Annulation based on 8-aminoquinoline assisted C–H activation: an emerging tool in N-heterocycle construction
Jie-Ping
Wan
,
Yi
Li
and
Yunyun
Liu
*
College of Chemistry and Chemical Engineering, Jiangxi Normal University, Nanchang 330022, P. R. China. E-mail: chemliuyunyun@jxnu.edu.cn
First published on 28th March 2016
Abstract
The synthesis of heterocyclic products via C–H activation has won impressive advances as a newly emerging synthetic strategy. Herein, the recent research advances in heterocycle synthesis employing 8-aminoquinoline directed C–H activation, either in the form of direct intramolecular C–H elaboration or domino reactions involving the C–H activation and other transformation-based annulation reactions are highlighted.
Since the pioneering work of Daugulis and co-workers on the C–H arylation of amides containing an N-quinolin-8-yl bidentate auxiliary,1 C–H activation reactions employing 8-aminoquinoline (AQ) as a directing group (DG) have rapidly evolved as the general protocol for the elaboration of alkyl, alkenyl and aryl C–H bonds, demonstrating the powerful application potential of this DG in C–H activation-based organic syntheses.2 According to the survey on the literature of transition metal-catalyzed and AQ-assisted C–H activation reactions and syntheses, the generation of linear structures was the main stream reaction pattern. On the other hand, the construction of a ring structure is known to be the crucial application area of cross coupling reactions,3 suggesting the high desire for developing efficient synthetic methodologies towards heterocycle synthesis via AQ-assisted C–H activation.
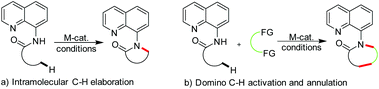
In recently years, following the rapid progress of AQ-assisted C–H activation in both the catalytic technology and in-depth mechanism understanding, the synthesis of heterocyclic products via AQ-assisted C–H activation has received significant advances. In general, ring construction via this AQ-assisted C–H activation can be achieved either by means of direct intramolecular C–H elaboration or domino reactions in the presence of other reaction partner(s) (Fig. 1). The seminal work on representative direct intramolecular C–H/N–H coupling annulation giving lactams via formal oxidative coupling reported by Chen,4 Kuninobu5 and Ge,6 has been introduced in Lei's recent reviews (Fig. 1a).3 Herein, the domino annulation reaction involving key C–H activation assisted by AQ, a different strategy of ring construction, is highlighted (Fig. 1b). In addition, the rather recent breakthrough in the direct intramolecular C–H amidation providing lactams is also updated.
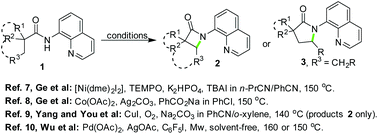
On the basis of their previous work on copper-catalyzed intramolecular C–H amidation, Ge and co-workers recently realized Ni-7 and Co-catalyzed8 intramolecular oxidative coupling of alkyl amides 1 for the synthesis of lactams 2 or 3, respectively (Scheme 1). These new catalytic approaches complemented the synthetic routes to these interesting products with an extended application scope. On the other hand, Yang and You et al.9 reported a similar reaction via oxidation using molecular oxygen and copper catalysis, and various β-lactams 2 were synthesized. The main feature of this alternative approach was the application of oxygen as a cleaner oxidant (Scheme 1). More recently, Wu and co-workers10a established a Pd/Ag co-catalyzed version of the reaction for the synthesis of 2 in the forms of both mono-cyclic and fused cyclic structures by employing C5F5I as a key additive. One of the notable points of the work was the excellent diastereoselectivity when the ring-fused lactams were synthesized (Scheme 1). This protocol was later successfully used for the stereoselective synthesis of bicyclic β-lactams using optically active starting materials.10b
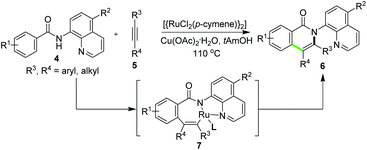
While direct intramolecular annulation provides a concise route to β- or γ-lactams, the synthesis of other ring structures, however, were not accessible by using this tactic. In this context, the intermolecular versions wherein a reaction partner was involved in the AQ-assisted C–H elaboration and subsequent annulation were more powerful in terms of diversity-oriented heterocycle synthesis. In 2014, Swamy and Allu11 reported the reactions of N-quinolin-8-yl-benzamides 4 with internal alkynes 5 for the synthesis of isoquinolones 6. The target products were proposed to be constructed via Ru-catalyzed aryl C–H alkenylation and reductive elimination through the possible key intermediate 7 (Scheme 2).
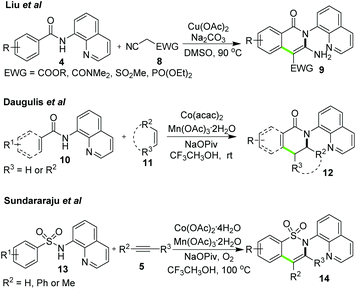
For the synthesis of similar isoquinolone derivatives, Liu et al.12 reported copper-catalyzed C–H alkylation-based domino reactions using cyano functionalized methylene substrates 8 as reaction partners of N-quinolin-8-yl-benzamides 4. The domino reactions provided amino functionalized isoquinolones 9 (Scheme 3). On the other hand, the direct synthesis of dihydroisoquinolinones 12 was realized by Daugulis et al.13 with domino reactions involving AQ-assisted C(sp2)–H alkenylation of N-AQ amides 10 and subsequent intramolecular addition using alkenes 11. The reactions proceeded smoothly at room temperature in the Co(acac)2/Mn(OAc)3 co-catalyst system with aerobic oxidation (Scheme 3). In addition, the application of N-AQ sulfonamides 13 which were structurally analogous with N-AQ benzamides was also successfully achieved enabling the synthesis of equivalent cyclic sultams 14via cobalt-catalyzed domino reactions of terminal/internal alkenes (Scheme 3).14
 | ||
| Scheme 3 Domino reactions involving AQ-assisted C–H activation for the synthesis of isoquinolinones and dihydroisoquinolinones. | ||
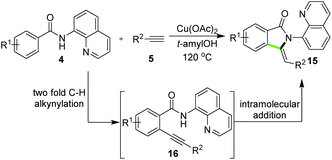
Besides the synthesis of six-membered heterocycles, the reactions of N-AQ-amides and alkynes also displayed applicability in the synthesis of five-membered ring systems in a diverse fashion. You and co-workers15 disclosed the synthesis of 3-methyleneisoindolin-1-ones 15via copper-catalyzed reactions of benzamides 4 and terminal alkynes 5. As outlined in Scheme 4, the catalysis of only Cu(OAc)2 allowed the construction of products 15via domino C–C and C–N bond formation, and the control experiment suggested that the C–H alkynylation generating intermediate 16 was a key transformation during the product formation (Scheme 4). Rather recently, a breakthrough on corresponding domino reactions involving C(sp3)–H bond alkynylation and annulation has been achieved for the synthesis of pyrrolidinones. As reported by Zhang and co-workers, the catalyst system consisting of Co(OAc)2·4H2O, Ag2CO3, TBAI, pyridine and Na2CO3 in PhCF3 enabled the direct synthesis of pyrrolidinones 18 by using amides 17 and terminal alkynes. Besides 18, the synthesis of isoindolinones 15 could also be synthesized under slightly modified cobalt-catalytic conditions using benzamides 4 (Scheme 5).16 More comprehensive exploration by the same group disclosed that copper catalyst Cu(OAc)2·H2O could also catalyze the reactions with 17 and 5 to provide 18 in the presence of Ag2CO3, TBAI in DMF. In addition, the approach of copper catalysis was applicable for the synthesis of 18 and 15 by employing alkynyl carboxylic acids as alternative substrates of terminal alkynes.17
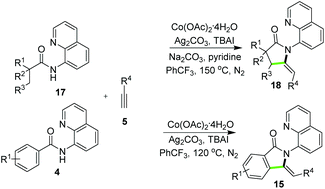 | ||
| Scheme 5 AQ-assisted C(sp3)–H activation for the domino synthesis of pyrrolidinones and 3-methyleneisoindolinones. | ||
Also in 2015, Ge and co-workers reported the Ni/Cu co-catalyzed C–H activation-based domino reactions of amides 4/17 with DMF as a facile method to access isoindoline-1,3-diones 19 and succinimides 20, respectively.18 In these reactions, DMF acted as the carbonyl source by generating iminium 21via catalytic oxidation with CuII/O2; on the other hand, the formation of Ni-complex 22 allowed the production of intermediate 23via direct C–H amination. The subsequent occurrence of other key intermediates 24–27 were proposed as crucial steps for yielding final products 19 or 20 (Scheme 6).
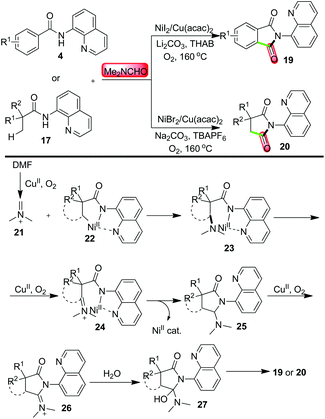 | ||
| Scheme 6 Synthesis of imides via Ni/Cu-co-catalyzed C–H carbonylation using DMF as the carbonyl source. | ||
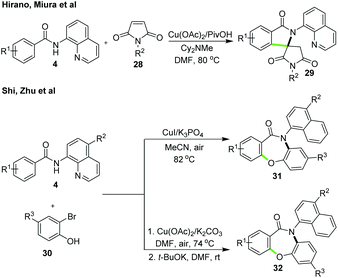
In the synthesis of more diverse N-containing cyclic scaffolds, other reaction partners have also been employed. Hirano and Miura et al.19 accomplished the domino reactions providing isoindolinone-spirosuccinimides 29via copper-catalyzed, AQ-assisted C–H olefination and intramolecular N-nucleophilic addition by using benzamides 4 and maleimides 28 (Scheme 7). On the other hand, by means of copper-catalyzed cascade reactions involving the key C–H esterification, Shi and Zhu et al.20 designed the synthesis of seven-membered polycyclic dibenzoxazepinones 31 and 32via the assemblies of benzamides 4 and o-bromophenols 30. The catalysis of CuI in the presence of K3PO4 gave normal products 31 in acetonitrile via domino C–H esterification and Goldberg-type C–N cross coupling. And the modified catalysis of Cu(OAc)2 in the presence of K2CO3 as well as the subsequent treatment with t-BuOK led to the formation of rearranged products 32via the sequence of C–H esterification and Smiles rearrangement (Scheme 7).
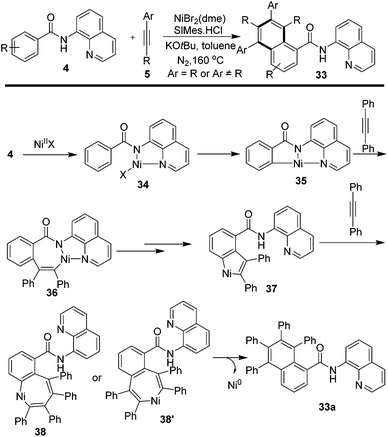
A different annulation approach leading to the construction of a naphthalene moiety was reported by Chatani et al.21via the reactions of N-quinolinyl benzamides 4 and internal alkynes. With the catalysis of NiBr2(dme), a class of napthamides 33 were readily synthesized by heating at 160 °C under nitrogen atmosphere. Mixed regioisomers of 33 were provided when unsymmetrical internal alkynes were employed. Based on the clues from related control experiments, the reactions were proposed to run via Ni-complex intermediates 34 and 35. And further incorporation to alkynes leading to intermediates 36 and 37 afforded seven-membered cyclic intermediate 38 or 38′ which yielded target product 33 by releasing Ni0. The regeneration of Ni0 to intermediates 35 with the assistance of alkyne enabled the recyclization of the nickel catalyst (Scheme 8).
Conclusions
In summary, by making use of AQ-assisted C–H activation, the syntheses of N-containing heterocycles have won important new advances by means of the strategies of both direct intramolecular C–H amidation and domino reactions consisting of C–H elaboration and other transformation-based annulation. While the recent advances in these research studies indicate the promising future of such synthetic methodologies, it should be pointed out that the limited diversity of products which is predetermined by the restricted reaction partners of AQ-amides is still the main challenge at the current stage. Therefore, in order to prompt C–H activation-based domino reaction as a general strategy toward heterocycle synthesis, searching for more different reaction partners for AQ-amides to enable the synthesis of more structurally diverse products constitutes the contemporary major task in this research area.Acknowledgements
Financial support from the National Natural Science Foundation of China (21562024 and 21562025) is gratefully acknowledged.Notes and references
- V. G. Zaitsev, D. Shabashov and O. Daugulis, J. Am. Chem. Soc., 2005, 127, 13154 CrossRef CAS PubMed.
- (a) M. Corbet and F. De Campo, Angew. Chem., Int. Ed., 2013, 52, 9896 CrossRef CAS PubMed; (b) G. Rouquet and N. Chatani, Angew. Chem., Int. Ed., 2013, 52, 11726 CrossRef CAS PubMed; (c) O. Daugulis, J. Roane and L. D. Tran, Acc. Chem. Res., 2015, 48, 1053 CrossRef CAS PubMed.
- J. Yuan, C. Liu and A. Lei, Chem. Commun., 2015, 51, 1394 RSC.
- G. He, S.-Y. Zhang, W. A. Nack, Q. Li and G. Chen, Angew. Chem., Int. Ed., 2013, 52, 11124 CrossRef CAS PubMed.
- Z. Wang, J. Ni, Y. Kuninobu and M. Kanai, Angew. Chem., Int. Ed., 2014, 53, 3496 CrossRef CAS PubMed.
- X. Wu, Y. Zhao, G. Zhang and H. Ge, Angew. Chem., Int. Ed., 2014, 53, 3706 CrossRef CAS PubMed.
- X. Wu, Y. Zhao and H. Ge, Chem. – Eur. J., 2014, 20, 9530 CrossRef CAS PubMed.
- X. Wu, K. Yang, Y. Zhao, H. Sun, G. Li and H. Ge, Nat. Commun., 2015, 6, 6462 CrossRef CAS PubMed.
- C. Wang, Y. Yang, D. Qin, Z. He and J. You, J. Org. Chem., 2015, 80, 8424 CrossRef CAS PubMed.
- (a) W.-W. Sun, P. Cao, R.-Q. Mei, Y. Li, Y.-L. Ma and B. Wu, Org. Lett., 2014, 16, 480 CrossRef CAS PubMed; (b) S.-J. Zhang, W.-W. Sun, P. Cao, X.-P. Dong, J.-K. Liu and B. Wu, J. Org. Chem., 2016, 81, 956 CrossRef CAS PubMed.
- S. Allu and K. C. K. Swamy, J. Org. Chem., 2014, 79, 3963 CrossRef CAS PubMed.
- W. Zhu, D. Zhang, N. Yang and H. Liu, Chem. Commun., 2014, 50, 10634 RSC.
- L. Grigorjeva and O. Daugulis, Org. Lett., 2014, 16, 4684 CrossRef CAS PubMed.
- D. Kalsi and B. Sundararaju, Org. Lett., 2015, 17, 6118 CrossRef CAS PubMed.
- J. Dong, F. Wang and J. You, Org. Lett., 2014, 16, 2884 CrossRef CAS PubMed.
- J. Zhang, H. Chen, C. Lin, Z. Liu, C. Wang and Y. Zhang, J. Am. Chem. Soc., 2015, 137, 12990 CrossRef CAS PubMed.
- J. Zhang, D. Li, H. Chen, B. Wang, Z. Liu and Y. Zhang, Adv. Synth. Catal., 2016, 358, 792 CrossRef CAS.
- X. Wu, Y. Zhao and H. Ge, J. Am. Chem. Soc., 2015, 137, 4924 CrossRef CAS PubMed.
- W. Miura, K. Hirano and M. Miura, Org. Lett., 2015, 17, 4034 CrossRef CAS PubMed.
- Y. Zhou, J. Zhu, B. Li, Y. Zhang, J. Feng, A. Hall and J. Shi, Org. Lett., 2016, 18, 380 CrossRef CAS PubMed.
- L. C. M. Castro, A. Obata, Y. Aihara and N. Chatani, Chem. – Eur. J., 2016, 22, 1362 CrossRef PubMed.
| This journal is © the Partner Organisations 2016 |