Carbamide promoted polyol synthesis and transmittance properties of silver nanocubes
Jing
Zhang
ab,
Qiang
Wang
*ab,
Xiaohui
Zhang
ab,
Jigang
Wang
ab,
Ming
Guo
ac,
Benjamin J.
Wiley
*d,
Chunhong
Li
e and
Changwen
Hu
*c
aLaboratory for Micro-sized Functional Materials & College of Elementary Education, Capital Normal University, Beijing, P. R. China. E-mail: qwchem@gmail.com
bDepartment of Chemistry, Capital Normal University, Beijing, P. R. China
cKey Laboratory of Cluster Science of Ministry of Education of China, The Institute for Chemical Physics and Department of Chemistry, Beijing Institute of Technology, Beijing, P. R. China. E-mail: cwhu@bit.edu.cn
dDepartment of Chemistry, Duke University, Durham, North Carolina, USA. E-mail: benjamin.wiley@duke.edu
eNational Laboratory for Superconductivity, Institute of Physics and Beijing National Laboratory for Condensed Matter Physics, Chinese Academy of Sciences, Beijing, P. R. China
First published on 18th January 2016
Abstract
In this work, silver (Ag) nanocubes with different sizes were rapidly synthesized with a modified HCl-based polyol approach by employing carbamide (CO(NH2)2) as the additive or promoter, which could shorten the reaction time from about 25 h to less than 4 h and the method could be confirmed as facile and robust. In the reaction system, the NH3 molecules play the role of aggregating Ag+ and [Ag(NH3)2]+ could gradually release Ag+, which in turn results in formation of a more homogeneous product in a short time (50 min–4 h). Some factors affecting the synthesis including the concentration, reaction time and agitator speed have also been investigated, which could be adjusted to control the size, morphology, purity and uniformity of the Ag nanocubes. A mechanism for the rapid synthesis of the Ag nanocubes was proposed. To overcome the lower repeatability of reported methods, we have supplied a robust method to synthesize Ag nanocubes and this procedure may provide a useful guide for the future synthesis of Ag or other metal nanoparticles. The transmittance properties of the different Ag nanocubes have also been detected, which demonstrated that the transmittance of the Au film coupled with the Ag nanocubes is very sensitive to not only the size of the Ag nanocubes but also the thickness of the polyelectrolyte molecular spacer layers.
Introduction
Metal nanocrystals have excellent applications in the biomedical, electronic, catalytic, and sensing fields.1 Silver nanoparticles (AgNPs) have been one of the most important nanostructural materials due to their fascinating properties and potential applications in many fields.2–5 It is well known that the intrinsic properties of metal nanoparticles are closely related with their size, shape, composition and crystallinity.6–11 As a result, the properties of the AgNPs could be controlled and adjusted by tailoring their particle shape and size. Over the past decade, AgNPs have been successfully synthesized with a variety of shapes, including spheres, spheroids, disks, rods, wires, stars, prisms, cuboctahedrons, right bipyramids, cubes etc.12–16 Among these morphologies, Ag nanocubes, especially those with sharp corners, have received increasing attention in recent years owing to their remarkable physicochemical properties in a wide range of applications involving surface plasmon resonance, surface-enhanced Raman scattering, metal-enhanced fluorescence, sacrificial templates, sensing, imaging, catalysis, and antimicrobial technology.17–22 Therefore, advancing the techniques to produce specific structures has become the focus of the research in recent years and has met with much success.23,24 In particular, the synthesis of noble-metal nanocrystals, such as Ag, in a solution phase has been continuously refined.24–26 As is known, the physicochemical properties of Ag nanocubes are heavily dependent on their size, shape or morphology. Over the past few decades, a number of methods have been applied to synthesize Ag nanocubes with different edge lengths. For example, Xia and co-workers synthesized silver nanocubes via polyol reduction in ethylene glycol (EG). The polyol method is an excellent method for producing nanocubes with a uniform size, but it is difficult for most of us to reproduce the experiment successfully.27–35 In addition to the polyol system, hydrothermal synthesis and some other strategies have also been reported for the synthesis of Ag nanocubes.36–38 Some further improved strategies have also been demonstrated to prepare Ag nanocubes successfully.39,40 Nevertheless, it remains a grand challenge to synthesize uniform Ag nanocubes rapidly and it is difficult to develop a robust and reliable method for generating Ag nanocubes with a uniform edge length and with high purity. Although a method using silver trifluoroacetate as the precursor that only requires 4 h and has very high reproducibility has been reported by the Xia group, the cost of silver trifluoroacetate is higher than that of AgNO3. More importantly than that, the salt of silver trifluoroacetate is irritating to the eyes, skin and respiratory system, and is very toxic to aquatic organisms.28Herein we report a further improved polyol approach to obtain monodispersed Ag nanocubes in a HCl-based polyol system with carbamide (CO(NH2)2) as the additive or promoter at 140 °C. The most significant of the improvements for the polyol method is that the reaction time could be reduced from about 25 h to less than 4 h for obtaining cubes of the same size by using silver nitrate as the precursor, which is more economic than using silver trifluoroacetate.33,34 The transmittance properties of the Ag nanocubes have been detected.
Experimental
Materials
Ethylene glycol (A.R.), PVP (Mr ≈ 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), HCl (36.0–38.0 wt%), AgNO3 (≥99.8%, A.R.), and (CO(NH2)2) (≥99%, A.R.). All of the materials and reagents were used directly without further purification. Distilled water was purified with a Milli-Q system (Millipore, Billerica, MA). A stirring hotplate with a temperature controller (Ms7-H550-Pro, Dragon Laboratory Instruments limited), and micropipettes (Eppendorf ResearchR Plus) were used.
000), HCl (36.0–38.0 wt%), AgNO3 (≥99.8%, A.R.), and (CO(NH2)2) (≥99%, A.R.). All of the materials and reagents were used directly without further purification. Distilled water was purified with a Milli-Q system (Millipore, Billerica, MA). A stirring hotplate with a temperature controller (Ms7-H550-Pro, Dragon Laboratory Instruments limited), and micropipettes (Eppendorf ResearchR Plus) were used.
Synthesis and characterization
In a typical synthesis, 5 ml of ethylene glycol was placed in a 20 ml vial, and heated with stirring in an oil bath at 140 °C for 1 h. HCl (1 ml of 3 mM in EG) was then quickly added. After at least 10 min, AgNO3 (3 ml of a 99.13 mM solution in EG) and poly(vinyl pyrrolidone) (PVP, 3 ml of a solution in EG (155 mM in terms of the repeating unit, Mr ≈ 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)) were simultaneously added to the stirring solution. After about 3 minutes, CO(NH2)2 (0.1 ml of 0.39 mM in EG) was added to the solution. All the reaction procedures could be finished in 4 h. All samples for morphology and structure analysis were washed with acetone and then with water to remove excess EG and PVP.
000)) were simultaneously added to the stirring solution. After about 3 minutes, CO(NH2)2 (0.1 ml of 0.39 mM in EG) was added to the solution. All the reaction procedures could be finished in 4 h. All samples for morphology and structure analysis were washed with acetone and then with water to remove excess EG and PVP.
The samples for the transmission electron microscopy (TEM) or scanning electron microscopy (SEM) studies were prepared by drying a drop of the aqueous suspension of the particles on a piece of carbon-coated copper grid or silicon wafer under ambient conditions. The ultraviolet visible (UV-Vis) extinction spectrum was obtained at room temperature on a Lambda 750S spectrometer (PerkinElmer). The solution was diluted 6 times with ethylalcohol before obtaining spectrum.
The film-coupled Ag nanocubes were prepared according to previously reported literature.41,42 Experimental transmittance measurements were performed on a SP2555 grating spectrophotometer with a detector and a photomultiplier tube. The resolution of the spectrum was 0.1 nm.
Results and discussion
The monodispersed Ag nanocubes could be accomplished by such traditional reactions as the introduction of CO(NH2)2 and the synthetic reactions are as follows.| 2HOCH2CH2OH → 2CH3CHO + 2H2O | (1) |
| Ag+ + Cl− = AgCl | (2) |
| 2Ag+ + 2CH3CHO → CH3CHO–OHCCH3 + 2Ag + 2H+ | (3) |
| 4HNO3 + 3Ag → 3AgNO3 + NO + 2H2O | (4) |
| CO(NH2)2 → NH3 + CO2 | (5) |
| Ag+ + 2NH3 → [Ag(NH3)2]+ | (6) |
| 2[Ag(NH3)2]+ + 2CH3CHO → CH3CHO–OHCCH3 + 2Ag + 2NH4+ | (7) |
In a typical polyol synthesis, the initial Ag nanoparticles are obtained by reducing AgNO3 with ethylene glycol through eqn (1) and (3) and a AgCl precipitate emerges with a higher reaction equilibrium constant (ksp = 1.8 × 10−10) caused by the relatively high concentration of chloride ions in the reaction mixture [eqn (2)].43,44 At the same time, nitric acid generated in situ activates a backward reaction that dissolves the initially formed silver [eqn (4)].45 Through the introduction of HCl, eqn (4) could be driven further to the right owing to the formation of more HNO3 from HCl and AgNO3.43,44 Upon addition of CO(NH2)2 into the solution, the CO(NH2)2 will decompose and release ammonia (NH3, eqn (5)), and the latter could combine quickly with Ag+ to form [Ag(NH3)2]+ [eqn (6)]. According to the Ksp of AgCl and the Ks of [Ag(NH3)2]+ (ks = 1.6 × 107), AgCl could be easily dissolved. In the reaction system, NH3 plays the important role of aggregating Ag+ and thus would accelerate eqn (4) and result in the second formation of Ag nanoparticles. The newly formed twinned crystals were etched by Cl−/O2, and only single-crystals were left.40 The higher the concentration of Ag+, the faster the single-crystals will come into being. With the proceeding of the reactions, [Ag(NH3)2]+ could gradually release Ag+ and eqn (7) will be carried out smoothly, which in turn results in forming a more homogeneous product.
In the reaction system, the NH3 molecules play the role of aggregating Ag+ and [Ag(NH3)2]+ could gradually release Ag+, which in turn results in formation of a more homogeneous product in a short time (50 min–4 h) as illustrated in Scheme 1. The synthesis of the Ag nanocubes involves two key processes: oxidative etching and growth.46 According to ref. 43 and 44, the initially formed Ag nanoparticles were resolved by HNO3 generated in situ. Then the Ag+ was reduced to form single crystals by the cooperation of EG and Cl−/O2, meanwhile, the single crystals could grow gradually. Obviously if the concentration of HNO3 is increased, the initially formed Ag nanoparticles will be rapidly dissolved, directly inducing an increase of the concentration of Ag+ and resulting in the accelerated reduction of Ag+ by EG, and the growth rate of the single crystal will speed up at the same time. In summary, enhancing oxidative etching and the growth process can reduce the reaction time for the synthesis of Ag nanocubes.
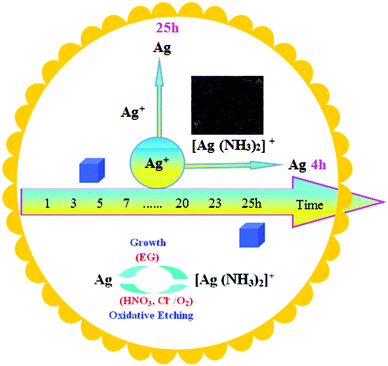 | ||
| Scheme 1 Schematic illustration of the reduction of a Ag salt precursor and the oxidation of a metal that occur simultaneously in a synthesis by introducing CO(NH2)2 as the additive. | ||
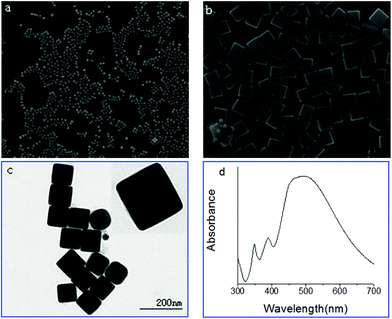
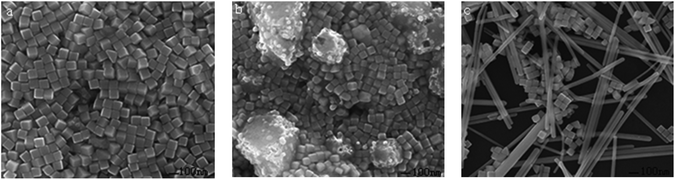
Fig. 1a–c demonstrate the SEM and TEM images of the Ag nanocubes from the improved polyol strategy in the presence of CO(NH2)2 as the additive or promoter, and the corresponding extinction spectrum of the same cubes is shown in Fig. 1d. Fig. 1a indicates that the product consists of a large quantity of nanocubes, which are uniform with sharp corners as illustrated in Fig. 1b. The cubes were 100 nm in edge length, which is the same as for those synthesized over 25 h. The TEM image displayed in Fig. 1c clearly shows the cubic shape of the sample. The samples showed an absorption band at about 500 nm (Fig. 1d), which proved that the number of peaks and the relative positions are consistent with a theoretical calculation on the basis of the reported literature.28,43,44
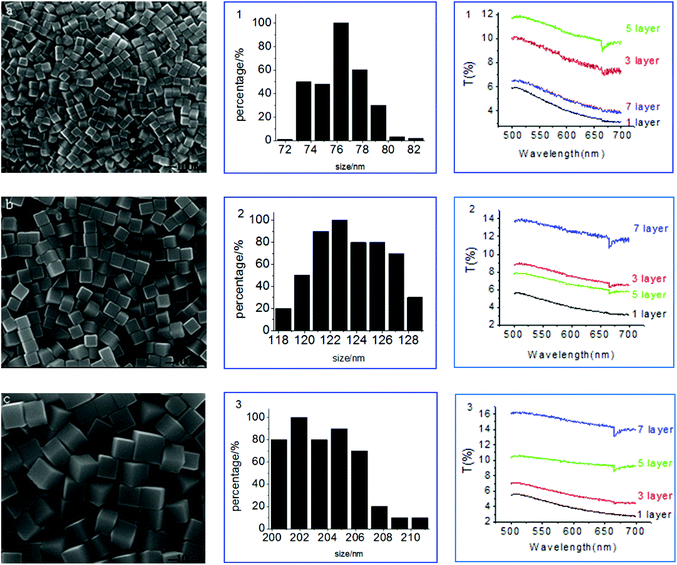
Fig. 2 shows the images and the size distribution of the Ag nanocubes when employing CO(NH2)2 as the additive or promoter. For the sample illustrated in Fig. 2a, the Ag nanocubes were synthesized at 140 °C with an agitation speed of 800 r min−1, and the whole reaction time was just 50 min. The size distribution of the Ag nanocubes in Fig. 2a is provided in graph 1, and we found that the size of the Ag nanocubes varied from 73 nm to 80 nm and the average size of them was about 76 nm. It is feasible to control the size of the Ag nanocubes by quenching the reaction at a different time. The reaction can also be quenched at 2 h (Fig. 2b) or 4 h (Fig. 2c) with similar conditions to Fig. 2a in our study, but the cubes would grow with the reaction time increase, as illustrated in graphs 2 and 3, respectively. It is easy to find that the size distribution of the Ag nanocubes in Fig. 2b is from 120 to 127 nm and the size distribution is from 200 to 207 nm for Fig. 2c. It could be clearly concluded that the Ag nanocubes grow with the increasing of the reaction time and that the Ag nanocubes were uniform and pure from the synthesis procedures of different times. In conclusion, Ag nanocubes with a uniform morphology could be synthesized rapidly, i.e. in less than 4 h, using a polyol process with CO(NH2)2 as the additive when absorbing EG.
Transmittance spectra corresponding to Fig. 2a–c are shown in Fig. 2 illustrations 1–3. The transmittance spectra were obtained from samples where the surface of the gold film was occupied by Ag nanocubes and the nanocube–film separation distance was modulated by polyelectrolyte (PE) molecular spacer layers of increasing thickness. From the illustrations, it can be concluded that the T% of the samples will mostly increase with the increasing number of PE layers.
Fig. 3 shows the T% of the Ag nanocubes with a size of 76 nm, 122 nm and 202 nm with different PE layers. At PE = 1 layer (Fig. 3a), the T% has no obvious change with the size increasing from 76 nm to 202 nm for the Ag nanocubes. But at PE = 3 layers (Fig. 3b), the T% shows a significant increase for the sizes of 76 nm, 122 nm and 202 nm of the Ag nanocubes, however the rate of increase of T% decreases with the increasing size of the Ag nanocubes. On increasing the PE layers to 5 layers, the T% for the sizes of 76 nm and 202 nm of the Ag nanocubes continues to increase, but the T% corresponding to the size of 122 nm is decreased slightly (Fig. 3c). At PE = 7 layers (Fig. 3d), the T% for the size of 76 nm of the Ag nanocubes is significantly reduced while the others still grow. This demonstrated that the transmittance of the Au film coupled with the Ag nanocubes is very sensitive to not only the size of the Ag nanocubes but also the thickness of the PE molecular spacer layers.41
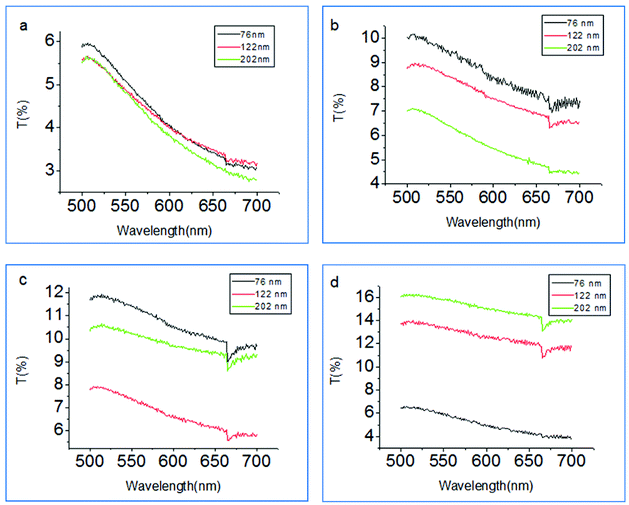 | ||
| Fig. 3 Transmittance spectra of the Ag nanocubes with a size of 76, 122 and 202 nm with different PE layers. (a) 1 layer, (b) 3 layers, (c) 5 layers, (d) 7 layers. | ||
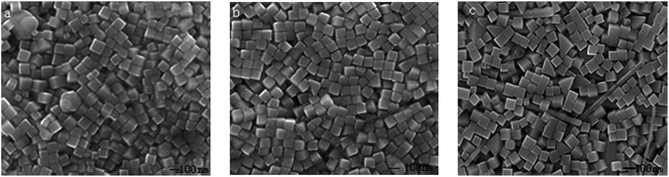
As shown in Fig. 4, the influence of the concentration of CO(NH2)2 on the geometry of the Ag nanocubes was also investigated. A series of experiments were conducted under the same reaction conditions except that the concentration of CO(NH2)2 in the EG solution was increased from 1.6 to 3.6 mM. It was found that the ideal concentration of CO(NH2)2 to obtain Ag nanocubes with good uniformity and in large quantities is 2.8 to 3.2 mM among these serial experiments. The emergence of irregular Ag nanoparticles in some of the products (shown in Fig. 4a–c) may probably be owing to the low concentration of [Ag(NH3)2]+ as a result of a low concentration of NH3 from decomposed CO(NH2)2. With increase of the concentration of CO(NH2)2, the growth rate of the single-crystal seeds could be kept at a constant basic rate. Hence, Ag nanocubes become the absolute component in the final products, illustrated by Fig. 4d and e. In the concentration range of 2.8 to 3.2 mM, NH3 and Ag+ would have a good aggregation rate and then [Ag(NH3)2]+ would gradually release Ag+, which maybe plays a key role not only in controlling the growth rate of the nanocubes within a stable range, but also further resulting in the reaction being carried out more smoothly and generating more homogeneous Ag nanocubes. The edge length of the Ag nanocubes is 90 nm in Fig. 4d, smaller than that in Fig. 4e. This phenomenon is ascribed to the increased growth rate of the nanocubes. In detail, the increase of the concentration of CO(NH2)2 can enhance the release rate of the Ag atoms from multiple twinned and single twinned seeds, which can improve the deposition rate of Ag atoms on the single-crystal seeds. This good situation, however, cannot be maintained on further increasing the concentration of CO(NH2)2. As shown in Fig. 4f, some small Ag nanocubes and some nanowires start to appear. This is probably due to that the increased introduction of CO(NH2)2 into the solution will improve the probability of the combination of NH3 and Ag+, and thus the chemical equilibrium is disrupted due to the relatively high concentration of [Ag(NH3)2]+.
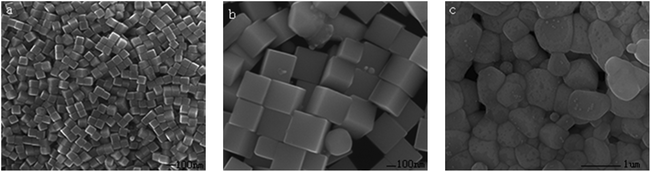
In order to further verify the key role of CO(NH2)2, the source of NH3 was replaced with NH3·H2O or NH4Cl. Fig. 5a shows the SEM image of a product synthesized using a HCl-based polyol method by employing CO(NH2)2 as the additive. Fig. 5b shows the SEM image of a product formed on replacing CO(NH2)2 with NH3·H2O using the same reaction conditions, and it can be seen that the sample contains large Ag nanocubes of about 150–250 nm in edge length and some irregular nanoparticles. It is believed that the high concentration of NH3 leads to such a quick etching and growth process that it results in the formation of big or irregular nanocubes. The more Ag atoms in the unit volume that exist, the more rapid the formation of the Ag nanocubes, and so bigger or more irregular Ag nanocubes would come into being.
When replacing CO(NH2)2 with NH4Cl at the same concentration, irregular nanoparticles could be observed, as shown in Fig. 5c. This is probably due to the high concentration of Cl− in the solution which will accelerate the combination of Cl− and Ag+, and thus the influence of AgCl cannot be ignored any more.47 The formation of AgCl could reduce the amount of free Ag+ at the initial stage and prevent the solution from being rapidly supersaturated with Ag seeds. As a result, nanocubes of a small size are formed due to the decreased deposition rate of Ag atoms. Meanwhile, a small amount of AgCl can account for the appearance of irregular nanoparticles in Fig. 5c, which probably serve as sites for the growth of twinned seeds like for the function of AgCl.48
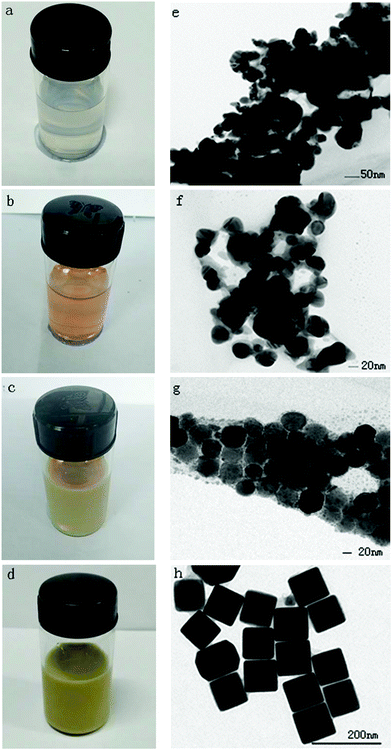
In order to validate our findings, we conducted follow-up observations using TEM as illustrated in Fig. 6. At the point of 3 min, the solution turned milky white (Fig. 6a), and this indicates the presence of AgCl (Fig. 6e). The solution turned melon yellow at about 20 min (Fig. 6b), which indicates that large AgCl nanoparticles dissolved because of the existence of NH3 and the initially formed Ag nanoparticles appeared (Fig. 6f). At the point of 2 h, the mixture turned orange mixed with green (Fig. 6c), which suggests that single-crystal nanoparticles had formed (Fig. 6g). The solution did not become colorless during the reaction process (at about 2 h) as reported in the literature, and this suggests that the NH3 can combine with Ag+ to form [Ag(NH3)2]+, which could accelerate the dissolving of the initially formed Ag nanoparticles and promote the formation of the second round of Ag nanoparticles.28 The solution became yellow (Fig. 6d) at 4 h, implying that cubes with a perfect shape and corners had formed (Fig. 6h). With the proceeding of the reactions, [Ag(NH3)2]+ could gradually release Ag+ and growth of the single nanoparticles will be carried out smoothly, which in turn results in forming a more homogeneous product. This observation proved that carbamide had accelerated the etching and growth process.
The concentration of HCl, AgNO3 and PVP was found to have an influence on the results when using CO(NH2)2 as the modifier. Firstly, the concentration of HCl was set at 0.25 mM and the reaction solution used was composed of 24.75 mM AgNO3, 38.75 mM PVP (calculated in terms of the repeating unit) and CO(NH2)2 (2.8 mM), which was heated in an oil bath at 140 °C, and then perfect cubes followed as a result (Fig. 7a). On doubling the concentration of HCl, though the Ag cubes were uniform with a size of about 80 nm, the product contained some very large particles (Fig. 7b). However, when the concentration of HCl was decreased to 0.125 mM, lots of nanowires could be found in the products (Fig. 7c). It is assumed that a high concentration of H+ ions enhanced the etching strength of Cl− and O2 through improving the standard potential of O2.34 As a result, a high concentration of H+ ions could result in formation of some very large Ag particles, indirectly forming cubes of a small size. In contrast, a lower concentration of Cl− resulted in incomplete etching of the twinned seeds and nanowires were inclined to form. It is believed that using 0.25 mM could produce uniform Ag nanocubes appropriately.
The influence of the concentration of AgNO3 and PVP has also been studied with a concentration of HCl of 0.25 mM and a concentration of CO(NH2)2 of 2.8 mM used for the reaction solutions. Fig. 8a shows the SEM image of the Ag nanocubes synthesized with a concentration of AgNO3 of 23.5 mM and PVP of 36.7 mM, and Fig. 8b shows the image of the Ag nanocubes synthesized with an increased concentration of AgNO3 of 24.75 mM and PVP of 38.75 mM. Contrasting Fig. 8a and b, it is easy to see that the cubes in Fig. 8b are more uniform and well-dispersed than those of Fig. 8a. When increasing the concentration of AgNO3 to 49.5 mM and PVP to 77.5 mM, some unexpected nanowires and trigonal bipyramidal particles formed (Fig. 8c). It was found that the concentration of HCl (0.25 mM) was higher than the concentration of AgNO3 and PVP for the sample in Fig. 8a and lower than that in Fig. 8c, but was most favorable for that of Fig. 8b. A higher concentration of H+ could produce less nuclei and easily result in larger particles, and a lower concentration of H+ reduces the etching strength of Cl−/O2 resulting in incomplete etching of the twined seeds, which formed the wires and trigonal bipyramids. It could be concluded that Ag nanocubes can be ideally synthesized with a concentration of AgNO3 of 24.75 mM, PVP of 38.75 mM, HCl of 0.25 mM and CO(NH2)2 of 2.8 mM used for the reaction system.
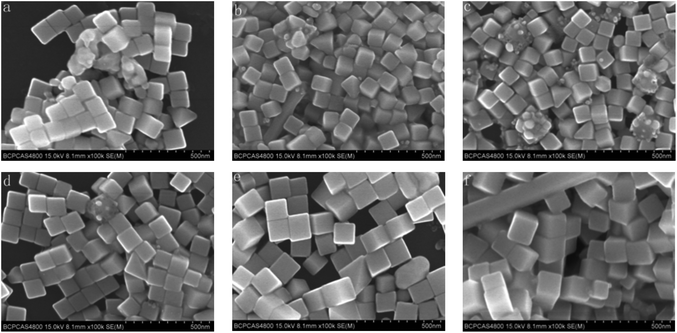
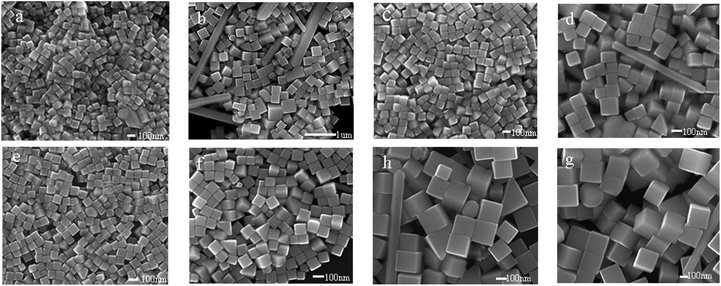
Using the same reaction time (by quenching the reactions simultaneously), the effect of the speed of the agitator has also been studied. Fig. 9 shows SEM images of the Ag nanocubes synthesized using a range of speeds from 500–800 r min−1, and the reaction solution was composed of AgNO3 (24.75 mM), PVP (38.75 mM, calculated in terms of the repeating unit), HCl (0.25 mM) and CO(NH2)2 (2.8 mM), which was heated in an oil bath at 140 °C. Fig. 9a and b show the SEM images of the Ag nanocubes synthesized with a stirring speed of 500 r min−1. It is easy to see that the cubes in Fig. 9a are uniform and well dispersed, but there are some rods blended with the Ag nanocubes in Fig. 9b. Based on sufficient experiments, it is believed that 500 r min−1 could produce perfect nanocubes but sometimes mixed with some wires in the product. When the stirring speed was accelerated to 650 r min−1, some irregular particles or rods could also be found in the resulting sample once in a while, as illustrated in Fig. 9c and d. When it was speeded up to 800 r min−1, uniform and pure Ag nanocubes could be fabricated repeatedly (shown in Fig. 9e and f). Nevertheless, when the speed was increasingly accelerated to 1000 r min−1, some unexpected particles could appear again (Fig. 9g and h). A low speed of 300 r min−1 had also been studied, however the reaction time became longer and no ideal cubes appeared. Perhaps the lower and faster speeds could be disturbing the formation and growth of the seeds of the nanocrystals, and the speed of 800 r min−1 is the best speed for use in the synthesis in our case.
Conclusion
In summary, uniform and pure monodispersed Ag nanocubes were successfully synthesized using a HCl-based polyol system with CO(NH2)2 as the additive or promoter at 140 °C, and the transmittance properties of the cubes were also detected. The influence of different factors on the synthesis of the Ag nanocubes was also studied systematically. The transmittance properties of the different Ag nanocubes were also detected, which demonstrated that the transmittance of the Au film coupled with the Ag nanocubes is very sensitive to not only the size of the Ag nanocubes but also the thickness of the polyelectrolyte molecular spacer layers. It has been proved that the CO(NH2)2 could enhance the etching and growth process. To overcome the lower repeatability of reported methods, we have supplied a robust method to synthesize Ag nanocubes and this procedure may provide a useful guide for the future synthesis of Ag nanoparticles. Therefore, a further improved polyol strategy with a high yield has been successfully demonstrated, which is facile, robust and reproducible. An extensive range of materials could be selected for use in the reaction, and there is no need to waste a long time in strictly controlling the experimental conditions to remove the water from the reaction solution. The most significant improvement of this polyol method is that the reaction time could be greatly shortened to less than 2 h for obtaining cubes of the same size. Considering the unique properties and wide application of Ag nanocubes, the improved polyol method should be of importance for using Ag nanocubes in the fields of both controllable synthesis and theoretical investigations or practical applications.Acknowledgements
This work was supported by the Natural Science Foundation of China (NSFC no. 21471103, 21471100, 51002180, 21001074, 20731002, 20871016, 10876002, 91022006 and 20973023), the 111 Project (B07012), the Project of Excellent Talents of Beijing (203135407707), and the Scientific Research Base Development Program of the Beijing Municipal Commission of Education.References
- Y. Xia, Y. Xiong, B. Lim and S. E. Skrabalak, Angew. Chem., Int. Ed., 2009, 48, 60–103 CrossRef CAS PubMed.
- Z. X. Ji, M. N. Ismail, D. M. Callahan Jr., E. Pandowo, Z. H. Cai, T. L. Goodrich, K. S. Ziemer, J. Warzywoda and A. Sacco Jr., Appl. Catal., B, 2011, 102, 323–328 CrossRef CAS.
- X. M. Lu, M. Rycenga and Y. N. Xia, Annu. Rev. Phys. Chem., 2009, 60, 167–192 CrossRef CAS PubMed.
- A. K. Singh, K. G. Gryczynski, S. Y. Park, M. Kim and A. Neogi, Solid State Commun., 2011, 151, 1405–1409 CrossRef CAS.
- J. Kolbe, A. Arp, F. Calderone, E. M. Meyer, W. Meyer, H. Schaefer and M. Stuve, Microelectron. Reliab., 2007, 47, 331–334 CrossRef CAS.
- D. K. Sharma, A. Ott, A. P. O'Mullane and S. K. Bhargava, Colloids. Surf., A, 2011, 386, 98–106 CrossRef CAS.
- R. Sancı and M. Volkan, Sens. Actuators, B, 2009, 139, 150–155 CrossRef.
- P. Etchegoin, L. F. Cohen, H. Hartigan, R. J. C. Brown, M. J. T. Milton and J. C. Gallop, J. Chem. Phys., 2003, 119, 5281–5289 CrossRef CAS.
- J. M. McLellan, A. Siekkinen, J. Y. Chen and Y. N. Xia, Chem. Phys. Lett., 2006, 427, 122–126 CrossRef CAS.
- K. L. Kelly, E. Coronado, L. L. Zhao and G. C. Schatz, J. Phys. Chem. B, 2003, 107, 668–677 CrossRef CAS.
- P. V. Kamat, J. Phys. Chem. B, 2002, 106, 7729–7744 CrossRef CAS.
- D. H. Zhang, X. H. Liu and X. Wang, J. Mol. Struct., 2011, 985, 82–88 CrossRef CAS.
- K. E. Korte, S. E. Skrabalak and Y. N. Xia, J. Mater. Chem., 2008, 18, 437–441 RSC.
- Y. N. Xia, Y. J. Xiong, B. Lim and S. E. Skrabalak, Angew. Chem., Int. Ed., 2009, 48, 6304–6308 CrossRef PubMed.
- L. L. Zhao, K. L. Kelly and G. C. Schatz, J. Phys. Chem. B, 2003, 107, 7343–7350 CrossRef CAS.
- A. Panacek, L. Kvıtek, R. Prucek, M. Kolar, R. Vecerova, N. Pizurova, V. K. Sharma, T. Nevecna and R. Zboril, J. Phys. Chem. B, 2006, 110, 16248–16253 CrossRef CAS PubMed.
- S. Nie and S. R. Emory, Science, 1997, 275, 1102–1106 CrossRef CAS PubMed.
- X. Xia, J. Zeng, B. McDearmon, Y. Zheng, Q. Li and Y. Xia, Angew. Chem., Int. Ed., 2011, 50, 12542–12546 CrossRef CAS PubMed.
- K. Aslan, M. Wu, J. R. Lakowicz and C. D. Geddes, J. Am. Chem. Soc., 2007, 129, 1524–1525 CrossRef CAS PubMed.
- A. Kumar, P. K. Vemula, P. M. Ajayan and G. John, Nat. Mater., 2008, 7, 236–241 CrossRef CAS PubMed.
- P. Christopher and S. Linic, ChemCatChem, 2010, 2, 78–83 CrossRef CAS.
- L. J. Sherry, S. H. Chang, G. C. Schatz, R. P. Van Duyne, B. J. Wiley and Y. Xia, Nano Lett., 2005, 5, 2034–2038 CrossRef CAS PubMed.
- C. H. Moran, M. Rycenga, Q. Zhang and Y. Xia, J. Phys. Chem. C, 2011, 115, 21852–21857 CAS.
- Y. Xia, Y. Xiong, B. Lim and S. E. Skrabalak, Angew. Chem., Int. Ed., 2009, 48, 60–103 CrossRef CAS PubMed.
- S. E. Skrabalak, L. Au, X. Li and Y. Xia, Nat. Protoc., 2007, 2, 2182–2190 CrossRef CAS PubMed.
- Q. Zhang, W. Li, C. Moran, J. Zeng, J. Chen, L.-P. Wen and Y. Xia, J. Am. Chem. Soc., 2010, 132, 11372–11378 CrossRef CAS PubMed.
- Y. Sun and Y. Xia, Science, 2002, 298, 2176–2179 CrossRef CAS PubMed.
- S. H. Im, Y. T. Lee, B. Wiley and Y. Xia, Angew. Chem., Int. Ed., 2005, 44, 2154–2157 CrossRef CAS PubMed.
- Y. T. Lee, S. H. Im, B. Wiley and Y. Xia, Chem. Phys. Lett., 2005, 411, 479–483 CrossRef CAS.
- A. R. Siekkinen, J. M. McLellan, J. Chen and Y. Xia, Chem. Phys. Lett., 2006, 432, 491–496 CrossRef CAS PubMed.
- S. E. Skrabalak, L. Au, X. Li and Y. Xia, Nat. Protoc., 2007, 2, 2182–2190 CrossRef CAS PubMed.
- S. E. Skrabalak, B. J. Wiley, M. Kim, E. V. Formo and Y. Xia, Nano Lett., 2008, 8, 2077–2081 CrossRef CAS PubMed.
- Q. Zhang, C. Cobley, L. Au, M. McKiernan, A. Schwartz, L.-P. Wen, J. Chen and Y. Xia, ACS Appl. Mater. Interfaces, 2009, 1, 2044–2048 CAS.
- R. Long, S. Zhou, B. J. Wiley and Y. Xiong, Chem. Soc. Rev., 2014, 43, 6288–6310 RSC.
- Q. Zhang, W. Li, L.-P. Wen, J. Chen and Y. Xia, Chem. – Eur. J., 2010, 16, 10234–10239 CrossRef CAS PubMed.
- D. Yu and V. W.-W. Yam, J. Am. Chem. Soc., 2004, 126, 13200–13201 CrossRef CAS PubMed.
- H. Chen, Y. Wang and S. Dong, Inorg. Chem., 2007, 46, 10587–10593 CrossRef CAS PubMed.
- Q. Zhang, C. Z. Huang, J. Ling and Y. F. Li, J. Phys. Chem. B, 2008, 112, 16990–16994 CrossRef CAS PubMed.
- Y. Ma, W. Li, J. Zeng, M. McKiernan, Z. Xie and Y. Xia, J. Mater. Chem., 2010, 20, 3586–3589 RSC.
- Y. Wang, Y. Zheng, C. Huang and Y. Xia, J. Am. Chem. Soc., 2013, 135, 1941–1951 CrossRef CAS PubMed.
- A. Moreau, C. Ciracı’, J. J. Mock, R. T. Hill, Q. Wang, B. J. Wiley and A. Chilkoti, Nature, 2012, 492, 86–89 CrossRef CAS PubMed.
- J. B. Lassiter, F. McGuire, J. J. Mock, C. Ciracì, R. T. Hill, B. J. Wiley, A. Chilkoti and D. R. Smith, Nano Lett., 2013, 13, 5866–5872 CrossRef CAS PubMed.
- C. Őzmetin, M. Çopur, A. Yartasi and M. M. Kocakerim, Chem. Eng. Technol., 2003, 23, 707–712 CrossRef.
- B. Blin, F. Fievet, D. Beaupère and M. Figlarz, Nouv. J. Chim., 1989, 13, 67–73 CAS.
- F. Fievet, J. P. Lagier and M. Figlarz, MRS Bull., 1989, 14, 29–34 CrossRef CAS.
- Y. Zheng, J. Zeng, A. Ruditskiy, M. Liu and Y. Xia, Chem. Mater., 2013, A–L Search PubMed.
- I. O. Sosa, C. Noguez and R. G. A. Barrer, J. Phys. Chem. B, 2003, 107, 6269–6275 CrossRef CAS.
- F. Wu, W. Wang, Z. Xu and F. Li, Sci. Rep., 2015, 1–7 Search PubMed.
| This journal is © the Partner Organisations 2016 |