Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of 1,1-diboronate esters by cobalt-catalyzed sequential hydroboration of terminal alkynes†
Ziqing
Zuo
and
Zheng
Huang
*
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, 345 Lingling Road, Shanghai, 200032, China. E-mail: huangzh@sioc.ac.cn
First published on 26th January 2016
Abstract
A cobalt complex of iminopyridine-oxazoline catalyzes sequential hydroboration of alkyl and aryl alkynes with pinacolborane to form 1,1-diboronate esters. The reactions proceed under mild conditions with high yields, high regioselectivity, and wide functional group tolerance. The synthetic utility of 1,1-di(boronates) is demonstrated by chemoselective monoarylation and stepwise diarylation through palladium-catalyzed Suzuki–Miyaura coupling reactions.
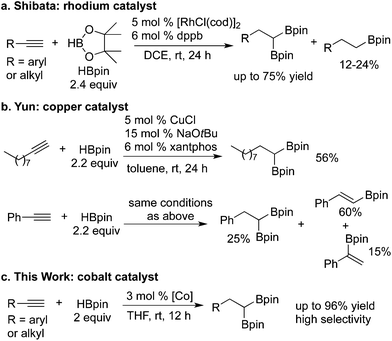
1,1-Organodiboronate esters are valuable synthetic intermediates for preparation of multifunctionalized molecules.1 Such 1,1-diboryl compounds can be used as coupling reagents for C–C bond formations through Suzuki–Miyaura reactions.2 Advantages of 1,1-diboronate esters over other 1,1-organodimetallic nucleophiles include their unique stability, operational simplicity, and non-toxicity.3,4 In addition, the boronate moiety can be readily converted into alcohol, amine, and other functional groups. Conventional, non-catalytic methods for synthesis of 1,1-diboronate esters involve reactions of lithiated reagents with bis(pinacolato)diboron,5 or hydroboration of terminal alkynes with a mixture of trichloride and trialkylsilane, followed by treatment with a suitable diol reagent.6 However, these methods suffer from poor functional-group compatibility, formation of waste inorganic salts, and multiple synthetic sequences. Recently, transition-metal-catalyzed methods have gained attention. For example, copper-catalyzed diborylation of 1,1-dibromoethane with bis(pinacolato)diboron formed 1,1-diborylethane in moderate yield.7,8 Hall2c and Yun9 reported copper-catalyzed enantioselective hydroboration of alkenylboron compounds with a 1,8-naphthalenediaminatoboryl substituent, furnishing 1,1-diboronate esters with high optical purity. Hartwig reported iridium-catalyzed diborylation of benzylic C–H bonds directed by a hydrosilyl group to form 1,1-benzyldiboronate esters.10 Platinum-catalyzed11 or metal-free12 carbene insertions into B–B bonds of diboron compounds have also been developed for preparation of 1,1-diboronate esters.
Due to high atom economy, easy access of starting materials, and mild reaction conditions, the catalytic sequential hydroboration of terminal alkynes is a synthetically useful approach to 1,1-diboronates. However, the sequential, regioselective hydroborations of the alkenylboronate intermediates are rare, and most reactions generate a regioisomeric mixture.13,14 In 2009, Shibata reported a rhodium-catalyzed sequential hydroboration of alkynes with pinacolborane (HBpin) to afford 1,1-diboronates with high regioselectivity, but in low to moderate yields; monoborylalkanes are formed in noticeable yields (12–24%) as the side-products via reduction of the alkenylboronate intermediates (Scheme 1a).15 More recently, Yun reported a copper(I)-catalyzed selective sequential hydroboration of alkyl alkynes with HBpin to form 1,1-diboronate esters, but reactions of aryl alkynes yield monoboryl and diboryl mixtures (Scheme 1b).16
Driven by our interest in developing base–metal catalyst systems for alkene hydrofunctionalizations,17 recently we and Lu independently reported iminopyridine-oxazoline (IPO) cobalt17e,18 and iron17g,19 complexes for asymmetric hydroboration/hydrosilylation of 1,1-disubstituted alkenes and ketones. Herein, we report that an IPO cobalt complex catalyzes regioselective sequential hydroboration of alkyl and aryl alkynes (Scheme 1c). Most reactions occur under mild conditions with high isolated yields. The method exhibits a broad substrate scope and wide functional group tolerance.
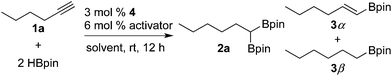
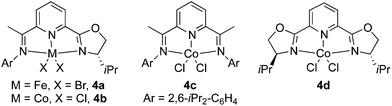
We commenced our studies by examining the reaction of 1-hexyne (1a) with HBpin (Table 1). When using 3 mol% of (IPO)FeBr2 (4a) as the catalyst precursor and 6 mol% of NaBHEt3 as the catalyst activator, the reaction of 1a with 2 equiv. of HBpin in THF at room temperature after 12 h gave 23% of the desired dual hydroboration product 2a, 28% of trans-monoborylalkene (3α), and 42% of monoborylalkane (3β) (entry 1). However, using the cobalt analogue (IPO)CoCl24b as the precatalyst led to the formation of 2a with very high selectivity and yield (96%) (entry 2). A control experiment with the catalyst activator, but without the precatalyst only gave 4% of 3α (entry 3). To evaluate the role of the ligand, reactions using the related cobalt complexes with bis(imino)pyridine (4c) and bis(oxazoline)pyridine (4d) ligands have been carried out. The former gave the desired product in low yield (11%), along with 39% of 3α and 43% of 3β (entry 5), whereas the latter gave 89% of 2a and 5% of 3β (entry 6). The addition of the catalyst activator is essential for the catalysis (entry 4), but it is not limited to NaBHEt3. The reaction using MeLi as the activator afforded the dual hydroboration product in a yield close to that using NaBHEt3. The reactions proceeded smoothly in other solvents, such as toluene, n-pentane, and diethyl ether, albeit with relatively low yield compared to that in THF (entries 7–10).
| Entry | Precatalyst | Activator | Solvent | Yieldb (%) | ||
|---|---|---|---|---|---|---|
| 2 | 3α | 3β | ||||
| a Reaction conditions: 1a (0.5 mmol), HBpin (1.0 mmol), 4 (3 mol%), and additive (6 mol%) in THF (2 mL) at RT. b GC yields using mesitylene as an internal standard (isolated yields in parentheses). | ||||||
| 1 | 4a | NaBHEt3 | THF | 23 | 28 | 42 |
| 2 | 4b | NaBHEt3 | THF | 96 (92) | <1 | 3 |
| 3 | None | NaBHEt3 | THF | 0 | 4 | 0 |
| 4 | 4b | — | THF | 0 | 3 | 0 |
| 5 | 4c | NaBHEt3 | THF | 11 | 39 | 43 |
| 6 | 4d | NaBHEt3 | THF | 89 | <1 | 5 |
| 7 | 4b | MeLi | THF | 95 (90) | <1 | 4 |
| 8 | 4b | MeLi | Toluene | 83 | <1 | 3 |
| 9 | 4b | MeLi | n-Pentane | 87 | <1 | 4 |
| 10 | 4b | MeLi | Et2O | 87 | <1 | 5 |
|
||||||
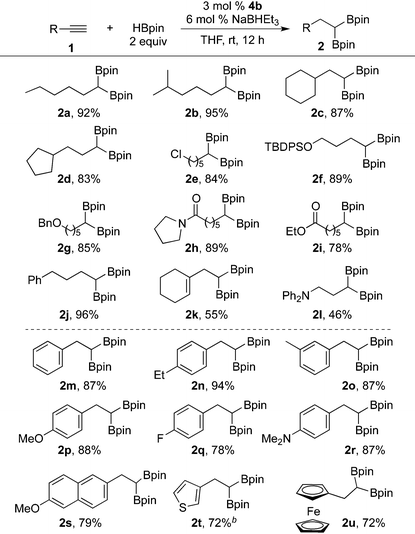
We next studied the scope and limitation of the protocol with (IPO)CoCl24b as the catalyst precursor, NaBHEt3 as the activator, and THF as the solvent (Table 2). Terminal aliphatic alkynes all reacted with HBpin to form the diboryl products selectively. Simple alkynes with linear and branched alkyl groups were converted to the corresponding 1,1-diboronate esters in high yields (2a–2d). A wide range of functional groups, such as chloride (2e), silicon ether (2f), benzyl ether (2g), amide (2h), ester (2i), and internal olefin (2k), can be tolerated. Phenyl-protected propargyl amine gave the desired product (2l) in moderate yield.
Reactions of terminal aryl alkynes also occurred efficiently. Substrates containing both electron-donating and -withdrawing substituents, such as alkyl (2n and 2o), methoxy (2p), fluoride (2q), and dimethylamino (2r) groups, afforded the 1,1-diboryl products in high isolated yields. Naphthyl- (2s), thienyl- (2t), and ferrocenyl-substituted acetylenes (2u) are suitable substrates for sequential hydroboration with exclusive terminal selectivity.
In situ monitoring of the cobalt-catalyzed reaction of 1-hexyne (1a) with 2 equiv. of HBpin provided insight into the catalytic process. As shown in Fig. 1, the reaction at the early stage gave 2a in low yield, but a substantial amount of 3α (e.g., 15 min, 67% of 3α, 8% of 2a). The intermediate 3α was gradually converted to 2a over the course of the reaction. The transformation was nearly complete in 3 h, furnishing 2a in 91% yield. Except for 2a, 3α, and a trace amount of 3β (<3%), no other products were detected by GC during the whole process. The results indicate that the reaction occurs via formation of trans-monoborylalkene (3α) as the intermediate, which undergoes subsequent hydroboration to give the 1,1-diboryl product.
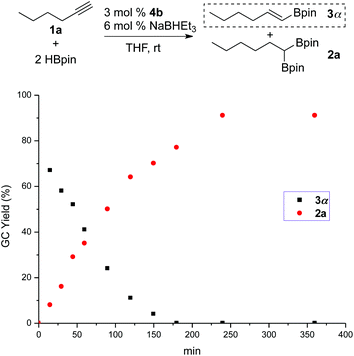 | ||
| Fig. 1 Profile of sequential hydroboration of 1-hexyne (1a) with 2 equiv. of HBpin catalyzed by 3 mol% 4b and 6 mol% NaBHEt3 in THF at room temperature. | ||
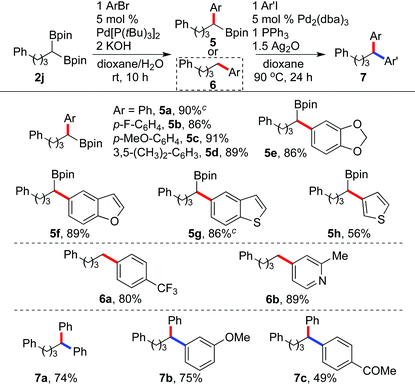
The synthetic utility of 1,1-diboronate esters was demonstrated by their applications to palladium-catalyzed Suzuki–Miyaura coupling reactions. Seminal work by Shibata showed that the adjacent boron atom in 1,1-diborylalkanes has a beneficial effect on the transmetallation step for coupling reactions.2a,8,10 Using Pd[P(tBu)3]2 as the catalyst and KOH as the base, we found that 1,1-diboryl compound 2j coupled selectively with various aryl bromides at room temperature, giving the monoarylation products in high yields (Table 3). O- and S-containing benzoheterocyclic (5e–5g) and heterocyclic (5h) bromides are also favorable substrates under the reaction conditions. Noteworthily, while the reaction with a p-F-substituted aryl bromide gave the benzyl boronate 5b in 86% isolated yield, under otherwise identical conditions, the coupling with a p-CF3-substituted aryl bromide afforded 80% of the protodeborylation product 6a. Furthermore, with 4-bromo-2-methylpyridine as the substrate, a similar transformation involving the combination of cross coupling and protodeborylation occurred to form 6b in 89% yield.20
| a Reaction conditions: 2j (0.22 mmol), ArBr (0.2 mmol), Pd[P(tBu)3]2 (5 mol%), and KOH aq. (40 μL, 10 M in H2O) in dioxane (1 mL) at RT. Isolated yields. b Reaction conditions: 5a (0.2 mmol), ArI (0.24 mmol), Pd2(dba)3 (5 mol%), PPh3 (0.2 mmol), and Ag2O (0.3 mmol) in dioxane (1 mL) at 90 °C. Isolated yields. c Carried out on 0.5 mmol scale. |
|---|
|
In addition, using a protocol developed by Crudden, the isolated secondary benzylic boronate esters could undergo subsequent cross couplings.21 For example, the reactions of 5a with aryl iodides catalyzed by Pd2(dba)2/PPh3 in the presence of Ag2O afforded the diarylation products (7a–c) in useful yields. Thus, the sequence of dual hydroboration and two-step cross coupling reactions provides a synthetically efficient approach to diarylmethane derivatives from simple alkynes.
In summary, we have developed a cobalt catalyst system for selective synthesis of 1,1-diboronates from terminal alkyl and aryl alkynes. Featuring the use of low-cost base–metal catalyst, 100% atom economy, mild reaction conditions, high conversion, wide substrate scope, and broad functional group compatibility, the cobalt-catalyzed alkyne sequential hydroboration could be an attractive route to 1,1-organodiboronate esters. We have also demonstrated that the dual hydroboration products are useful synthetic intermediates for chemoselective Suzuki–Miyaura coupling reactions.
Conflict of InterestThe authors declare no competing financial interest.
Acknowledgements
We gratefully acknowledge the financial support from the National Basic Research Program of China (2015CB856600), the National Natural Science Foundation of China (no. 21422209, 21272255, 21432011, and 21421091), and the CAS/SAFEA International Partnership Program for Creative Research Teams.References
- (a) D. S. Matteson and R. J. Moody, Organometallics, 1982, 1, 20–28 CrossRef; (b) K. Endo, M. Hirokami and T. Shibata, J. Org. Chem., 2010, 75, 3469–3472 CrossRef CAS PubMed; (c) K. Endo, A. Sakamoto, T. Ohkubo and T. Shibata, Chem. Lett., 2011, 40, 1440–1442 CrossRef CAS; (d) K. Hong, X. Liu and J. P. Morken, J. Am. Chem. Soc., 2014, 136, 10581–10584 CrossRef CAS PubMed; (e) Z. Q. Zhang, C. T. Yang, L. J. Liang, B. Xiao, X. Lu, J. H. Liu, Y. Y. Sun, T. B. Marder and Y. Fu, Org. Lett., 2014, 16, 6342–6345 CrossRef CAS PubMed; (f) J. R. Coombs, L. Zhang and J. P. Morken, Org. Lett., 2015, 17, 1708–1711 CrossRef CAS PubMed; (g) H.-Y. Sun, K. Kubota and D. G. Hall, Chem. – Eur. J., 2015, 21, 19186–19194 CrossRef CAS PubMed.
- (a) K. Endo, T. Ohkubo, M. Hirokami and T. Shibata, J. Am. Chem. Soc., 2010, 132, 11033–11035 CrossRef CAS PubMed; (b) K. Endo, T. Ohkubo and T. Shibata, Org. Lett., 2011, 13, 3368–3371 CrossRef CAS PubMed; (c) J. C. H. Lee, R. McDonald and D. G. Hall, Nat. Chem., 2011, 3, 894–899 CrossRef CAS PubMed; (d) K. Endo, T. Ishioka, T. Ohkubo and T. Shibata, J. Org. Chem., 2012, 77, 7223–7231 CrossRef CAS PubMed; (e) K. Endo, T. Ohkubo, T. Ishioka and T. Shibata, J. Org. Chem., 2012, 77, 4826–4831 CrossRef CAS PubMed; (f) H. Li, Z. Zhang, X. Shangguan, S. Huang, J. Chen, Y. Zhang and J. Wang, Angew. Chem., Int. Ed., 2014, 53, 11921–11925 CrossRef CAS PubMed; (g) C. Sun, B. Potter and J. P. Morken, J. Am. Chem. Soc., 2014, 136, 6534–6537 CrossRef CAS PubMed; (h) J. C. Lee, H. Y. Sun and D. G. Hall, J. Org. Chem., 2015, 80, 7134–7143 CrossRef CAS PubMed; (i) S. Xu, X. Shangguan, H. Li, Y. Zhang and J. Wang, J. Org. Chem., 2015, 80, 7779–7784 CrossRef CAS PubMed.
- (a) A. Hirai, M. Nakamura and E. Nakamura, J. Am. Chem. Soc., 1999, 121, 8665–8666 CrossRef CAS; (b) I. Marek, Chem. Rev., 2000, 100, 2887–2900 CrossRef CAS PubMed; (c) S. Matsubara, K. Oshima and K. Utimoto, J. Organomet. Chem., 2001, 617–618, 39–46 CrossRef CAS; (d) J. F. Normant, Acc. Chem. Res., 2001, 34, 640–644 CrossRef CAS PubMed; (e) V. M. Dembitsky, H. Abu Ali and M. Srebnik, Appl. Organomet. Chem., 2003, 17, 327–345 CrossRef CAS; (f) P. Langer and W. Freiberg, Chem. Rev., 2004, 104, 4125–4150 CrossRef CAS PubMed; (g) F. Foubelo and M. Yus, Curr. Org. Chem., 2005, 9, 459–490 CrossRef CAS.
- (a) M. Shimizu and T. Hiyama, Proc. Jpn. Acad., Ser. B, 2008, 84, 75–85 CrossRef CAS; (b) J. Takaya and N. Iwasawa, ACS Catal., 2012, 2, 1993–2006 CrossRef CAS.
- (a) T. Hata, H. Kitagawa, H. Masai, T. Kurahashi, M. Shimizu and T. Hiyama, Angew. Chem., Int. Ed., 2001, 40, 790–792 CrossRef CAS; (b) T. Kurahashi, T. Hata, H. Masai, H. Kitagawa, M. Shimizu and T. Hiyama, Tetrahedron, 2002, 58, 6381–6395 CrossRef CAS; (c) M. Shimizu, C. Nakamaki, K. Shimono, M. Schelper, T. Kurahashi and T. Hiyama, J. Am. Chem. Soc., 2005, 127, 12506–12507 CrossRef CAS PubMed; (d) M. Shimizu, M. Schelper, I. Nagao, K. Shimono, T. Kurahashi and T. Hiyama, Chem. Lett., 2006, 35, 1222–1223 CrossRef CAS.
- R. Soundararajan and D. S. Matteson, Organometallics, 1995, 14, 4157–4166 CrossRef CAS.
- H. Ito and K. Kubota, Org. Lett., 2012, 14, 890–893 CrossRef CAS PubMed.
- C.-T. Yang, Z.-Q. Zhang, H. Tajuddin, C.-C. Wu, J. Liang, J.-H. Liu, Y. Fu, M. Czyzewska, P. G. Steel, T. B. Marder and L. Liu, Angew. Chem., Int. Ed., 2012, 51, 528–532 CrossRef CAS PubMed.
- X. Feng, H. Jeon and J. Yun, Angew. Chem., Int. Ed., 2013, 52, 3989–3992 CrossRef CAS PubMed.
- S. H. Cho and J. F. Hartwig, Chem. Sci., 2014, 5, 694–698 RSC.
- (a) H. Abu Ali, I. Goldberg and M. Srebnik, Organometallics, 2001, 20, 3962–3965 CrossRef; (b) H. Abu Ali, I. Goldberg, D. Kaufmann, C. Burmeister and M. Srebnik, Organometallics, 2002, 21, 1870–1876 CrossRef; (c) A. J. Wommack and J. S. Kingsbury, Tetrahedron Lett., 2014, 55, 3163–3166 CrossRef CAS.
- (a) H. Li, L. Wang, Y. Zhang and J. Wang, Angew. Chem., Int. Ed., 2012, 51, 2943–2946 CrossRef CAS PubMed; (b) H. Li, X. Shangguan, Z. Zhang, S. Huang, Y. Zhang and J. Wang, Org. Lett., 2014, 16, 448–451 CrossRef CAS PubMed; (c) A. B. Cuenca, J. Cid, D. García-López, J. J. Carbó and E. Fernández, Org. Biomol. Chem., 2015, 13, 9659–9664 RSC.
- (a) D. J. Pasto, J. Am. Chem. Soc., 1964, 86, 3039–3047 CrossRef CAS; (b) V. V. R. Rao, S. K. Agarwal, I. Mehrotra and D. Devaprabhakara, J. Organomet. Chem., 1979, 166, 9–16 CrossRef CAS; (c) P. Nguyen, R. B. Coapes, A. D. Woodward, N. J. Taylor, J. M. Burke, J. A. K. Howard and T. B. Marder, J. Organomet. Chem., 2002, 652, 77–85 CrossRef CAS; (d) R. B. Coapes, F. E. S. Souza, R. L. Thomas, J. J. Hall and T. B. Marder, Chem. Commun., 2003, 614–615 RSC; (e) J. Ramírez, A. M. Segarra and E. Fernández, Tetrahedron: Asymmetry, 2005, 16, 1289–1294 CrossRef.
- (a) H. C. Brown and S. K. Gupta, J. Am. Chem. Soc., 1975, 97, 5249–5255 CrossRef CAS; (b) C. A. Brown and R. A. Coleman, J. Org. Chem., 1979, 44, 2328–2329 CrossRef CAS; (c) C. E. Tucker, J. Davidson and P. Knochel, J. Org. Chem., 1992, 57, 3482–3485 CrossRef CAS; (d) S. Pereira and M. Srebnik, Organometallics, 1995, 14, 3127–3128 CrossRef CAS; (e) X. He and J. F. Hartwig, J. Am. Chem. Soc., 1996, 118, 1696–1702 CrossRef CAS; (f) S. Pereira and M. Srebnik, Tetrahedron Lett., 1996, 37, 3283–3286 CrossRef CAS; (g) T. Ohmura, Y. Yamamoto and N. Miyaura, J. Am. Chem. Soc., 2000, 122, 4990–4991 CrossRef CAS; (h) M. Hoshi, K. Shirakawa and M. Okimoto, Tetrahedron Lett., 2007, 48, 8475–8478 CrossRef CAS; (i) D. M. Khramov, E. L. Rosen, A. Joyce, P. D. Vu, V. M. Lynch and C. W. Bielawski, Tetrahedron, 2008, 64, 6853–6862 CrossRef CAS; (j) C. Gunanathan, M. Holscher, F. Pan and W. Leitner, J. Am. Chem. Soc., 2012, 134, 14349–14352 CrossRef CAS PubMed; (k) B. Sundararaju and A. Furstner, Angew. Chem., Int. Ed., 2013, 52, 14050–14054 CrossRef CAS PubMed; (l) H. E. Ho, N. Asao, Y. Yamamoto and T. Jin, Org. Lett., 2014, 16, 4670–4673 CrossRef CAS PubMed; (m) J. V. Obligacion, J. M. Neely, A. N. Yazdani, I. Pappas and P. J. Chirik, J. Am. Chem. Soc., 2015, 137, 5855–5858 CrossRef CAS PubMed.
- K. Endo, M. Hirokami and T. Shibata, Synlett, 2009, 1331–1335 CrossRef CAS.
- S. Lee, D. Li and J. Yun, Chem. – Asian J., 2014, 9, 2440–2443 CrossRef CAS PubMed.
- (a) L. Zhang, D. Peng, X. Leng and Z. Huang, Angew. Chem., Int. Ed., 2013, 52, 3676–3680 CrossRef CAS PubMed; (b) Z. Huang and L. Zhang, Synlett, 2013, 1745–1747 CrossRef; (c) D. Peng, Y. Zhang, X. Du, L. Zhang, X. Leng, M. D. Walter and Z. Huang, J. Am. Chem. Soc., 2013, 135, 19154–19166 CrossRef CAS PubMed; (d) L. Zhang, Z. Zuo, X. Leng and Z. Huang, Angew. Chem., Int. Ed., 2014, 53, 2696–2700 CrossRef CAS PubMed; (e) L. Zhang, Z. Zuo, X. Wan and Z. Huang, J. Am. Chem. Soc., 2014, 136, 15501–15504 CrossRef CAS PubMed; (f) Y. Cao, Y. Zhang, L. Zhang, D. Zhang, X. Leng and Z. Huang, Org. Chem. Front., 2014, 1, 1101–1106 RSC; (g) Z. Zuo, L. Zhang, X. Leng and Z. Huang, Chem. Commun., 2015, 51, 5073–5076 RSC; (h) L. Zhang and Z. Huang, J. Am. Chem. Soc., 2015, 137, 15600–15603 CrossRef CAS PubMed; (i) X. Jia and Z. Huang, Nat. Chem., 2016, 8, 157–161 CAS.
- (a) J. Chen, T. Xi, X. Ren, B. Cheng, J. Guo and Z. Lu, Org. Chem. Front., 2014, 1, 1306–1309 RSC; (b) J. Guo, J. Chen and Z. Lu, Chem. Commun., 2015, 51, 5725–5727 RSC.
- (a) J. Chen, T. Xi and Z. Lu, Org. Lett., 2014, 16, 6452–6455 CrossRef CAS PubMed; (b) J. Chen, B. Cheng, M. Cao and Z. Lu, Angew. Chem., Int. Ed., 2015, 54, 4661–4664 CrossRef CAS PubMed.
- Using aryl iodides as the electrophiles, NaOH as the base, THF/H2O as the solvent, Hartwig and co-workers showed that cross coupling with 1,1-benzyldiboronate esters was accompanied by protodeborylation. Also see ref. 10.
- D. Imao, B. W. Glasspoole, V. S. Laberge and C. M. Crudden, J. Am. Chem. Soc., 2009, 131, 5024–5025 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and product characterization. See DOI: 10.1039/c5qo00426h |
| This journal is © the Partner Organisations 2016 |