a) N. Selander, B. T. Worrell, S. Chuprakov, S. Velaparthi and V. V. Fokin, J. Am. Chem. Soc., 2012, 134, 14670 CrossRef CAS PubMed; (b) J. C. Culhane and V. V. Fokin, Org. Lett., 2011, 13, 4578 CrossRef CAS PubMed; (c) J. S. Alford and H. M. L. Davies, Org. Lett., 2012, 14, 6020 CrossRef CAS PubMed; (d) N. Grimster, L. Zhang and V. V. Fokin, J. Am. Chem. Soc., 2010, 132, 2510 CrossRef CAS PubMed; (e) S. Chuprakov, S. W. Kwok, L. Zhang, L. Lercher and V. V. Fokin, J. Am. Chem. Soc., 2009, 131, 18034 CrossRef CAS PubMed.
- (a) S. Chuprakov, J. A. Malik, M. Zibinsky and V. V. Fokin, J. Am. Chem. Soc., 2011, 133, 10352 CrossRef CAS PubMed; (b) S. Chuprakov, B. T. Worrell, N. Selander, R. K. Sit and V. V. Fokin, J. Am. Chem. Soc., 2014, 136, 195 CrossRef CAS PubMed; (c) T. Miura, T. Tanaka, T. Biyajima, A. Yada and M. Murakami, Angew. Chem., Int. Ed., 2013, 52, 3883 CrossRef CAS PubMed; (d) T. Miura, T. Biyajima, T. Fujii and M. Murakami, J. Am. Chem. Soc., 2012, 134, 194 CrossRef CAS PubMed.
- (a) A. Boyer, Org. Lett., 2014, 16, 1660 CrossRef CAS PubMed; (b) T. Miura, Y. Funakoshi, M. Morimoto, T. Biyajima and M. Murakami, J. Am. Chem. Soc., 2012, 134, 17440 CrossRef CAS PubMed; (c) N. Selander, B. T. Worrell and V. V. Fokin, Angew. Chem., Int. Ed., 2012, 51, 13054 CrossRef CAS PubMed.
- (a) S. Chuprakov, F. W. Hwang and V. Gevorgyan, Angew. Chem., Int. Ed., 2007, 46, 4757 CrossRef CAS PubMed; (b) T. Horneff, S. Chuprakov, N. Chernyak, V. Gevorgyan and V. V. Fokin, J. Am. Chem. Soc., 2008, 130, 14972 CrossRef CAS PubMed; (c) T. Miura, M. Yamauchi and M. Murakami, Chem. Commun., 2009, 1470 RSC; (d) B. Chattopadhyay and V. Gevorgyan, Org. Lett., 2011, 13, 3746 CrossRef CAS PubMed; (e) M. Zibinsky and V. V. Fokin, Angew. Chem., Int. Ed., 2013, 52, 1507 CrossRef CAS PubMed; (f) S. Chuprakov, S. W. Kwok and V. V. Fokin, J. Am. Chem. Soc., 2013, 135, 4652 CrossRef CAS PubMed; (g) B. T. Parr, S. A. Green and H. M. L. Davies, J. Am. Chem. Soc., 2013, 135, 4716 CrossRef CAS PubMed; (h) J. E. Spangler and H. M. L. Davies, J. Am. Chem. Soc., 2013, 135, 6802 CrossRef CAS PubMed; (i) Y. Shi and V. Gevorgyan, Org. Lett., 2013, 15, 5394 CrossRef CAS PubMed; (j) T. Miura, T. Tanaka, K. Hiraga, S. G. Stewart and M. Murakami, J. Am. Chem. Soc., 2013, 135, 13652 CrossRef CAS PubMed; (k) B. T. Parr and H. M. L. Davies, Angew. Chem., Int. Ed., 2013, 52, 10044 CrossRef CAS PubMed; (l) T. Miura, T. Tanaka, A. Yada and M. Murakami, Chem. Lett., 2013, 42, 1308 CrossRef CAS; (m) H. Shang, Y. Wang, Y. Tian, J. Feng and Y. Tang, Angew. Chem., Int. Ed., 2014, 53, 5662 CrossRef CAS PubMed; (n) J. S. Alford and H. M. L. Davies, J. Am. Chem. Soc., 2014, 136, 10266 CrossRef CAS PubMed; (o) D. J. Jung, H. J. Jeon, J. H. Kim, Y. Kim and S. Lee, Org. Lett., 2014, 16, 2208 CrossRef CAS PubMed; (p) C.-E. Kim, S. Park, D. Eom, B. Seo and P. H. Lee, Org. Lett., 2014, 16, 1900 CrossRef CAS PubMed; (q) R.-Q. Ran, J. He, S.-D. Xiu, K.-B. Wang and C.-Y. Li, Org. Lett., 2014, 16, 3704 CrossRef CAS PubMed; (r) T. Miura, Y. Funakoshi, T. Tanaka and M. Murakami, Org. Lett., 2014, 16, 2760 CrossRef CAS PubMed; (s) F. Medina, C. Besnard and J. Lacour, Org. Lett., 2014, 16, 3232 CrossRef CAS PubMed; (t) D. J. Lee, H. S. Han, J. Shin and E. J. Yoo, J. Am. Chem. Soc., 2014, 136, 11606 CrossRef CAS PubMed; (u) X. Ma, S. Pan, H. Wang and W. Chen, Org. Lett., 2014, 16, 4554 CrossRef CAS PubMed; (v) Y.-Z. Zhao, H.-B. Yang, X.-Y. Tang and M. Shi, Chem. – Eur. J., 2015, 21, 3562 CrossRef CAS PubMed; (w) T. Miura, Y. Fujimoto, Y. Funakoshi and M. Murakami, Angew. Chem., Int. Ed., 2015, 54, 9967 CrossRef CAS PubMed.
- (a) J. S. Alford, J. E. Spangler and H. M. L. Davies, J. Am. Chem. Soc., 2013, 135, 11712 CrossRef CAS PubMed; (b) E. E. Schultz and R. Sarpong, J. Am. Chem. Soc., 2013, 135, 4696 CrossRef CAS PubMed; (c) Y. Shi and V. Gevorgyan, Org. Lett., 2013, 15, 5394 CrossRef CAS PubMed; (d) T. Miura, Y. Funakoshi and M. Murakami, J. Am. Chem. Soc., 2014, 136, 2272 CrossRef CAS PubMed; (e) B. Rajagopal, C.-H. Chou, C.-C. Chung and P.-C. Lin, Org. Lett., 2014, 16, 3752 CrossRef CAS PubMed; (f) E. E. Schultz, V. N. G. Lindsay and R. Sarpong, Angew. Chem., Int. Ed., 2014, 53, 9904 CrossRef CAS PubMed; (g) J. Fu, H. Shen, Y. Chang, C. Li, J. Gong and Z. Yang, Chem. – Eur. J., 2014, 20, 12881 CrossRef CAS PubMed; (h) J.-M. Yang, C.-Z. Zhu, X.-Y. Tang and M. Shi, Angew. Chem., Int. Ed., 2014, 53, 5142 CAS; (i) Y.-S. Zhang, X.-Y. Tang and M. Shi, Chem. Commun., 2014, 50, 15971 RSC; (j) X.-Y. Tang, Y.-S. Zhang, L. He, Y. Wei and M. Shi, Chem. Commun., 2015, 51, 133 RSC; (k) Y. Jiang, X.-Y. Tang and M. Shi, Chem. Commun., 2015, 51, 2122 RSC; (l) Y.-S. Zhang, X.-Y. Tang and M. Shi, Org. Chem. Front., 2015, 2, 1516 RSC; (m) J. He, Y. Shi, W. Cheng, Z. Man, D. Yang and C.-Y. Li, Angew. Chem., Int. Ed., 2016, 55, 4557 CrossRef CAS PubMed; (n) R. Sun, Y. Jiang, X.-Y. Tang and M. Shi, Chem. – Eur. J., 2016, 22, 5727 CrossRef CAS PubMed.
- (a) Y. Jiang, R. Sun, Q. Wang, X.-Y. Tang and M. Shi, Chem. Commun., 2015, 51, 16968 RSC; (b) K. Chen, Z.-Z. Zhu, Y.-S. Zhang, X.-Y. Tang and M. Shi, Angew. Chem., Int. Ed., 2014, 53, 6645 CrossRef CAS PubMed.
- For recent reviews on N-sulfonyl ketenimine chemistry: (a) S. H. Kim, S. H. Park, J. H. Choi and S. Chang, Chem. – Asian J., 2011, 6, 2618 CrossRef CAS PubMed; (b) P. Lu and Y. Wang, Synlett, 2010, 165 CAS; (c) E. J. Yoo and S. Chang, Curr. Org. Chem., 2009, 13, 1766 CrossRef CAS; (d) P. Lu and Y. Wang, Chem. Soc. Rev., 2012, 41, 5687 RSC.
- (a) S. H. Cho, E. J. Yoo, I. Bae and S. Chang, J. Am. Chem. Soc., 2005, 127, 16046 CrossRef CAS PubMed; (b) E. J. Yoo, I. Bae, S. H. Cho, H. Han and S. Chang, Org. Lett., 2006, 8, 1347 CrossRef CAS PubMed; (c) I. Bae, H. Han and S. Chang, J. Am. Chem. Soc., 2005, 127, 2038 CrossRef CAS PubMed; (d) S. L. Cui, J. Wang and Y. Wang, Org. Lett., 2007, 9, 5023 CrossRef CAS PubMed; (e) S. L. Cui, J. Wang and Y. Wang, Tetrahedron, 2008, 64, 487 CrossRef CAS; (f) E. J. Yoo and S. Chang, Org. Lett., 2008, 10, 1163 CrossRef CAS PubMed; (g) S. H. Cho and S. Chang, Angew. Chem., Int. Ed., 2008, 47, 2836 CrossRef CAS PubMed; (h) J. Kim, Y. Lee, J. Lee, Y. Do and S. Chang, J. Org. Chem., 2008, 73, 9454 CrossRef CAS PubMed; (i) J. Y. Kim, S. H. Kim and S. Chang, Tetrahedron Lett., 2008, 49, 1745 CrossRef CAS; (j) J. She, Z. Jiang and Y. Wang, Synlett, 2009, 2023 CAS; (k) J. Wang, J. Wang, Y. Zhu, P. Lu and Y. Wang, Chem. Commun., 2011, 47, 3275 RSC; (l) Z. Chen, D. Zheng and J. Wu, Org. Lett., 2011, 13, 848 CrossRef CAS PubMed; (m) L. Sun, Y. Zhu, P. Lu and Y. Wang, Org. Lett., 2013, 15, 5894 CrossRef CAS PubMed; (n) H.-D. Xu, Z.-J. Jia, K. Xu, M. Han, S.-N. Jiang, J. Cao, J.-C. Wang and M.-H. Shen, Angew. Chem., Int. Ed., 2014, 53, 9284 CrossRef CAS PubMed.
- (a) A. Van Camp, D. Gossens, M. Moya-Portuguez, J. Marchand-Brynaert and L. Ghosez, Tetrahedron Lett., 1980, 21, 3081 CrossRef CAS; (b) M. Whiting and V. V. Fokin, Angew. Chem., Int. Ed., 2006, 45, 3157 CrossRef CAS PubMed; (c) W. Lu, W. Z. Song, D. Hong, P. Lu and Y. Wang, Adv. Synth. Catal., 2009, 351, 1768 CrossRef CAS; (d) S.-Y. Li, Y. Luo and J. Wu, Org. Lett., 2011, 13, 4312 CrossRef CAS PubMed; (e) Y. Xing, H. Zhao, Q. Shang, J. Wang, P. Lu and Y. Wang, Org. Lett., 2013, 15, 2668 CrossRef CAS PubMed; (f) S. Li, Y. Luo and J. Wu, Org. Lett., 2011, 13, 3190 CrossRef CAS PubMed.
- R. Sun, D.-. H. Zhang and M. Shi, Synlett, 2014, 2293 CAS.
- For the example of base-promoted 1,2-rearrangement of N-sulfonyl-1,2,3-triazoles: M. Yamauchi, T. Miura and M. Murakami, Heterocycles, 2010, 80, 177 CrossRef CAS.
- For the example of synthesis of 2-substituted-1,2,3-triazoles: J. Kalisiak, K. B. Sharpless and V. V. Fokin, Org. Lett., 2008, 10, 3171 CrossRef CAS PubMed.
- For several examples on the applications of bis(dialkylamino)methane: (a) B. E. Love, J. Org. Chem., 2007, 72, 630 CrossRef CAS PubMed; (b) Y. Xie, J. Hu, Y. Wang, C. Xia and H. Huang, J. Am. Chem. Soc., 2012, 134, 20613 CrossRef CAS PubMed; (c) Y. Xie, J. Hu, P. Xie, B. Qian and H. Huang, J. Am. Chem. Soc., 2013, 135, 18327 CrossRef CAS PubMed; (d) J. Hu, Y. Xie and H. Huang, Angew. Chem., Int. Ed., 2014, 53, 7272 CrossRef CAS PubMed; (e) G. Qin, L. Li, J. Li and H. Huang, J. Am. Chem. Soc., 2015, 137, 12490 CrossRef CAS PubMed.
- The crystal data of 3ha have been given in CCDC 1465122.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization data of new compounds. CCDC 1465122. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6qo00102e |
| This journal is © the Partner Organisations 2016 |