A new methylviologen-templated zinc gallophosphate zeolite with photo-/thermochromism, fluorescent and photoelectric properties†
Chunyao
Tao
,
Junbiao
Wu
,
Yan
Yan
,
Chao
Shi
and
Jiyang
Li
*
State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, Changchun 130012, P. R. China. E-mail: lijiyang@jluedu.cn; Fax: +(86)431-85168614
First published on 21st January 2016
Abstract
A new zinc gallophosphate zeolite [Zn5.8Ga14.2(PO4)20F3.2(H3O)3]|C12H14N2|3·3H2O (noted as JU104) with a USI zeotype structure has been solvothermally synthesized by using in situ generated methylviologen (MV) as the template. JU104 has two-dimensional interconnected 12-ring and 10-ring channels running along the [100] and [0![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0] direction, respectively. It exhibits dual thermochromism and photochromism with a broad photo-response range (UV light to visible light) and a long-lived charge-separated state, as well as variable fluorescent and photoelectric properties. Moreover, the multi photo/thermo-induced colors occurring in JU104 make it unusual as compared to other inorganic photo/thermochromism compounds. The MV-templated JU104 zeolite may function as a new efficient electron-transfer system for potential applications in photo/thermochromism and solar energy conversion.
0] direction, respectively. It exhibits dual thermochromism and photochromism with a broad photo-response range (UV light to visible light) and a long-lived charge-separated state, as well as variable fluorescent and photoelectric properties. Moreover, the multi photo/thermo-induced colors occurring in JU104 make it unusual as compared to other inorganic photo/thermochromism compounds. The MV-templated JU104 zeolite may function as a new efficient electron-transfer system for potential applications in photo/thermochromism and solar energy conversion.
Introduction
Zeolites are an important class of inorganic nanoporous materials with three-dimensional (3D) crystalline frameworks composed of TO4 tetrahedra (T = Si, Al, P, etc.).1,2 These materials have wide applications as catalysts, adsorbents and ion-exchangers in petroleum refining, the petrochemical industry, and the fine chemical industry.3–5 Besides these traditional applications, zeolites have also emerged as advanced functional materials applied in the fields of luminescence, electricity, magnetism, medicine, proton conduction, carbon dots, microelectronics, etc.6–8 These new properties will promise more exciting advances in the application of zeolite materials.Photochromic materials have attracted considerable attention because of their potential applications in protection, decoration, displays, memory, switches, photography, photometry, photomechanics and so on.9–11 More recently, photochromism as an emerging property has also been found in zeolite materials by introducing photochromic organic species, methylviologen (MV), as the templates and/or electron-transfer intermediates. Methylviologen has been introduced into zeolite materials including Na-MOR, zeolite-L, Naβ, NaZSM-5, NaX and NaY through ion-exchange to achieve a long-lived charge separation for solar energy conversion, but the amounts of methylviologen dications are limited in these zeolites which affects their resulting properties.12–17 JU101 is the only known photochromic zeolite based on the MV template, which exhibits interesting photochromism, thermochromism, and a tuneable photovoltaic activity.18
A previous study reveals that the photochromic properties of MV are strongly influenced by the electron-transfer pathway between the surrounding anionic framework and MV2+ cations. Therefore, the cooperation between various inorganic zeolite frameworks and the organic photochromic molecules can make it possible to generate more new photochromic materials with unusual properties, such as a distinct photo-responsive range from visible light, UV to X-ray and different photochromic colorations, as well as photoluminescence and photoelectricity.19 However, little success has been achieved in the field of zeolite materials although several photochromic open-framework and metal–organic framework (MOF) materials have been reported involving MV as templates or ligands.20–24
Herein, we present a new photochromic zinc gallophosphate zeolite JU104 ([Zn5.8Ga14.2(PO4)20F3.2(H3O)3]|C12H14N2|3·3H2O) synthesized by the utilization of MV as the template. JU104 is the second MV-templated zeolite, which possesses the USI zeotype topology with intersecting 10-ring and 12-ring channels. The MV-templated JU104 exhibits photochromism with a photo-response from visible to UV light and thermochromism with a thermal response temperature of 250 °C. Interestingly, it displays multi photo/thermo-induced colors that rarely occur in other photo/thermochromism compounds. Also, JU104 shows variable fluorescent and photoelectric properties in response to light and heating.
Experimental section
Materials
The source materials used in the synthesis of JU104 are zinc acetate dihydrate (Zn(OAc)2·2H2O, 99.0%, Xilong Chemical Reagent Co., Ltd), gallium(III) oxide (Ga2O3, 99.999%, Sinopharm Chemical Reagent Co., Ltd), phosphorous acid (H3PO3, 98.0%, J&K Chemical Technology), 4,4′-bipyridine (C10H8N2·2H2O, 98.0%, Shanghai Kefeng Industry & Commerce Co., Ltd), hydrofluoric acid (HF, 40%, Beijing Chemical Works), deionized water (H2O, 100%, Milli-Q Integral Water purification system) and anhydrous methanol (CH3OH, 99.5% Tianjin Guangfu Fine Chemical Research Institute). The reagents and solvents employed have been used as received without further purification.Synthesis of JU104
JU104 was synthesized from a reaction mixture of Zn(OAc)2·2H2O (0.11 g, 0.5 mmol), Ga2O3 (0.187 g, 1 mmol), H3PO3 (0.164 g, 2 mmol), 4,4′-bipyridine (0.192 g, 1 mmol) and HF (0.1 ml, 2.25 mmol) in a mixture solvent of methanol (5 ml) and water (2 ml) with a molar composition of 1.0 ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 Ga2O3
2.0 Ga2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.0 H3PO3
4.0 H3PO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 4,4′-bipyridine
2.0 4,4′-bipyridine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5HF
4.5HF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 248.0 CH3OH
248.0 CH3OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 222.0 H2O at 170 °C for 7 days in a Teflon-lined stainless steel autoclave. The pale yellow crystals of JU104 (yields: about 6%) mixed with an amorphous phase were filtered and washed with distilled water and ethanol, and then dried at room temperature. Note that the initially added H3PO3 was oxidized into PO43− anions in this reaction system, and JU104 could not be produced in the presence of H3PO4. This indicates that the utilization of H3PO3 is important for the synthesis of the target product, and the role of H3PO3 in the synthesis needs to be further investigated. MV2+ cations were formed by an in situ reaction of 4,4′-bipyridine and methanol under acidic conditions, which has been observed in previous reports.18,23,24 The existence of MV2+ cations was confirmed by a high resolution liquid chromatography-mass spectrum (Fig. S1†). The crystals of JU104 were selected from the mixture product for the following characterization.
222.0 H2O at 170 °C for 7 days in a Teflon-lined stainless steel autoclave. The pale yellow crystals of JU104 (yields: about 6%) mixed with an amorphous phase were filtered and washed with distilled water and ethanol, and then dried at room temperature. Note that the initially added H3PO3 was oxidized into PO43− anions in this reaction system, and JU104 could not be produced in the presence of H3PO4. This indicates that the utilization of H3PO3 is important for the synthesis of the target product, and the role of H3PO3 in the synthesis needs to be further investigated. MV2+ cations were formed by an in situ reaction of 4,4′-bipyridine and methanol under acidic conditions, which has been observed in previous reports.18,23,24 The existence of MV2+ cations was confirmed by a high resolution liquid chromatography-mass spectrum (Fig. S1†). The crystals of JU104 were selected from the mixture product for the following characterization.
Characterization
Powder X-ray diffraction (PXRD) data were collected on a Rigaku D/max-2550 diffractometer with Cu Kα radiation (λ = 1.5418 Å). The infrared spectrum was collected on IFS-66 V/S from Bruker. Thermogravimetric (TG) analysis was conducted on a STA 449C analyzer in air with a heating rate of 10 °C min−1 from RT to 1200 °C. It gave an obvious weight loss of ca. 19.68 wt% from RT to 1200 °C (Fig. S2†). Inductively coupled plasma (ICP) analysis was performed on a Perkin-Elmer Optima 3300DV spectrometer, giving Zn 9.07%, Ga 24.14%, P 15.96%. Elemental analysis was performed on a Perkin-Elmer 2400 elemental analyzer, found C 10.99%, H 1.50% and N 2.19% (calcd Zn 9.48%, Ga 24.74%, P 15.50%, C 10.80%, H 1.42%, N 2.10% and F 1.52%). F− ion selective electrode analysis was performed on a Mettler Toledo instrument to confirm the existence of F− ions (found: 1.62%) in the compound. High resolution liquid chromatography-mass spectroscopy (LC-HRMS) was performed on a HPLC: Agilent 1290 and HRMS: MicroTOF-Q II (Bruker Daltonics, Bremen, Germany). The UV/Vis absorption spectra were recorded at room temperature on a Shimadzu U-4100 spectrophotometer. Electron paramagnetic resonance (EPR) spectroscopy was conducted on a JEOL JES-FA200 EPR spectrometer. Photoluminescence spectra of the samples were obtained from a Fluoromax-4 spectrofluorometer (Horiba). The fluorescence quantum yield was recorded on FLS920 (Edinburgh Instrument). The surface photovoltage (SPV) measurement system was composed of a monochromatic light source, a lock-in amplifier (SR830-DSP) with a light chopper (SR540), a sample cell and a computer. A 500 W xenon lamp and a double-prism monochromator provided the monochromatic light. In the photovoltaic cell, the powder sheet was directly sandwiched between two blank indium tin oxide (ITO) electrodes. Field-induced surface photovoltage spectroscopy (FISPS) is a supplement to the SPV spectroscopy method. In FISPS, the external electric fields were applied between the two electrodes.Single-crystal structure determination
A suitable single crystal of JU104 with dimensions of 0.20 × 0.18 × 0.17 mm3 was selected for single-crystal X-ray diffraction analysis. The intensity data were collected on a Bruker SMART APEXII CCD diffractometer by oscillation scans using graphite-monochromated Mo Kα radiation (λ = 0.71073 Å) at a temperature of 23 ± 2 °C. Cell refinement and data reduction were accomplished with the SAINT processing program.25 The structure was solved in the space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) by direct methods and refined by the full matrix least-squares technique with the SHELXTL crystallographic software package.26 The heaviest atoms of Zn/Ga, P and O could be unambiguously located, but the C, N and F atoms were not located due to the highly disordered structure. Therefore, the SQUEEZE routine of PLATON was used to remove the diffraction contribution from guests to produce a set of guest-free diffraction intensities. The existence of guest species in JU104, such as MV2+ cations, F− ions and H2O molecules will be determined by the compositional and TGA analyses. All non-hydrogen atoms were refined anisotropically. Experimental details for the structure determination are presented in Tables S1 and S2, ESI.†
by direct methods and refined by the full matrix least-squares technique with the SHELXTL crystallographic software package.26 The heaviest atoms of Zn/Ga, P and O could be unambiguously located, but the C, N and F atoms were not located due to the highly disordered structure. Therefore, the SQUEEZE routine of PLATON was used to remove the diffraction contribution from guests to produce a set of guest-free diffraction intensities. The existence of guest species in JU104, such as MV2+ cations, F− ions and H2O molecules will be determined by the compositional and TGA analyses. All non-hydrogen atoms were refined anisotropically. Experimental details for the structure determination are presented in Tables S1 and S2, ESI.†
Simulation method
As the positions of C and N atoms are highly disordered, computer simulation was used to determine the positions of MV2+ cations in the pores of JU104. Calculations were performed in Materials Studio software 4.0 by using the Universal force field.27 The crystal symmetry of JU104 was decreased to P1 to fit the location of MV2+ cations. Based on compositional and TG analyses, there are about 1.5 MV2+ cations in one unit cell, so 6 MV2+ cations were put in the pores of four unit cells, and the positions of MV2+ cations were optimized by using energy minimization with fixing of the inorganic framework.Results and discussion
Structure of JU104
JU104 crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . The asymmetric unit of JU104 contains five unique M sites (M = Ga or Zn) and five unique P sites, in which one of the M sites is split (Fig. S3†). All of the M and P atoms are tetrahedrally connected with oxygen atoms. The average P–O distance is 1.522 Å, and the average M–O distance is 1.844 Å which ranges from the ideal Ga–O distance (1.82 Å) and Zn–O distance (1.95 Å), thus the Zn and Ga sites in the structure cannot be distinguished through single crystal X-ray diffraction. The ratio of Zn/Ga is about 2/5 determined by ICP analysis.
. The asymmetric unit of JU104 contains five unique M sites (M = Ga or Zn) and five unique P sites, in which one of the M sites is split (Fig. S3†). All of the M and P atoms are tetrahedrally connected with oxygen atoms. The average P–O distance is 1.522 Å, and the average M–O distance is 1.844 Å which ranges from the ideal Ga–O distance (1.82 Å) and Zn–O distance (1.95 Å), thus the Zn and Ga sites in the structure cannot be distinguished through single crystal X-ray diffraction. The ratio of Zn/Ga is about 2/5 determined by ICP analysis.
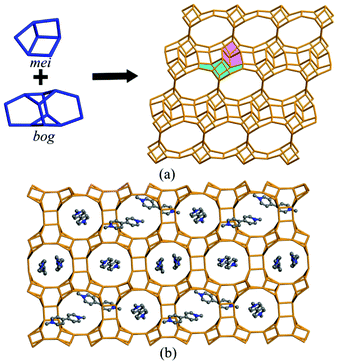
The connection of MO4 (M = Ga, Zn) and PO4 tetrahedra constructs the anionic [Zn5.8Ga14.2(PO4)20]5.8− framework of JU104, and MV2+ cations, F− ions and protonated H3O+ cations are located in the pores to achieve the charge balance (Fig. 1a). Two composite building units (CBUs): mei and bog can be found in the framework of JU104, which are connected to each other through sharing edges to form the three-dimensional (3D) framework structure. The structure of JU104 possesses the USI zeotype topology. It has a 2D interconnected channel system with 10-ring channels running along the [0![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0] direction and 12-ring channels running along the [100] direction. About 1.5 MV2+ cations are located in one unit cell of JU104 on the basis of compositional and TG analyses, and their positions are theoretically simulated by using Materials Studio Software (Fig. 1b).27 So far, the cobalt gallium phosphate IM-6 is the only known USI zeolite, which is synthesized by using N-substituted piperazine as the template.28 The different organic templates used here result in the distinct framework composition of JU104.
0] direction and 12-ring channels running along the [100] direction. About 1.5 MV2+ cations are located in one unit cell of JU104 on the basis of compositional and TG analyses, and their positions are theoretically simulated by using Materials Studio Software (Fig. 1b).27 So far, the cobalt gallium phosphate IM-6 is the only known USI zeolite, which is synthesized by using N-substituted piperazine as the template.28 The different organic templates used here result in the distinct framework composition of JU104.
Photochromism and thermochromism
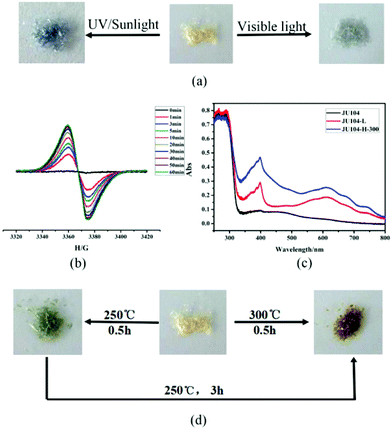
JU104 is the second MV-templated zeolite material, which is distinct from JU101 that has a novel zeotype structure with 8- and 10-ring channels. Thus, its chromic behaviours are also different. Under the irradiation of 20 W UV light, some of the pale yellow crystals of JU104 change to light grey after 10 min, and finally all samples transform to steel grey crystals (noted as JU104-L) with increasing irradiation time (Fig. 2a). Upon irradiation of sunlight, a rapid color transformation can be observed after 5 min, yielding steel grey crystals of JU104-L after 0.5 h. When JU104 is placed under visible light (λ > 400 nm) for 1 h, the crystals become light grey (Fig. 2a). This indicates that JU104 are photo-sensitive to UV and visible light. Such an extended photo-response range may be ascribed to the short electron-transfer pathway between the anionic-framework donor and the MV2+ acceptor in JU104, i.e. about 3.20 Å based on the simulation results. This value is smaller than that of MV2+-containing zinc gallophosphate zeolite JU101 (3.28 Å) which is also sensitive to visible light.18 However, the photochromism coloration of JU104 and JU101 is different. Under photoirradiation, JU101 crystals change from colorless to blue, while the color change of JU104 is from pale yellow to light grey (under visible light) or steel grey (under UV light). This result demonstrates that the required threshold energy for photochromism is mainly determined by the electron transfer pathway between the MV2+ cations and the inorganic framework, and the photoinduced color change of MV2+ is strongly influenced by the surrounding inorganic anionic framework and the packing of guest cations in the host framework.In situ time dependent electron paramagnetic resonance (EPR) spectroscopy at room temperature shows that there is almost no EPR signal for the as-synthesized JU104, while a signal at g = 2.0050 is detected after the UV light irradiation of JU104 for 1 min, and its intensity increases with increasing irradiation time (Fig. 2b). This phenomenon can be attributed to the generation of the MV˙+ radicals reduced from MV2+ cations after photoirradiation. As shown in Fig. 2c, two absorption bands at 400 nm (sharp) and 615 nm (broad) associated with MV˙+ radicals are also observed for JU104-L in the UV/Vis absorption spectra. This indicates that the electron transfer occurs between the anionic zeolite framework and the MV2+ cations, generating MV˙+ radicals upon photoirradiation. Such a phenomenon has been observed previously in the MV-containing compounds.18,20–24,29,30
Interestingly, JU104 also displays thermochromic behavior. As shown in Fig. 2d, black green crystals (noted as JU104-H-250) are observed when JU104 is heated at 250 °C for 0.5 h, while kelly green crystals (noted as JU104-H-300) are produced upon heating JU104 at 300 °C for 0.5 h. On further heating JU104-H-250 at 250 °C for 3 h, black green crystals can transform into kelly green crystals. The different thermochromic color may be caused by the loss of a part of guest water molecules upon heating. The generation of MV˙+ radicals upon heating is revealed by UV/Vis absorption spectra and EPR (Fig. 2c and S4†), indicating that thermochromic transformation also arises from the electron transfer process.
As we know, thermochromism rarely occurs in crystalline solid materials because high temperatures facilitate charge-recombination. To the best of our knowledge, the only known dual photochromic and thermochromic solid crystalline materials are zeolite JU101, two MV-based MOFs and a solid-state coordination compound.18,21,22,31 These compounds all exhibit similar color change under irradiation and heating. Compared with JU101 (300 °C), JU104 has a lower thermal-response temperature (250 °C). Moreover, it also exhibits a distinct color change for thermochromism and photochromism. Under visible light and UV irradiation, JU104 crystals change from pale yellow to light grey and steel grey, respectively. Upon heating, pale yellow crystals of JU104 can transform into black green or kelly green crystals depending on the temperature and heating time. Such multi photo/thermoinduced colours observed in JU104 make it unusual for other inorganic photo/thermochromism compounds, which may have potential applications in detection/sensing. Moreover, the framework of JU104 has good thermal stability, and the inorganic framework and the MV cations remain unchanged under photochromism and thermochromism (300 °C) (Fig. S5†).
The photochromism and thermochromism of JU104 are reversible but they need several weeks or even months. There is an obvious color change after JU104-L is kept in the dark for 6 weeks, and steel grey crystals finally change to pale yellow crystals when JU104-L is kept in the dark for 4 months. Correspondingly, the EPR signal associated with MV˙+ radicals obviously decreases or disappears (Fig. S6†). As for JU104-H-300, the EPR signal disappears after placing it in the dark for about 4 months, but the color of JU104-H-300 is only a little faded (Fig. S4†). This indicates that JU104-H-300 is more difficult to bleach as compared to JU104-L.
The slow reversible photochromic and thermochromic transformation shows that JU104 has a long-lived charge-separated state upon photoirradiation and heating, which may be used in the conversion process from solar energy to chemical energy.32 This phenomenon has been observed in JU101 and two reported MV-based MOFs.18,21,22 The reason may also be the dense packing of MV2+ cations in the 10-ring and 12-ring pores of JU104, which prevents the contact of the generated MV˙+ radicals with framework oxygen atoms.
Photoluminescence
JU104 shows strong photoluminescence (Fig. 3) in the solid state at room temperature. The maximum fluorescent emission is observed at 410 nm upon 365 nm excitation. As with MV-containing JU99, the luminescence of JU104 may be ascribed to the charge transfer from the anionic framework to MV2+ cations.16 The fluorescence quantum yield of JU104 is 12.70%, which is comparable to the metal activator-free luminescent open-framework metal phosphates, such as NTHU-3 (10%), NTHU-10 (11%) and NTHU-14 (8%–10%).33–35 Notably, the UV/visible light irradiation and heating treatment have a regulating effect on the photoluminescence of JU104; the emission intensity of JU104 largely decreases after UV/visible light irradiation and heating (Fig. 3).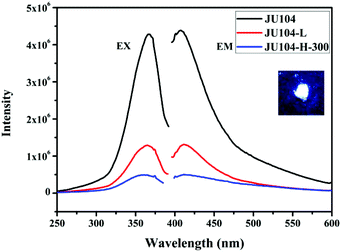 | ||
| Fig. 3 The excitation and emission spectra of JU104, JU104-L and JU104-H-300. Inset is the image of JU104 under 365 nm UV light in the dark. | ||
Photovoltaic properties
Studies on the steady state surface photovoltage spectroscopy (SPV) show that JU104 also possesses photovoltaic properties. When illuminated without an external electric field, an SPV response in the range of 300–350 nm (max. 325 nm) is observed for JU104 (Fig. 4). As the positive electric-field strengthens (0.3 V and 0.5 V), the intensity of the SPV response increases according to field-induced surface photovoltage spectroscopy. It suggests that JU104 had the properties of a semiconductor. The intensity of the SPV response slightly decreased for JU104 after light irradiation or heating (Fig. 4). This indicates that the formation of MV˙+ on irradiation and heating treatment weakens the photogeneration of excess carriers and their spatial separation. The detailed mechanism of the intriguing photovoltaic properties of JU104 needs further investigation.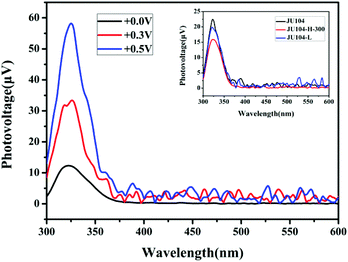 | ||
| Fig. 4 FISPS of JU104 under different positive electric-fields. Inset: SPV spectra of JU104, irradiated JU104-L and heated JU104-H-300. | ||
Conclusions
In summary, a new multifunctional zeolite, zinc galliumphosphate JU104 templated by MV has been successfully synthesized. It possesses a USI zeotype topology with two-dimensional interconnected 10-ring and 12-ring channel systems. As a photo-/thermochromic material, JU104 exhibits an extended photo-response range from UV light to visible light, a long-lived charge-separated state and multiform photo-/thermochromic coloration. JU104 also presents luminescence and photoelectric properties, which can be tunable by light irradiation or heating treatment. This work further demonstrates that the utilization of MV as a template may provide more opportunities to synthesize novel photo/thermo-responsive materials.Acknowledgements
We thank the National Natural Science Foundation of China (no.: 21271081) for financial support.Notes and references
- Y. Li and J. Yu, Chem. Rev., 2014, 114, 7268–7316 CrossRef CAS PubMed.
- J. Li, A. Corma and J. Yu, Chem. Soc. Rev., 2015, 44, 7112–7127 RSC.
- A. Corma, Chem. Rev., 1997, 97, 2373–2420 CrossRef CAS PubMed.
- M. E. Davis, Nature, 2002, 417, 813–821 CrossRef CAS PubMed.
- Y. Li, X. Li, J. Liu, F. Duan and J. Yu, Nat. Commun., 2015, 6, 8328–8336 CrossRef CAS PubMed.
- Y. Mu, Y. Wang, Y. Li, J. Li and J. Yu, Chem. Commun., 2015, 51, 2149–2151 RSC.
- Y. Sun, Y. Yan, Y. Wang, Y. Li, J. Li and J. Yu, Chem. Commun., 2015, 51, 9317–9319 RSC.
- Y. Wang, Y. Li, Y. Yan, J. Xu, B. Guan, Q. Wang, J. Li and J. Yu, Chem. Commun., 2013, 49, 9006–9008 RSC.
- I. Yildiz, E. Deniz and F. M. Raymo, Chem. Soc. Rev., 2009, 38, 1859–1867 RSC.
- M. Irie, T. Fukaminato, T. Sasaki, N. Tamai and T. Kawai, Nature, 2002, 420, 759–760 CrossRef CAS PubMed.
- M.-S. Wang, G. Xu, Z.-J. Zhang and G.-C. Guo, Chem. Commun., 2010, 46, 361–376 RSC.
- K. B. Yoon, Chem. Rev., 1993, 93, 321–339 CrossRef CAS.
- P. K. Dutta and M. Severance, J. Phys. Chem. Lett., 2011, 2, 467–476 CrossRef CAS.
- M. Alvaro, H. García, S. García, F. Márquez and J. C. Scaiano, J. Phys. Chem. B, 1997, 101, 3043–3051 CrossRef CAS.
- B. Hennessy, S. Megelski, C. Marcolli, V. Shklover, C. Bärlocher and G. Calzaferri, J. Phys. Chem. B, 1999, 103, 3340–3351 CrossRef CAS.
- E. L. Clennan, Coord. Chem. Rev., 2004, 248, 477–492 CrossRef CAS.
- K. T. Ranjit and L. Kevan, J. Phys. Chem. B, 2002, 106, 1104–1109 CrossRef CAS.
- J. Wu, C. Tao, Y. Li, J. Li and J. Yu, Chem. Sci., 2015, 6, 2922–2927 RSC.
- R. Pardo, M. Zayat and D. Levy, Chem. Soc. Rev., 2011, 40, 672–687 RSC.
- P.-C. Jhang, N.-T. Chuang and S.-L. Wang, Angew. Chem., Int. Ed., 2010, 49, 4200–4204 CrossRef CAS PubMed.
- H. Chen, G. Zheng, M. Li, Y. Wang, Y. Song, C. Han, J. Dai and Z. Fu, Chem. Commun., 2014, 50, 13544–13546 RSC.
- Y. Zeng, Z. Fu, H. Chen, C. Liu, S. Liao and J. Dai, Chem. Commun., 2012, 48, 8114–8116 RSC.
- J. Wu, Y. Yan, B. Liu, X. Wang, J. Li and J. Yu, Chem. Commun., 2013, 49, 4995–4997 RSC.
- J. Wu, C. Tao, Y. Li, Y. Yan, J. Li and J. Yu, Chem. Sci., 2014, 5, 4237–4241 RSC.
- SAINT, Bruker AXS Inc., 5465 East Cheryl Parkway, Madison, WI, 53711-5373, USA, 2000 Search PubMed.
- SHELXTL, Bruker AXS Inc., 5465 East Cheryl Parkway, Madison, WI, 53711-5373, USA, 2000 Search PubMed.
- Materials Studio software 4.0.
- L. Josien, A. Simon-Masseron, V. Gramlich, J. Patarin and L. Rouleau, Chem. – Eur. J., 2003, 9, 856–861 CrossRef CAS PubMed.
- G. Xu, G.-C. Guo, M.-S. Wang, Z.-J. Zhang, W.-T. Chen and J.-S. Huang, Angew. Chem., Int. Ed., 2007, 46, 3249–3251 CrossRef CAS PubMed.
- G. Xu, G.-C. Guo, J.-S. Guo, S.-P. Guo, X.-M. Jiang, C. Yang, M.-S. Wang and Z.-J. Zhang, Dalton Trans., 2010, 39, 8688–8692 RSC.
- P.-X. Li, M.-S. Wang, L.-Z. Cai, G.-E. Wang and G.-C. Guo, J. Mater. Chem. C, 2015, 3, 253–256 RSC.
- Y. Kim, A. Das, H. Zhang and P. K. Dutta, J. Phys. Chem. B, 2005, 109, 6929–6932 CrossRef CAS PubMed.
- Y.-T. Huang, Y.-L. Lai, C.-H. Lin and S.-L. Wang, Green Chem., 2011, 13, 2000–2003 RSC.
- S.-H. Huang and S.-L. Wang, Angew. Chem., Int. Ed., 2011, 50, 5319–5322 CrossRef CAS PubMed.
- H.-L. Huang and S.-L. Wang, Angew. Chem., Int. Ed., 2015, 54, 965–968 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Crystallographic data, LC-HRMS spectrum, TGA, thermal ellipsoids of JU104 given at 50% probability, ESR spectroscopy, PXRD and IR spectra for JU104. CCDC 1432335. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5qi00283d |
| This journal is © the Partner Organisations 2016 |