Rapid, green and inexpensive synthesis of high quality UiO-66 amino-functionalized materials with exceptional capability for removal of hexavalent chromium from industrial waste†
Sofia
Rapti
a,
Anastasia
Pournara
a,
Debajit
Sarma
b,
Ioannis T.
Papadas
c,
Gerasimos S.
Armatas
c,
Youssef S.
Hassan
d,
Mohamed H.
Alkordi
d,
Mercouri G.
Kanatzidis
b and
Manolis J.
Manos
*a
aDepartment of Chemistry, University of Ioannina, 45110 Ioannina, Greece. E-mail: emanos@cc.uoi.gr
bDepartment of Chemistry, Northwestern University, Evanston, IL 60208, USA
cDepartment of Materials Science and Technology, University of Crete, 71003 Heraklion, Greece
dZewail City of Science and Technology, Center for Materials Science, Sheikh Zayed Dist. 6th of October, 12588, Giza, Egypt
First published on 1st February 2016
Abstract
We describe a new synthetic method for the isolation of the UiO-66 amino-functionalized material (called metal organic resin-1, MOR-1) and its composite with alginic acid (HA). MOR-1 can be prepared in high yield (∼70%) and purity within an hour via a reflux reaction of ZrCl4 and 2-amino-terephthalic acid in acifidied aqueous solution, whereas addition of sodium alginate to the fine suspension of MOR-1 resulting from the reflux synthesis affords the MOR-1-HA composite. This inexpensive, green and fast preparation method results in UiO-66 amino-functionalized materials (MOR-1 and MOR-1-HA) of the same quality and microporous features as those of compounds isolated with the slower solvothermal synthesis involving toxic and costly organic solvents. Field Emission-Scanning Electron Microscopy (FE-SEM) studies revealed that MOR-1 consists of spongy nanoparticles (150–300 nm in size), whereas MOR-1-HA nanoparticles are relatively compact. Thus, for the first time we could visualize the effect of alginic acid partially coating the surface of the MOR particles. The composite prepared by this method can be successfully utilized as a stationary phase, mixed with sand, in an anion-exchange column. The column shows excellent hexavalent chromium sorption properties and can be easily regenerated and reused several times with almost no loss of its initial Cr(VI) removal capacity. Remarkably, this ion exchange column is capable of eliminating Cr(VI) ions from chrome plating wastewater samples, thus indicating its potential for applications in industrial wastewater treatment.
Introduction
Porous metal organic frameworks (MOFs)1 with functional groups and ion-exchange properties can be considered as a new generation of ion-exchange resins, since they combine the binding groups of organic resins and a highly ordered porous structure not existing in the conventional amorphous organic polymers. Such MOFs can thus adequately be referred to as metal–organic resins (MORs). MORs, although they show excellent batch ion-exchange properties,2 are not suitable for use as stationary phases in ion-exchange columns which are required for practical applications such as the treatment of industrial wastewater. These materials are commonly isolated as very light powders that form fine suspensions in water and thus can flow out of the prepared columns, precluding their utilization in ion-exchange columns as is. To overcome this drawback of MORs, we are targeting on the isolation of engineered forms of these materials. We recently reported a composite form of a MOR, based on the UiO-66 amino-functionalized material (MOR-1, Fig. 1) and alginic acid (HA).3 In contrast to MOR-1, the MOR-1-HA material is not easily dispersed in water, since MOR particles are covered by an alginic acid shell. The composite containing MOR with protonated amino-groups and labile extra-framework Cl− ions exhibited highly efficient ion-exchange properties for the hexavalent chromium Cr(VI). The choice of hexavalent chromium was based on its profound adverse effects, being a well-known carcinogen and one of the most dangerous pollutants for water resources.4,5 Detailed batch ion-exchange studies revealed high absorption capacity, rapid sorption kinetics and exceptional selectivity of the composite sorbent for Cr(VI) in the presence of various competitive ions (e.g. Cl−, Br−, NO3−, SO42−). Importantly, columns with a mixture of MOR-1-HA/sand as the stationary phase and only 1 wt% MOR-1-HA content showed very good capability to lower moderate and trace Cr(VI) concentrations well-below the acceptable safe limits, even in the presence of high concentrations of competitive anions. These columns exhibit relatively high breakthrough capacities, excellent regeneration capability and reusability.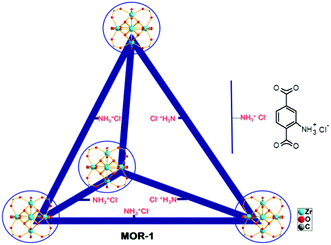 | ||
| Fig. 1 The structure of (protonated) MOR-1 material represented as a tetrahedral cage.6 | ||
The above results indicated the high potential of MOR-1-HA for practical use in the remediation of Cr(VI)-contaminated wastewater. For a mass production and extensive use of the sorbent in water treatment industry, a relatively fast, high yield, inexpensive and green (as possible) synthetic method of the material is required. Unfortunately, MOR-1, the basic component of the composite material, is usually isolated by a solvothermal reaction which lasts for several hours and involves high amounts of toxic and expensive organic solvents (DMF, DMAc etc.).6 Recently, it has been shown that the UiO-66 amino-functionalized material can be isolated within 24 h via hydrothermal synthesis under acidic conditions. The obtained product, however, is not clearly microporous as that isolated by typical solvothermal reactions; instead, it exhibits both microporous and mesoporous structural features.7
Here we report a new synthetic method for the isolation of purely microporous and highly crystalline MOR-1 and its composite form MOR-1-HA, which involves acidified water as a solvent and is completed within an hour. The obtained materials show exceptional capability to absorb hexavalent chromium in a diverse range of experimental conditions, including the pH of the solution and the presence of competitive ions. Importantly, the composite MOR-1-HA is particularly suitable to be used in an ion-exchange column, showing excellent Cr(VI) absorption properties. In addition, for the first time we show that the MOR-1-HA column is efficient for the decontamination of industrial (chrome plating) Cr(VI) wastewater samples. Considering the relatively low cost, fast and environmentally friendly synthesis method of the MOR-1-HA reported here, the MOR-1-HA column seems promising for real-world applications in the field of environmental remediation.
Results and discussion
Synthesis of MOR-1 and MOR-1-HA composite
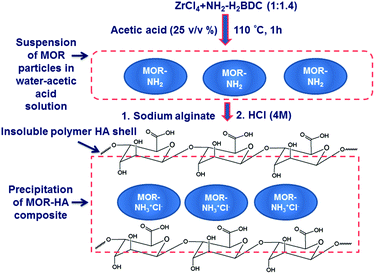
In our previous work, a MOR-1-HA composite was prepared via a three-step procedure involving (a) the synthesis of MOR-1via a solvothermal reaction in DMF/HCl solution, (b) encapsulation of MOR-1 by calcium alginate (CA) resulting in the MOR-1-CA composite and (c) formation of the MOR-1-HA material by the treatment of MOR-1-CA with HCl acid.3 Alternatively, we could prepare the MOR-1-HA by addition of HCl acid in a water suspension of MOR-1 and sodium alginate (SA).3As mentioned above, the material would be more attractive for applications if it can be synthesized by a fast and inexpensive synthesis method involving minimal quantities of organic solvents. Recently, it was shown that a UiO-66-amino functionalized type metal organic framework can be isolated with a reflux reaction of almost equimolar Zr(NO3)4 and NH2-H2BDC (2-amino-terephthalic acid) in water–acetic acid solution.7 However, the reported material showed structural characteristics that differ from those of the compound isolated from the reaction with DMF.6 It was thus challenging to isolate a UiO-66-amino functionalized material hydrothermally (i.e.MOR-1) with the same features as those of the well-known material prepared by solvothermal reaction. We have attempted this synthesis by modifying the reported reflux synthesis method (Fig. 2 and 3). We employed the same Zr4+ source (i.e. ZrCl4) and ligand to metal salt molar ratio (∼1.4) as those used in the solvothermal synthesis of UiO-66-NH2BDC, with the difference that in our synthesis the solvent was a mixture of water and acetic acid (25 v/v% acetic acid). This reaction resulted in a fine suspension of the MOR which formed in less than 1 h. The product of this reaction, isolated via centrifugation, contained impurities (Fig. S1†), probably some amount of unreacted organic ligand. Indeed, treating the product with HCl acid solution, which can dissolve the NH2-H2BDC ligand, resulted in the isolation of pure MOR-1 in ∼70% yield. After we established the method for the isolation of the high quality UiO-66 amino-functionalized material, the next step was the preparation of the MOR-1-HA composite. Fortunately, the isolation of the composite did not involve the separation process via centrifugation followed for MOR-1, which is time-consuming and not attractive for large scale synthesis of materials. Actually, the isolation of materials forming colloidal solutions requires the use of a flocculation agent that causes agglomeration of the particles thus simplifying the separation process.8 Such a flocculation agent could be sodium alginate, which in acidic environment is transformed to alginic acid. The latter forms an insoluble polymer shell around MOR particulates, resulting in the precipitation-easy separation of the solid from the solution (Fig. 2 and 3). Indeed, by adding sodium alginate to the MOR-1 water–acetic acid suspension, the MOR-1-HA is readily precipitated and can be isolated via simple filtration (Fig. 3). Only a small amount of sodium alginate is required for the isolation of the composite material and thus, the composite shows almost identical properties to those of the pristine MOR material. Specifically, the composite isolated contains alginic acid in an amount up to 2.1 wt%. (see the Experimental section, ESI†). The obtained product is further treated with HCl acid to dissolve the unreacted NH2-H2BDC ligand and complete the protonation of the amino-functional groups of the material. We have also performed studies for the formation of the material vs. the reaction time. The results indicated that (a) a significant amount of MOR-1-HA is formed within only 5 min and (b) an hour of reflux reaction is enough to achieve the maximum possible yield for the isolation of the MOR-1-HA composite material (Fig. S2†).
Characterization of MOR-1 and MOR-1-HA materials
Field Emission-Scanning electron microscopy (FE-SEM) images showed that both MOR-1 and MOR-1-HA materials are composed of aggregated polyhedral nanoparticles with a size ∼150–300 nm, see Fig. 4. High-magnification images reveal that the nanoparticles of MOR-1 are spongy with relatively large voids, whereas those of MOR-1-HA contain significantly smaller pores on their surface. Presumably, this is due to the fact that a thin layer of alginic acid covers the large pores on the surface of MOR-1 nanoparticles thus creating the denser nanoparticles of the composite. Therefore, MOR-1-HA isolated in a relatively compact form is much less dispersed in water and thus, can be successfully utilized in columns (see below). Note that no clear shape and size of nanoparticles could be observed by SEM studies of MOR-1 isolated from the solvothermal reaction in DMF and the corresponding MOR-1-HA material.3 Thus, in this case, we could not observe differences in morphology between MOR-1 and MOR-1-HA particles. Here, for the first time, we could visualize the clear differences between MOR-1 and MOR-1-HA materials, which may explain their different capabilities to form or not a fine suspension in water.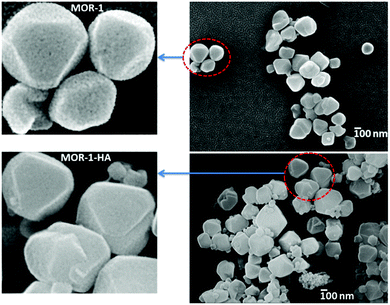 | ||
| Fig. 4 FE-SEM images for MOR-1 and MOR-1-HA and enlarged views of some particles of these materials. | ||
Powder X-ray diffraction (PXRD) studies indicate that MOR-1 isolated by 1 h reflux reaction and after its purification with HCl acid shows the typical structure of UiO-66 type materials (Fig. 5A).6 Elemental (C,H,N), energy dispersive spectroscopy (EDS) (indicating Zr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cl molar ratio ∼ 1) and thermogravimetric analysis (TGA) data (Fig. S3†) indicate the formula [Zr6O4(OH)4(NH3+-BDC)6]Cl6·35H2O for MOR-1. PXRD (Fig. 5A) and EDS (revealing Zr
Cl molar ratio ∼ 1) and thermogravimetric analysis (TGA) data (Fig. S3†) indicate the formula [Zr6O4(OH)4(NH3+-BDC)6]Cl6·35H2O for MOR-1. PXRD (Fig. 5A) and EDS (revealing Zr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cl molar ratio ∼ 1) data for the composite sample confirm its close similarity to the pristine MOR-1 solid. TGA data were also used for the determination of the water content of the composite material (∼19 water molecules, Fig. S4†).
Cl molar ratio ∼ 1) data for the composite sample confirm its close similarity to the pristine MOR-1 solid. TGA data were also used for the determination of the water content of the composite material (∼19 water molecules, Fig. S4†).
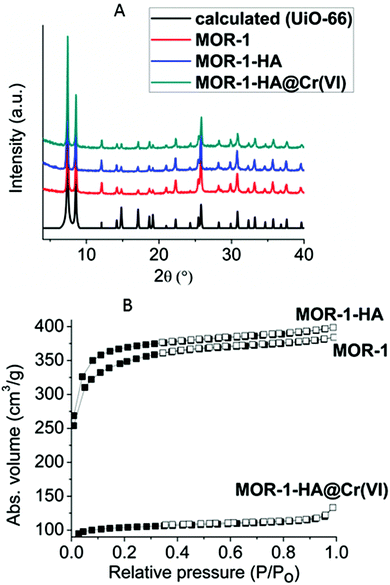 | ||
| Fig. 5 (A) PXRD patterns of MOR-1, MOR-1-HA, MOR-1-HA@Cr(VI) and the calculated pattern for UiO-66. (B) Nitrogen sorption isotherms at 77 K for MOR-1, MOR-1-HA and MOR-1-HA@Cr(VI). | ||
Nitrogen physisorption measurements recorded at 77 K for the activated MOR-1 and MOR-1-HA revealed type-I adsorption isotherms, characteristic of microporous solids (Fig. 5B). The Brunauer–Emmett–Teller (BET) surface areas of the MOR-1 and MOR-1-HA were determined to be 1097 (Langmuir 1638 m2 g−1) and 1182 (Langmuir 1670 m2 g−1) m2 g−1 respectively. These values fall within the range of surface areas found for amino-functionalized UiO-66 type materials prepared with solvothermal reactions.6 CO2 adsorption isotherms at 1 bar and 273 K indicated a sorption capacity of ∼4.4 mmol g−1 for both samples (Fig. S5†). Analysis of CO2 adsorption data with the density functional theory (DFT) suggests that both MOR-1 and MOR-1-HA consist of a microporous network with the pore size in the range of 8–9 Å (Fig. S5†). Interestingly, the MOR-1 and MOR-1-HA polymers presented here show significantly higher surface area and CO2 sorption capacity compared to those (BET = 833 m2 g−1, Langmuir = 1073 m2 g−1; CO2 sorption capacity = 2.8 mmol g−1 at 273 K) for the reported UiO-66 amino-functionalized compound prepared by a hydrothermal reaction.7 Furthermore, the type-I shape of the isotherms of MOR-1 and MOR-1-HA are indicative of the predominantly microporous structure, whereas the reported UiO-66-NH2BDC solid isolated hydrothermally showed a combination of type-I and type-IV isotherms revealing the existence of both micro and mesoporosity.7 Thus, here we propose, for the first time, a hydrothermal synthesis approach that yields amino-functionalized UiO-66-type materials with the same characteristics as those found in the UiO-66-NH2BDC compound prepared with typical solvothermal reaction.6
Isolation and characterization of Cr(VI)-containing materials
The isolation of the Cr(VI)-loaded composite material [MOR-1-HA@Cr(VI)] is achieved by treating it with a Cr2O72− water solution for ∼1 h. An anion-exchange reaction takes place as represented by the following equation: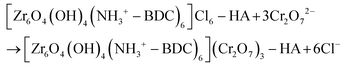 | (1) |
EDS analysis shows no Cl in the Cr(VI)-exchanged material. Furthermore, the analytical data from ICP-MS, EDS and UV-Vis spectroscopy indicate a Zr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cr molar ratio of ∼1, which is consistent with the replacement of 6 Cl− by 3 Cr2O72− anions. The PXRD data of MOR-1-HA@Cr(VI) indicate that the structure of the UiO-66 type framework is preserved after the ion-exchange process (Fig. 5A). The presence of Cr(VI) ions was also evidenced by IR, which shows a characteristic peak at 924 cm−1 attributed to the anti-symmetric CrVIO3-stretch (Fig. S6†). Furthermore, the XPS data showed the presence of Cr2p1/2 and Cr2p3/2 core-level signals. The main components of these peaks correspond to binding energies (588.1 and 579.2 eV), which are characteristic of Cr in the (VI) valence state (Fig. S7†). The insertion of Cr(VI) species into the pores was also demonstrated by the substantially smaller BET surface area of Cr(VI) exchanged samples compared to that of the pristine composite material. Thus, after the Cr(VI) exchange process, the surface area drops from 1182 m2 g−1 for MOR-1-HA to 298 m2 g−1 for MOR-1-HA@Cr(VI) (Fig. 5B).
Cr molar ratio of ∼1, which is consistent with the replacement of 6 Cl− by 3 Cr2O72− anions. The PXRD data of MOR-1-HA@Cr(VI) indicate that the structure of the UiO-66 type framework is preserved after the ion-exchange process (Fig. 5A). The presence of Cr(VI) ions was also evidenced by IR, which shows a characteristic peak at 924 cm−1 attributed to the anti-symmetric CrVIO3-stretch (Fig. S6†). Furthermore, the XPS data showed the presence of Cr2p1/2 and Cr2p3/2 core-level signals. The main components of these peaks correspond to binding energies (588.1 and 579.2 eV), which are characteristic of Cr in the (VI) valence state (Fig. S7†). The insertion of Cr(VI) species into the pores was also demonstrated by the substantially smaller BET surface area of Cr(VI) exchanged samples compared to that of the pristine composite material. Thus, after the Cr(VI) exchange process, the surface area drops from 1182 m2 g−1 for MOR-1-HA to 298 m2 g−1 for MOR-1-HA@Cr(VI) (Fig. 5B).
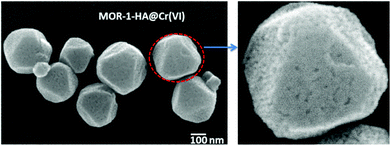
Finally, the FE-SEM studies indicate that the nanoparticles of MOR-1-HA@Cr(VI) retain the polyhedral shape of the MOR-1-HA particles (Fig. 6); however, the MOR-1-HA@Cr(VI) nanoparticles contain some defects and relatively large pores on their surface, which presumably resulted from the ion-exchange process.
Batch ion exchange studies
Fitting of the isotherm data with the Langmuir equation10 (Fig. 7, Fig. S8 and Table S1†) revealed a maximum sorption capacity of 280 ± 19 mg Cr2O72 per g of MOR-1-HA, which corresponds to a capacity of 286.0 ± 19.4 Cr2O72− mg per g of MOR-1 considering that the composite contains ∼97.9% MOR-1. This sorption capacity is consistent with the absorption of 3.1 ± 0.2 moles of Cr2O72− per formula unit of the MOR ([Zr6O4(OH)4(NH3+-BDC)6]Cl6·xH2O, x ∼ 19 for the MOR component of the composite), which is close to its maximum sorption capacity (3 moles per formula unit). Fitting of the isotherm data can also be done using the Freundlich model (Fig. S8 and Table S1†).
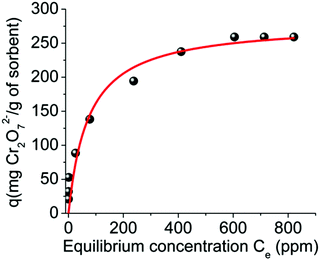 | ||
| Fig. 7 Equilibrium Cr2O72− sorption data for MOR-1-HA material (pH ∼ 3). The solid line represents the fitting of the data with the Langmuir model (see ESI†). | ||
The high efficiency of the composite for dichromate sorption is also revealed by the values of the distribution coefficient Kd calculated by using the following equation:
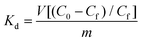 | (2) |
We focused our ion-exchange studies on the composite and not on the pristine MOR, since only the composite form is suitable for column ion-exchange (see below). For comparison, however, we have also determined the isotherm Cr2O72− sorption data for the MOR-1 material (Fig. S8 and Table S1†). The results revealed a maximum sorption capacity of 321 ± 16 Cr2O72− mg per g of MOR-1, which is slightly higher than that found for the composite. Thus, the presence of alginic acid in such a small quantity (∼2 wt%) in the composite results in a minor differentiation of the ion-exchange properties of the metal organic material.
Kinetic studies
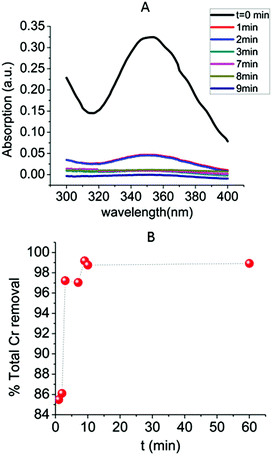
The kinetics of the Cr2O72− absorption by MOR-1-HA was also investigated (Fig. 8) The results indicated that this sorption process is quite fast, with ∼85.5% of the initial Cr2O72− content (C0 = 21.2 ppm, pH ∼ 3) removed within only 1 min MOR-1-HA/solution contact. After 3 min the Cr2O72− removal percentage increased to 97.2%, whereas the ion exchange reached its equilibrium within 9 min and more than 99% Cr2O72− sorption was observed. The kinetic data can be roughly fitted (Fig. S9†) with the Lagergren's first order equation:| qt = qe[1 − exp(−KLt)] | (3) |
The rapid Cr(VI) sorption kinetics observed for MOR-1-HA results from its highly porous structure allowing fast diffusion of ions within the pores and the strong Cr(VI)–amine group interactions.3
Variable pH studies
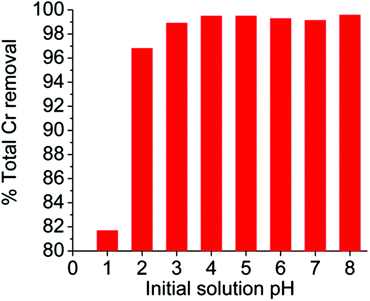
Although we focused more on the Cr(VI) sorption under acidic conditions that are usually observed in industrial waste, we have also studied the Cr(VI) ion exchange by MOR-1-HA in a relatively wide pH range (1–8). The results indicated that the material is capable of absorbing Cr(VI) from highly acidic to alkaline solutions (Fig. 9). In particular, it shows 97–99.6% Cr(VI) removal capacities in the pH range 2–8 (initial total chromium concentration = 10.2 ppm), whereas even at pH ∼ 1 MOR-1-HA displays a high Cr(VI) removal capacity (∼82%).Selectivity studies
Competitive Cr2O72−/Cl−, Cr2O72−/Br−, Cr2O72−/NO3− and Cr2O72−/ SO42− sorption experiments were also performed for MOR-1-HA. Cl−, Br− and NO3− ions have no effect on the dichromate (initial concentration 0.25 mM, pH ∼ 3) sorption by MOR-1-HA (UV-Vis data are shown in Fig. S10†). Thus, high dichromate removal capacities ∼98–99% and excellent Kd values ∼ 1.0–2.2 × 105 mL g−1 were observed even in the presence of a large excess of Cl−, Br− and NO3− ions (Cl−, Br−, NO3− concentration = 2.5 mM). SO42−, as a divalent cation, is expected to be a stronger competitor for dichromate ion exchange. Still, even in the presence of a ten-fold excess of SO42− ions (concentration = 2.5 mM), a significant dichromate removal capacity (∼55%) was obtained (Fig. S8†). The selectivity of MOR-1-HA for dichromate anions was explained on the basis of strong O3CrVI⋯NH2 interactions.3Column ion exchange studies
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 proved to be highly effective for Cr(VI) sorption and also showed a stable and relatively fast water flow (see below and ref. 3). The fact that columns with high efficiency for Cr(VI) removal contain only a small quantity of the sorbent (∼1% by weight) and the main component (99% by weight) of the stationary phase is sand, an abundant and very low cost material, is economically attractive.
100 proved to be highly effective for Cr(VI) sorption and also showed a stable and relatively fast water flow (see below and ref. 3). The fact that columns with high efficiency for Cr(VI) removal contain only a small quantity of the sorbent (∼1% by weight) and the main component (99% by weight) of the stationary phase is sand, an abundant and very low cost material, is economically attractive.
Prior to the breakthrough sorption experiments, we have tested MOR-1 and MOR-1-HA/sand columns (with a sorbent to sand mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) concerning the capability of the sorbent to remain fixed in the stationary phase and the flow rate for the column. SEM studies, presented above, indicate that the MOR-1 particles are porous and spongy and thus, it is expected to be easily dispersed in water. Indeed, MOR-1 (even mixed with an inert material as sand) is gradually removed from the column, since it forms fine water suspension (Fig. 10). Thus, clearly MOR-1 is not suitable to be used for column sorption applications. The composite material, however, consists of relatively compact MOR particles partially coated by the insoluble alginic acid shell (see the SEM images above) and thus, it has limited capability to form suspensions in water. As a result, the effluents flowing out of the composite/sand columns are clear solutions (Fig. 10).
100) concerning the capability of the sorbent to remain fixed in the stationary phase and the flow rate for the column. SEM studies, presented above, indicate that the MOR-1 particles are porous and spongy and thus, it is expected to be easily dispersed in water. Indeed, MOR-1 (even mixed with an inert material as sand) is gradually removed from the column, since it forms fine water suspension (Fig. 10). Thus, clearly MOR-1 is not suitable to be used for column sorption applications. The composite material, however, consists of relatively compact MOR particles partially coated by the insoluble alginic acid shell (see the SEM images above) and thus, it has limited capability to form suspensions in water. As a result, the effluents flowing out of the composite/sand columns are clear solutions (Fig. 10).
The flow rate for the columns should also be taken into account. Sorbents that result in column clogging are not desirable for applications.12 Thus, we investigated the flow rate of the MOR-1-HA/sand column. We observed that the water flow through the MOR-1-HA/sand column was stable over several runs and relatively fast (1.2–1.4 mL min−1). MOR-1-HA particles are of uniform (polyhedral) shape (Fig. 4) and thus, they can be distributed evenly in the column allowing a continuous and stable water flow. Thus, the MOR-1-HA/sand column seems promising in terms of immobilization of the sorbent in the stationary phase and flow rate.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
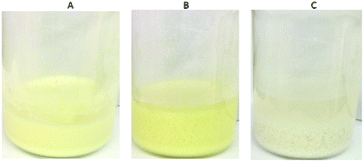
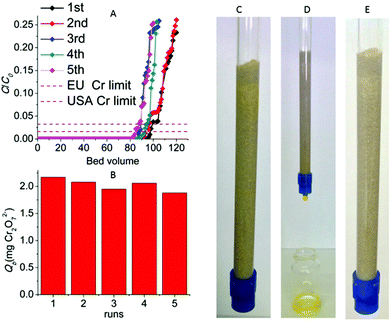
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) column and initial Cr2O72− concentration of 6.4 ppm (pH ∼ 3) revealed that 97 bed volumes (bed volume = bed height (cm) × cross sectional area (cm2) = 3.5 mL) of the effluent samples (bed volumes) show a total Cr content ≤30 ppb, significantly below the acceptable safety limits defined by USA-EPA (100 ppb) and EU (50 ppb),5Fig. 11A and B. The column can be regenerated by washing it with HCl acid solution (4 M). The regeneration process can be visually observed by the decolourization of the (yellow-colored) Cr(VI) loaded column (Fig. 11C–E). After the regeneration, the column can be reused showing only a small decrease (∼4) of bed volumes with the Cr content below the Cr safety limits. Even after a fifth run the column displays a high number of bed volumes (84) with total Cr concentration ≤30 ppb. The breakthrough capacity Qb (mg) of this ion-exchange column can be determined by the equation:
100) column and initial Cr2O72− concentration of 6.4 ppm (pH ∼ 3) revealed that 97 bed volumes (bed volume = bed height (cm) × cross sectional area (cm2) = 3.5 mL) of the effluent samples (bed volumes) show a total Cr content ≤30 ppb, significantly below the acceptable safety limits defined by USA-EPA (100 ppb) and EU (50 ppb),5Fig. 11A and B. The column can be regenerated by washing it with HCl acid solution (4 M). The regeneration process can be visually observed by the decolourization of the (yellow-colored) Cr(VI) loaded column (Fig. 11C–E). After the regeneration, the column can be reused showing only a small decrease (∼4) of bed volumes with the Cr content below the Cr safety limits. Even after a fifth run the column displays a high number of bed volumes (84) with total Cr concentration ≤30 ppb. The breakthrough capacity Qb (mg) of this ion-exchange column can be determined by the equation:| Qb = C0Vb | (4) |
The number of bed volumes passed through the column till the breakpoint concentration (i.e. total Cr concentration ≤50 ppb) was 97, 93, 87, 92 and 84 for the 1st–5th column runs respectively. Thus, the Qb values for the different runs of the specific MOR-1-HA/sand column and Cr2O72− initial concentration of 6.4 ppm were calculated to be 2.17 (1st run), 2.08(2nd run), 1.95 (3rd run), 2.06(4th run) and 1.88 (5th run) mg Cr2O72− (Fig. 11).
We have also examined the effect of the initial Cr(VI) concentration on the breakthrough sorption capacity of this MOR-1-HA/sand column. We have observed that breakthrough capacities of 2.06–2.25 and 2.25–2.34 mg Cr2O72− were obtained for initial dichromate concentrations of 53.5 and 25.7 ppm respectively (Fig. S11†). A similar breakthrough capacity is observed independently of the initial Cr(VI) concentration, thus emphasizing the reproducible sorption results obtained with the MOR-1-HA/sand column.
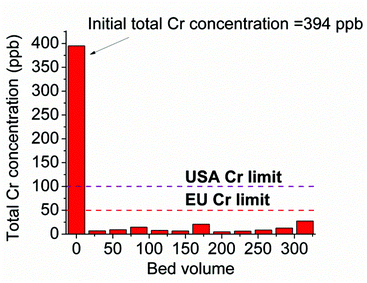
In addition, we tested the performance of the MOR-1-HA/sand column for the decontamination of solutions containing low Cr levels, which however are above the safety limits. Thus, 1.1 L (∼315 bed volumes) of a solution with a total Cr concentration of 394 ppb (pH ∼ 3) was passed through the MOR-1-HA/sand column. ICP-MS analysis for the Cr content of the effluents collected indicated that the Cr concentrations were 7–27 ppb (Fig. 12), which are well-below the EU and USA-EPA acceptable limits. These results indicate the exceptional capability of the MOR-1-HA/sand column to remediate water contaminated with extremely low Cr levels. Note that wastewater with quite low Cr concentrations (<1000 ppb) is not easy to be treated with common methods such as precipitation.14 Thus, the development of new technologies that are effective for such low Cr levels is particularly desirable.
At this point, we may compare the performance of the MOR-1-HA/sand column (MOR-1-HA to sand mass ratio = 1/100), containing MOR-1-HA prepared by reflux synthesis/SA addition with that of the corresponding column with a composite isolated from solvothermally prepared MOR-1.3 The latter displays a breakthrough capacity of 1.55–1.68 mg Cr2O72− (number of bed volume till the breakpoint concentration = 74–80, 1 bed volume = 3.5 mL, initial Cr2O72− concentration = 6 ppm), which is lower than that (1.88–2.17 mg Cr2O72− for initial dichromate concentration of 6.4 ppm) of the column with MOR-1-HA isolated from reflux synthesis-SA addition. Thus, high quality MOR-1-HA can be prepared by a fast, low cost and environmentally friendly synthesis and at the same time, it shows improved column Cr(VI) sorption properties.
Column sorption of chrome plating wastewater
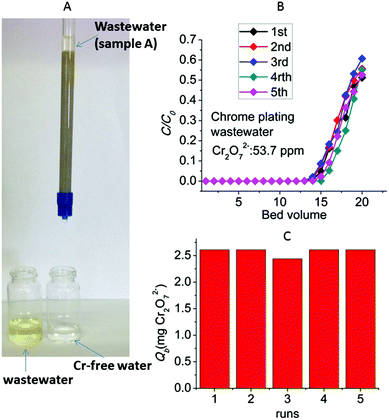
A common type of Cr(VI)-containing industrial waste is the chrome plating wastewater. Such Cr(VI)-bearing water is generated by rinsing the plated parts upon their removal from the plating bath.15 It should be noted that the Cr(VI) concentration in metal plating effluents may vary from very high to moderate or low levels depending on the amount of water used in the rinsing step of the metal plating process. Thus, Cr(VI) concentrations greater than 1000 and lower than 10 ppm have been reported for metal plating waste.9b,16,17Encouraged by the above excellent column sorption results, we decided to test the performance of the column for the removal of Cr(VI) from chrome plating wastewater. A metal plating company (located in North Greece) provided us with two different types of hexavalent chromium waste: one sample (A) contained dichromate ions in very high concentration (4855 ppm, pH ∼ 1.6) and the second sample (B) was a neutral pH solution with lower Cr(VI) content (analysis of the Cr(VI) concentration of this solution was done after adjusting its pH to ∼3, see below).
Sample A was too concentrated to be treated with our laboratory-scale ion exchange columns. Thus, Cr(VI)-contaminated wastewater was prepared by diluting the original sample A to ∼54 ppm of Cr2O72− (pH ∼ 3.5 after the dilution). The column is very efficient to decontaminate such wastewater, something that can be seen even with the naked eye (Fig. 13A). The breakthrough curves (Fig. 13B) obtained from five column runs (with regeneration of the column after each run) indicate 13–14 bed volumes with total Cr concentration <50 ppb (EU defined acceptable Cr limit) and a breakthrough capacity of 2.44–2.63 mg Cr2O72− (Fig. 13C). These breakthrough capacity values are similar to those obtained for the experiments with the laboratory prepared dichromate solutions.
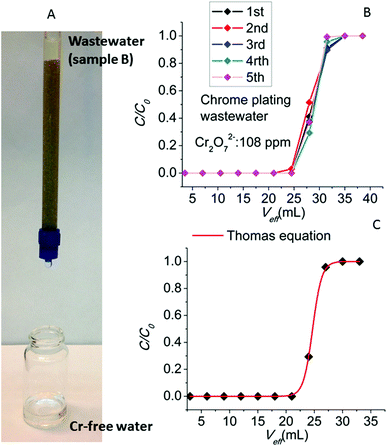
We have also studied the column ion-exchange properties with sample B supplied by the metal plating company. Prior to the sorption experiments, the pH of sample B was adjusted to ∼3 in order to enable the UV-Vis analysis of Cr(VI) as Cr2O72− anions (at neutral pH there is equilibrium between chromate and dichromate anions4) and allow us to compare the results for the synthetic dichromate solutions. The UV-Vis data for the sample B revealed a concentration of Cr2O72− of 108 ppm. The decontamination of the wastewater sample after its treatment with the MOR-1-HA/sand column was apparent even with the naked eye (Fig. 14A). Five runs of the column with the sample B (Fig. 14B) revealed the breakthrough capacities of 2.268–2.646 mg, relatively close to those observed for the experiments with the sample A and the laboratory prepared solutions. In these column ion-exchange experiments, due to the relatively high initial Cr(VI) concentration of sample B, the sorbent rapidly reaches its complete saturation with Cr(VI).
The MOR-1-HA/sand column performance for the decontamination of sample B can be very well modelled by the Thomas equation:13
 | (5) |
Finally, another important feature of the breakthrough curve is the degree of column utilization defined as the ratio of breakthrough to total column sorption capacity.12,13 For practical applications, it is desirable to achieve a degree of column utilization as close as possible to unity. For the column ion exchange experiments with the wastewater sample B, the degree of column utilization (%) lies in the range of 78–89%, thus revealing the highly efficient performance of the MOR-1-HA sand column.
Conclusions
In conclusion, a new rapid, green and low cost synthetic method for MOR-1 and its composite MOR-1-HA material was developed, which involves 1 h reflux reaction in water–acetic acid solvent (yielding MOR-1) and addition of sodium alginate (SA) to the resulting suspension of MOR-1 under reflux conditions (yielding MOR-1-HA). Our hydrothermal synthesis approach, for the first time, afforded UiO-66 type amino-functionalized materials which are purely microporous as the well-known UiO-66-NH2BDC compound prepared by the solvothermal method. It is important that we can prepare high quality UiO-66 amino-functionalized compounds very fast via an inexpensive reflux synthesis in acidified water, since such materials are of great interest not only for their Cr(VI) sorption capacity but also for their gas sorption and photocatalytic properties, post-synthetic chemistry etc. Furthermore, the presented synthetic method reveals the usefulness of sodium alginate (SA), being (a) the precursor for the formation of the alginic acid component of the composite and (b) a flocculation agent for the easy separation of MOR-1 from its fine suspension in water. Thus, SA could be useful for the large scale synthesis and facile isolation of a number of metal organic materials forming colloidal water solutions such as various UiO-66, UiO-67 analogues, MOFs of the MIL-family etc.Detailed batch Cr(VI) sorption studies for the MOR-1-HA composite isolated by the new method revealed its exceptional capability to absorb Cr(VI) under various conditions. This sorbent is particularly capable to be used in ion-exchange columns. It consists of relatively compact and polyhedral nanoparticles that can be uniformly distributed in the column allowing a stable flow rate. In addition, this sorbent, due to the coating of MOR-1 particles by alginic acid, is not easily dispersed in water (in contrast to the pristine MOR-1 material) and thus, it can be immobilized in the stationary phase of the column. Thus, an ion-exchange column containing MOR-1-HA showed relatively high Cr(VI) sorption capacities reproducibly as well as excellent regeneration capability and reusability. Compared to the columns with the composite isolated from solvothermally prepared MOR-1, the column with MOR-1-HA synthesized by the new method exhibits an improved performance. Importantly, this column is highly efficient for the removal of Cr(VI) not only from laboratory prepared solutions but also from industrial wastewater samples. Overall, the results presented here indicate that the MOR-1-HA ion-exchange column could be inexpensive, considering the relatively low cost of the new synthetic method for MOR-1-HA, and also promising for the remediation of real-world wastewater. The next step of this research could be thus the development of large scale MOR-1-HA columns and their application in wastewater treatment plants.
Acknowledgements
We thank the metal plating company (A. Nestorides&Sons, Sindos, Thessaloniki, Greece) for providing us with the chrome plating wastewater samples. We also acknowledge Dr M. Kaliva and Ms A. Manousaki (FORTH-IESL) for performing the FE-SEM measurements. The work performed at Northwestern University was supported by National Science Foundation grant DMR-1410169.References
- (a) M. Eddaoudi, D. B. Moler, H. L. Li, B. L. Chen, T. M. Reineke, M. O'Keeffe and O. M. Yaghi, Acc. Chem. Res., 2001, 34, 319 CrossRef CAS PubMed; (b) G. Ferey, Chem. Soc. Rev., 2008, 37, 191 RSC; (c) S. Horike, S. Shimomura and S. Kitagawa, Nat. Chem., 2009, 1, 695 CrossRef CAS PubMed; (d) D. Bradshaw, J. B. Claridge, E. J. Cussen, T. J. Prior and M. J. Rosseinsky, Acc. Chem. Res., 2005, 38, 273 CrossRef CAS PubMed; (e) O. K. Farha and J. T. Hupp, Acc. Chem. Res., 2010, 43, 1166 CrossRef CAS PubMed; (f) S. T. Zheng, T. Wu, J. A. Zhang, M. Chow, R. A. Nieto, P. Y. Feng and X. H. Bu, Angew. Chem., Int. Ed., 2010, 49, 5362 CrossRef CAS PubMed; (g) J. M. Taylor, T. Komatsu, S. Dekura, K. Otsubo, M. Takata and H. Kitagawa, J. Am. Chem. Soc., 2015, 137, 11498 CrossRef CAS PubMed.
- (a) H. H. Fei, M. R. Bresler and S. R. J. Oliver, J. Am. Chem. Soc., 2011, 133, 11110 CrossRef CAS PubMed; (b) H. H. Fei, C. S. Han, J. C. Robins and S. R. J. Oliver, Chem. Mater., 2013, 25, 647 CrossRef CAS; (c) H. R. Fu, Z. X. Xu and J. Zhang, Chem. Mater., 2015, 27, 205 CrossRef CAS; (d) X. X. Li, H. Y. Xu, F. Z. Kong and R. H. Wang, Angew. Chem., Int. Ed., 2013, 52, 13769 CrossRef CAS PubMed; (e) P. F. Shi, B. Zhao, G. Xiong, Y. L. Hou and P. Cheng, Chem. Commun., 2012, 48, 8231 RSC; (f) Q. Zhang, J. Yu, J. Cai, L. Zhang, Y. Cui, Y. Yang, B. Chen and G. Qian, Chem. Commun., 2015, 51, 14732 RSC.
- S. Rapti, A. Pournara, D. Sarma, I. T. Papadas, G. S. Armatas, A. C. Tsipis, T. Lazarides, M. G. Kanatzidis and M. J. Manos, Chem. Sci., 2016 10.1039/C5SC03732H , Advance Article.
- (a) M. Costa and C. B. Klein, Crit. Rev. Toxicol., 2006, 36, 155 CrossRef CAS PubMed; (b) F. Brito, J. Ascanio, S. Mateo, C. Hernandez, L. Araujo, P. Gili, P. MartinZarza, S. Dominguez and A. Mederos, Polyhedron, 1997, 16, 3846 CrossRef.
- (a) USA-EPA. Chromium in drinking water, http://water.epa.gov/drink/info/chromium/; (b) European Commission. Council Directive 98/83/EC on the quality of water intended for human consumption, http://eur lex.europa.eu/legal content/EN/TXT/?uri=CELEX:31998L0083.
- (a) J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850 CrossRef PubMed; (b) M. J. Katz, Z. J. Brown, Y. J. Colon, P. W. Siu, K. A. Scheidt, R. Q. Snurr, J. T. Hupp and O. K. Farha, Chem. Commun., 2013, 49, 9449 RSC; (c) M. Kandiah, M. H. Nilsen, S. Usseglio, S. Jakobsen, U. Olsbye, M. Tilset, C. Larabi, E. A. Quadrelli, F. Bonino and K. P. Lillerud, Chem. Mater., 2010, 22, 6632 CrossRef CAS.
- Z. Hu, Y. Peng, Z. Kang, Y. Qian and D. Zhao, Inorg. Chem., 2015, 54, 4862 CrossRef CAS PubMed.
- M. A. Hughes, Solid-Liquid Separation, ed. L. Svarovsky, Butterworth-Heinemann, 2000, pp. 104–128 Search PubMed.
- (a) M. Bosnic, J. Buljan, R. P. Daniels and S. Rajamani, Pollutants in tannery effluent, published online: 2003. http://leatherpanel.org/sites/default/files/publications-attachments/polutants.pdf; (b) F. Akbal and S. Camci, Desalination, 2011, 269, 214 CrossRef CAS.
- (a) M. J. Manos, N. Ding and M. G. Kanatzidis, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 3696 CrossRef CAS PubMed; (b) M. J. Manos and M. GKanatzidis, J. Am. Chem. Soc., 2009, 131, 6599 CrossRef CAS PubMed; (c) M. J. Manos and M. G. Kanatzidis, Chem. – Eur. J., 2009, 15, 4779 CrossRef CAS PubMed; (d) M. J. Manos and M. G. Kanatzidis, J. Am. Chem. Soc., 2012, 134, 16441 CrossRef CAS PubMed; (e) M. J. Manos, V. G. Petkov and M. G. Kanatzidis, Adv. Funct. Mater., 2009, 19, 1087 CrossRef CAS; (f) J. L. Mertz, Z. H. Fard, C. D. Malliakas, M. J. Manos and M. G. Kanatzidis, Chem. Mater., 2013, 25, 2116 CrossRef CAS; (g) D. Sarma, C. D. Malliakas, K. S. Subrahmanyam, S. M. Islam and M. G. Kanatzidis, Chem. Sci., 2016, 7, 1121 RSC.
- A. Benhammou, A. Yaacoubi, L. Nibou and B. Tanouti, J. Colloid Interface Sci., 2005, 282, 320 CrossRef CAS PubMed.
- A. A. Zagorodni, Ion Exchange Materials, Properties and Applications, Elsevier, 2007, pp. 243–281 Search PubMed.
- V. J. Inglezakis and S. G. Poulopoulos, Adsorption, Ion Exchange and Catalysis. Design of Operations and Environmental Applications, Elsevier, 2006 Search PubMed.
- N. P. Cheremisinoff, Handbook of water and water treatment technologies, Butterworth-Heinemann, 2002, p. 83 Search PubMed.
- N. V. Mandich and D. L. Snyder, Electrodeposition of Chromium, Modern Electroplating, 5th edn, 2010, pp. 205–249 Search PubMed.
- V. Boddu, K. H. Abburi, J. L. Talbott and E. Smith, Environ. Sci. Technol., 2003, 37, 4449 CrossRef CAS PubMed.
- L.-Y. Chang, Chrome reduction and heavy metals removal from wastewater – A pollution prevention approach, Proceedings of WM-01 Conference, Tucson, AZ, February 25-March 1, 2001 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, thermal analysis, CO2 sorption data and pore size distribution, UV-Vis data, breakthrough curves, and fitting of batch and column Cr(VI) sorption data. See DOI: 10.1039/c5qi00303b |
| This journal is © the Partner Organisations 2016 |