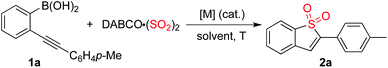Copper(I)-catalyzed sulfonylation of (2-alkynylaryl)boronic acids with DABSO†
Runyu
Mao
a,
Danqing
Zheng
a,
Hongguang
Xia
*b and
Jie
Wu
*ac
aDepartment of Chemistry, Fudan University, 220 Handan Road, Shanghai 200433, China. E-mail: jie_wu@fudan.edu.cn
bDepartment of Biochemistry and Molecular Biology, Zhejiang University School of Medicine, Hangzhou 310058, China. E-mail: hongguangxia@zju.edu.cn
cState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 354 Fenglin Road, Shanghai 200032, China
First published on 21st March 2016
Abstract
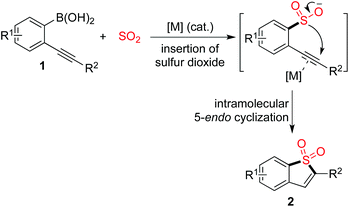
The scaffold of benzo[b]thiophene 1,1-dioxides can be easily constructed through a copper(I)-catalyzed insertion of sulfur dioxide into (2-alkynylaryl)boronic acids. The reaction proceeds in the presence of 10 mol% copper(I) acetate in DMF at 100 °C with high efficiency, leading to benzo[b]thiophene 1,1-dioxides in good to excellent yields. The sulfonyl group can be easily introduced via insertion of sulfur dioxide and the subsequent intramolecular 5-endo cyclization affords the core of benzo[b]thiophene 1,1-dioxide.
It is well known that sulfones are widely present in pharmaceutical and agrochemical molecules.1,2 Among cyclic sulfonyl compounds, the scaffold of benzo[b]thiophene 1,1-dioxide has attracted much attention. It was reported that a benzo[b]thiophene 1,1-dioxide derivative could act as an absorbance and fluorescence switch, leading to a multi-addressable system in the presence of ions, protons and light.3 Additionally, compounds with the benzo[b]thiophene 1,1-dioxide core have been applied in the drug discovery process as inhibitors of hepatitis C virus NS5B polymerase, as well as carbonic anhydrase inhibitors for the inhibition of cytosolic/tumor-associated carbonic anhydrase isozymes.4 Moreover, benzo[b]thiophene 1,1-dioxides are useful synthons for the generation of complex molecules in organic synthesis.5 Therefore, it is highly desirable to provide efficient routes to diverse benzo[b]thiophene 1,1-dioxides.
Introduction of the sulfonyl unit into small molecules via insertion of sulfur dioxide is promising and attractive in organic synthesis, due to the enormous scale of annual production.6 In the past few years, much attention has been paid to this area, and advancements in the insertion of sulfur dioxide into small molecules have been witnessed.7–10 For instance, transition-metal-catalyzed or metal-free aminosulfonylation via a three-component reaction of aryl electrophiles, sulfur dioxide and hydrazines could afford N-aminosulfonamides.8a,j In most cases, DABCO-bis(sulfur dioxide) (DABSO) was used as the source of sulfur dioxide. This reagent was developed by Willis and co-workers to solve the handling problem of toxic gaseous sulfur dioxide.8a Sulfones could be generated through the reaction of organometallic reagents (such as Grignard reagents, organolithium reagents, organosilane reagents, organoboronic acids, and organozinc reagents) with sulfur dioxide and various electrophiles.9,10 For example, Toste and co-workers described the synthesis of sulfones through a gold(I)-catalyzed sulfination of arylboronic acids via insertion of sulfur dioxide.10c Willis subsequently reported a similar result using a palladium catalyst in the transformation.10d During the reaction process, a metal sulfinate was believed to be the key intermediate, which was generated via coupling of the arylboronic acid with sulfur dioxide. This intermediate then acted as a nucleophile to react with an electrophile (such as an alkyl halide, epoxide, or aryliodonium salt), providing the corresponding sulfones. Prompted by these results, we envisioned that benzo[b]thiophene 1,1-dioxides could be produced via the reaction of (2-alkynylaryl)boronic acids 1 with sulfur dioxide under appropriate conditions (Scheme 1). We conceived that the metal catalyst would play a dual role by (1) promoting the coupling of the arylboronic acid with sulfur dioxide to afford the metal sulfinate, and (2) activating the triple bond of the alkyne, thus facilitating the intramolecular attack of the metal sulfinate on the alkyne. Although benzo[b]thiophene 1,1-dioxides could also be generated from the reaction of 2-alkynylaryldiazonium tetrafluoroborates with sulfur dioxide in the presence of morpholin-4-amine,8m the advantages of the proposed route are obvious: (1) it avoids the utilization of 2-alkynylaryldiazonium tetrafluoroborates as starting materials, which are unstable and unsuitable for storage; and (2) it avoids the use of large amounts of morpholin-4-amine, which is much more atom economic.
Due to its operational ease and availability, DABCO·(SO2)2 (DABSO) was selected as the source of sulfur dioxide.7 So far, bench-stable solid DABSO has been widely utilized as a source of sulfur dioxide. Encouraged by recent advancements in the insertion of sulfur dioxide, we therefore started to explore the practicability of the projected route as shown in Scheme 1.
Initially, the reaction of (2-(p-tolylethynyl)phenyl)boronic acid 1a with DABSO was selected as the model for reaction development (Table 1). As mentioned above, it was anticipated that the metal catalyst would play a dual role of promoting the coupling of the arylboronic acid with sulfur dioxide as well as activating the triple bond of the alkyne. Thus, palladium salt was used as the catalyst at the outset. To our surprise, the desired product 2a was not detected when the reaction was catalyzed by 10 mol% palladium acetate in the presence of sodium acetate in 1,4-dioxane at 80 °C (Table 1, entry 1). Since the power of cooperative catalysis has been demonstrated,11 copper(I) bromide was added to the catalytic system with the expectation that it might promote the transformation (Table 1, entry 2). However, the result was the same. A similar result was observed when the solvent was changed to toluene or 1,2-dichloroethane (Table 1, entries 3 and 4). To our delight, the corresponding benzo[b]thiophene 1,1-dioxide 2a was obtained in 19% yield when the reaction was carried out in acetonitrile (Table 1, entry 5). Gratifyingly, the yield of product 2a was increased to 43% when DMF was used as the solvent (Table 1, entry 6). An inferior result was observed when the reaction temperature was changed to 60 °C (Table 1, entry 7). A better yield was obtained when the reaction was performed at 100 °C (Table 1, entry 8). The benzo[b]thiophene 1,1-dioxide 2a was isolated in 20% yield when palladium acetate was replaced by palladium chloride (Table 1, entry 9). Interestingly, a 64% yield of 2a was obtained in a control experiment without the addition of palladium catalyst (Table 1, entry 10). This result means that the copper catalyst is effective for the coupling of arylboronic acid with sulfur dioxide and meanwhile could activate the triple bond of the alkyne to facilitate the subsequent intramolecular 5-endo cyclization. We further screened other bases in this reaction. A similar yield was obtained when potassium acetate was used as a base (Table 1, entry 11). The yield could not be improved when potassium carbonate or sodium carbonate were employed (Table 1, entries 12 and 13). Since DMF could be regarded as a strong Lewis base, a control experiment was performed in the absence of base (Table 1, entry 14). Interestingly, the corresponding product 2a was generated without loss of yield (66%). We subsequently surveyed other copper catalysts. It was found that copper(I) acetate was the best choice (Table 1, entries 15–18), leading to the expected product in 75% yield. A similar yield was obtained when the amount of DABSO was decreased to 0.7 equivalents (Table 1, entry 19). To our delight, the desired product 2a was afforded in 91% yield when 2.0 equivalents of DABCO·(SO2)2 were employed in the reaction (Table 1, entry 20).
| Entry | [M] | Base | Solvent | T (°C) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: (2-(p-tolylethynyl)phenyl)boronic acid 1a (0.2 mmol), DABSO (0.2 mmol), palladium catalyst (10 mol%), copper catalyst (10 mol%), base (1.5 equiv.), solvent (2.0 mL), N2. b Isolated yield based on (2-(p-tolylethynyl)phenyl)boronic acid 1a. c In the presence of 0.7 equivalents of DABSO. d In the presence of 2.0 equivalents of DABSO. | |||||
| 1 | Pd(OAc)2 | NaOAc | Dioxane | 80 | n.d. |
| 2 | Pd(OAc)2/CuBr | NaOAc | Dioxane | 80 | n.d. |
| 3 | Pd(OAc)2/CuBr | NaOAc | PhMe | 80 | n.d. |
| 4 | Pd(OAc)2/CuBr | NaOAc | DCE | 80 | n.d. |
| 5 | Pd(OAc)2/CuBr | NaOAc | MeCN | 80 | 19 |
| 6 | Pd(OAc)2/CuBr | NaOAc | DMF | 80 | 43 |
| 7 | Pd(OAc)2/CuBr | NaOAc | DMF | 60 | 37 |
| 8 | Pd(OAc)2/CuBr | NaOAc | DMF | 100 | 55 |
| 9 | PdCl2/CuBr | NaOAc | DMF | 100 | 20 |
| 10 | CuBr | NaOAc | DMF | 100 | 64 |
| 11 | CuBr | KOAc | DMF | 100 | 60 |
| 12 | CuBr | K2CO3 | DMF | 100 | 51 |
| 13 | CuBr | Na2CO3 | DMF | 100 | 54 |
| 14 | CuBr | — | DMF | 100 | 66 |
| 15 | CuCl | — | DMF | 100 | 61 |
| 16 | Cu2O | — | DMF | 100 | 45 |
| 17 | CuOAc | — | DMF | 100 | 75 |
| 18 | Cu(OAc)2 | — | DMF | 100 | 56 |
| 19c | CuOAc | — | DMF | 100 | 73 |
| 20d | CuOAc | — | DMF | 100 | 91 |
The scope of the sulfonylation reaction of (2-alkynylaryl)boronic acids 1 with DABSO was then investigated under the above optimized reaction conditions (10 mol% copper(I) acetate, DMF, 100 °C). The results are shown in Table 2. A range of (2-alkynylaryl)boronic acids 1 were coupled with DABSO efficiently, leading to the corresponding benzo[b]thiophene 1,1-dioxides 2 in good to excellent yields. (2-Alkynylaryl)boronic acids 1 bearing electron-donating or electron-withdrawing groups on the aromatic ring were all good partners for the reaction with sulfur dioxide under the standard conditions. For instance, fluoro-substituted benzo[b]thiophene 1,1-dioxide 2l was generated in 95% yield. (2-Alkynylaryl)boronic acids 1 bearing different substituents attached to the triple bond were examined as well, and reacted efficiently to afford the corresponding products. The substituents included aryl, alkyl, and heterocyclic groups. For example, the reaction of thiophenyl-substituted (2-alkynylaryl)boronic acid provided the desired product 2h in 63% yield. It was noteworthy that the presence of a sulfur atom in the thiophenyl group did not deactivate the copper(I) catalyst.
A plausible mechanism was proposed, as shown in Scheme 2. We reasoned that a transmetallation of (2-alkynylaryl)boronic acid 1 with the copper catalyst would occur first, which would afford the intermediate A. Then, sulfur dioxide would be incorporated, leading to intermediate B. In the meantime, the presence of copper catalyst would activate the triple bond of intermediate B, which would then facilitate the subsequent intramolecular 5-endo cyclization to produce the corresponding benzo[b]thiophene 1,1-dioxide 2.
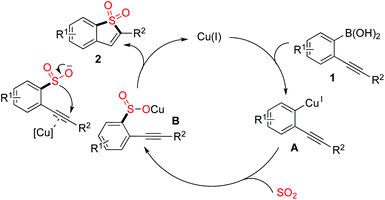 | ||
| Scheme 2 A plausible mechanism for the sulfonylation reaction of (2-alkynylaryl)boronic acids 1 with DABSO. | ||
In conclusion, we have described a facile route to benzo[b]thiophene 1,1-dioxides via a copper(I)-catalyzed insertion of sulfur dioxide into (2-alkynylaryl)boronic acids. The reaction proceeds in the presence of 10 mol% copper(I) acetate in DMF at 100 °C with high efficiency, leading to benzo[b]thiophene 1,1-dioxides in good to excellent yields. The sulfonyl group could be easily introduced with the formation of the benzo[b]thiophene 1,1-dioxide scaffold via insertion of sulfur dioxide and intramolecular 5-endo cyclization.
Financial support from the National Natural Science Foundation of China (no. 21372046 and 21532001) is gratefully acknowledged.
Notes and references
- (a) Y. Harrak, G. Casula, J. Basset, G. Rosell, S. Plescia, D. Raffa, M. G. Cusimano, R. Pouplana and M. D. Pujol, J. Med. Chem., 2010, 53, 6560 CrossRef PubMed; (b) D. A. Smith and R. M. Jones, Curr. Opin. Drug Discovery Dev., 2008, 11, 72 Search PubMed; (c) Y. Noutoshi, M. Ikeda, T. Saito, H. Osada and K. Shirasu, Front. Plant Sci., 2012, 3, 245 CrossRef PubMed.
- (a) M. Bartholow, Top 200 Drugs of 2011, Pharmacy Times, http://www.pharmacytimes.com/publications/issue/2012/July2012/Top-200-Drugs-of-2011, accessed on Jan 9, 2013; (b) For a list of top drugs by year, see: http://cbc.arizona.edu/njardarson/group/top-pharmaceuticals-poster, accessed on Jan 9, 2013; (c) J. Drews, Science, 2000, 287, 1960 CrossRef PubMed.
- (a) S. Chen, Y. Yang, Y. Wu, H. Tian and W. Zhu, J. Mater. Chem., 2012, 22, 5486 RSC; (b) S. Chen, L.-J. Chen, H.-B. Yang, H. Tian and W. Zhu, J. Am. Chem. Soc., 2012, 134, 13596 CrossRef PubMed.
- (a) S. H. Kim, M. T. Tran, F. Ruebsam, A. X. Xiang, B. Ayida, H. McGuire, D. Ellis, J. Blazel, C. V. Tran and D. E. Murphy, Bioorg. Med. Chem. Lett., 2008, 18, 4181 CrossRef PubMed; (b) A. Innocenti, R. Villar, V. Martinez-Merino, M. J. Gil, A. Scozzafava, D. Vullo and C. T. Supuran, Bioorg. Med. Chem. Lett., 2005, 15, 4872 CrossRef PubMed; (c) R. Villar, I. Encio, M. Migliaccio, M. J. Gil and V. Martinez-Merino, Bioorg. Med. Chem., 2004, 12, 963 CrossRef PubMed.
- (a) N. V. Lakshmi, P. Thirumurugan, C. Jayakumar and P. T. Perumal, Synlett, 2010, 955 Search PubMed; (b) N. Malatesti, A. N. Boa, S. Clark and R. Westwood, Tetrahedron Lett., 2006, 47, 5139 CrossRef; (c) K. Bougrin, M. Soufiaoui, A. Loupy and P. Jacquault, New J. Chem., 1995, 19, 213 Search PubMed.
- (a) S. D. Burke, in Encyclopedia of Reagents for Organic Synthesis, ed. L. A. Paquette, Wiley, Chichester, 1995, vol. 7, p. 4688 Search PubMed; (b) Z. Florjańczyk and D. Raducha, Pol. J. Chem., 1995, 69, 481 Search PubMed; (c) P. Vogel, M. Turks, L. Bouchez, D. Markovic, A. Varela-Álvarez and J. A. Sordo, Acc. Chem. Res., 2007, 40, 931 CrossRef CAS PubMed; (d) Ullmann's Encyclopedia of Industrial Chemistry, ed. B. Elvers, S. Hawkins and W. Russey, VCH, Weinheim, Germany, 5th edn, 1994, vol. A25 Search PubMed.
- For reviews: (a) G. Liu, C. Fan and J. Wu, Org. Biomol. Chem., 2015, 13, 1592 RSC; (b) P. Bisseret and N. Blanchard, Org. Biomol. Chem., 2013, 11, 5393 RSC; (c) A. S. Deeming, E. J. Emmett, C. S. Richards-Taylor and M. C. Willis, Synthesis, 2014, 2701 Search PubMed.
- (a) B. Nguyen, E. J. Emmet and M. C. Willis, J. Am. Chem. Soc., 2010, 132, 16372 CrossRef CAS PubMed; (b) E. J. Emmet, C. S. Garcia-Rubia, B. Nguyen, A. B. Garcia-Rubia, R. Hayter and M. C. Willis, Org. Biomol. Chem., 2012, 10, 4007 RSC; (c) L. Martial, Synlett, 2013, 1595 Search PubMed; (d) W. Li, H. Li, P. Langer, M. Beller and X.-F. Wu, Eur. J. Org. Chem., 2014, 3101 CrossRef; (e) W. Li, M. Beller and X.-F. Wu, Chem. Commun., 2014, 50, 9513 RSC; (f) X. Wang, L. Xue and Z. Wang, Org. Lett., 2014, 16, 4056 CrossRef CAS PubMed; (g) A. S. Deeming, C. J. Russell and M. C. Willis, Angew. Chem., Int. Ed., 2015, 54, 1168 CrossRef CAS PubMed; (h) S. Ye and J. Wu, Chem. Commun., 2012, 48, 7753 RSC; (i) S. Ye and J. Wu, Chem. Commun., 2012, 48, 10037 RSC; (j) D. Zheng, Y. An, Z. Li and J. Wu, Angew. Chem., Int. Ed., 2014, 53, 2451 CrossRef CAS PubMed; (k) S. Ye, H. Wang, Q. Xiao, Q. Ding and J. Wu, Adv. Synth. Catal., 2014, 356, 3225 CrossRef CAS; (l) Y. An, D. Zheng and J. Wu, Chem. Commun., 2014, 50, 11746 RSC; (m) Y. Luo, X. Pan, C. Chen, L. Yao and J. Wu, Chem. Commun., 2015, 51, 180 RSC; (n) D. Zheng, Y. Li, Y. An and J. Wu, Chem. Commun., 2014, 50, 8886 RSC; (o) A. S. Tsai, J. M. Curto, B. N. Rocke, A. R. Dechert-Schmitt, G. K. Ingle and V. Mascitti, Org. Lett., 2016, 18, 508 CrossRef CAS PubMed; (p) W. Zhang and M. Luo, Chem. Commun., 2016, 52, 2980 RSC.
- (a) C. C. Chen and J. Waser, Org. Lett., 2015, 17, 736 CrossRef CAS PubMed; (b) A. S. Deeming, C. J. Rusell, A. J. Hennessy and M. C. Willis, Org. Lett., 2014, 16, 150 CrossRef CAS PubMed; (c) B. N. Rocke, K. B. Bahnck, M. Herr, S. Lavergne, V. Mascitti, C. Perreault, J. Polivkova and A. Shavnya, Org. Lett., 2014, 16, 154 CrossRef CAS PubMed; (d) E. J. Emmett, B. R. Hayter and M. C. Willis, Angew. Chem., Int. Ed., 2013, 52, 12679 CrossRef CAS PubMed.
- (a) E. J. Emmett, B. R. Hayter and M. C. Willis, Angew. Chem., Int. Ed., 2014, 53, 10204 CrossRef CAS PubMed; (b) A. Shavnya, S. B. Coffey, A. C. Smith and V. Mascitti, Org. Lett., 2013, 15, 6226 CrossRef CAS PubMed; (c) M. W. Johnson, S. W. Bagley, N. P. Mankad, R. G. Bergman, V. Mascitti and F. D. Toste, Angew. Chem., Int. Ed., 2014, 53, 4404 CrossRef CAS PubMed; (d) A. Shavnya, K. D. Hesp, V. Mascitti and A. C. Smith, Angew. Chem., Int. Ed., 2015, 54, 13571 CrossRef CAS PubMed; (e) A. S. Deeming, C. J. Russell and M. C. Willis, Angew. Chem., Int. Ed., 2016, 55, 747 CrossRef CAS PubMed; (f) D. Zheng, R. Mao, Z. Li and J. Wu, Org. Chem. Front., 2016, 3, 359 RSC.
- For selected recent reviews on cooperative catalysts, see: (a) A. Ajamian and J. L. Gleason, Angew. Chem., Int. Ed., 2004, 43, 3754 CrossRef CAS PubMed; (b) J. M. Lee, Y. Na, H. Han and S. Chang, Chem. Soc. Rev., 2004, 33, 302 RSC; (c) J. C. Wasilke, O. S. J. Brey, R. T. Baker and G. C. Bazan, Chem. Rev., 2005, 105, 1001 CrossRef CAS PubMed; (d) D. Enders, C. Grondal and M. R. Huttl, Angew. Chem., Int. Ed., 2007, 46, 1570 CrossRef CAS PubMed; (e) C. J. Chapman and C. G. Frost, Synthesis, 2007, 1 CAS; (f) A. M. Walji and D. W. C. MacMillan, Synlett, 2007, 1477 CAS; (g) C. Wang and Z. Xi, Chem. Soc. Rev., 2007, 36, 1395 RSC; (h) Z. Shao and H. Zhang, Chem. Soc. Rev., 2009, 38, 2745 RSC; (i) C. Zhong and X. Shi, Eur. J. Org. Chem., 2010, 2999 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6qo00070c |
| This journal is © the Partner Organisations 2016 |