Defect-driven oxygen reduction reaction (ORR) of carbon without any element doping†
Xiaojun
Zhao
abc,
Xiaoqin
Zou
b,
Xuecheng
Yan
a,
Christopher L.
Brown
a,
Zhigang
Chen
d,
Guangshan
Zhu
b and
Xiangdong
Yao
*a
aQueensland Micro- and Nanotechnology Centre, Griffith University, Nathan QLD 4111, Australia. E-mail: x.yao@griffith.edu.au
bState Key Laboratory of Inorganic Synthesis & Preparative Chemistry, Jilin University, Changchun 130012, China
cClinical Pharmacology Center, Research Institute of Translational Medicine, The First Bethune Hospital of Jilin University, Changchun 130061, China
dSchool of Mechanical and Mining Engineering, The University of Queensland, St. Lucia, Queensland 4072, Australia
First published on 6th January 2016
Abstract
A porous carbon (PC) material, containing carbon and oxygen only, was synthesized via carbonisation of a Zn-MOF (IRMOF-8) at 950 °C. Interestingly, the derived materials of this reaction exhibit excellent electrocatalytic activity, molecular selectivity and long-term durability. The fact that this material, which is effectively a “pure” carbon, lacking any elemental doping, exhibits excellent oxygen reduction reaction (ORR) activity suggests that a mechanism not dependent on elemental doping is being utilised. We suggest the formation of defects arising from the removal of Zn atoms as a consequence of the calcination procedure play the critical role in this process.
The development of clean energy supplies and the management of environmental pollution are serious challenges to the world's sustainable development and the progress of human society. The continued use of non-renewal energy sources such as those derived from fossil fuels are both environmentally polluting and their stocks will not meet future energy demands. Therefore, it is imperative that we as a society develop clean and renewable energy sources.1 One approach to this problem is the introduction of the hydrogen economy, in which fuel cells are a fundamental component. There are two critical issues associated with the use of these devices, unit cost and operational lifetime; the two being interrelated.2–5 One of the main costs arises from the materials used in the traditional fuel cell's anodic and cathodic catalytic electrodes that typically use expensive Pt-based materials. The rates of reaction at the two electrodes are not equal as the oxygen reduction reaction (ORR) at the cathode is more than six orders slower than hydrogen oxidation at the anode, accordingly development of the cathodic ORR catalyst is more critical2 and forms the focus of this research. Whilst the current range of Pt-based catalysts displays excellent performance, the metal is scarce and accordingly very expensive. Consequently, numerous efforts have been devoted to the design and development of new catalysts that either reduce the amount of Pt required2,5,6 or completely replace the Pt with other materials4,5,7 such as non-precious metals supported by carbon or completely metal-free catalysts.
Among the metal-free catalysts for ORR, porous carbon (PC) materials have attracted enormous interest. They exhibit high chemical stability and possess long lifetimes in the basic or acidic reaction media; an environment that may make metals leach from the surface of more traditional metal-based electrocatalysts resulting in the loss of electrocatalytic activity.8 Doped carbon nanotube systems possessing ORR activity have previously been reported in which the catalytic activity arises from the doped sp2 carbon by heteroatoms (N and B),9 or other elements such as P and S.10 It has been proposed that the heteroatoms resident within the carbon sp2 framework induces charged sites that are favorable for O2 adsorption and that these sites also provide the electrons required for subsequent O2 reduction.9,11 Additionally, the high surface area and volume resident within PC materials are beneficial for the process as they also provide efficient dispersion of the active sites.1h,12
So far, heteroatom-doping has been the only reported mechanism in carbon materials facilitating the 4-electron pathway for the reduction of oxygen.
There are a few papers published recently, regarding to the role of defects on ORR in carbon nanotubes (CNTs).13 However, CNTs contain the catalyst metals such as Fe, Co, Ni, which cannot be fully removed by purification. So the ORR activity is arguable, and the race amount of Fe, Co and/or Ni is regarded as key contributions to ORR activity of CNTs.14 Therefore, the investigation of real metal free catalyst for ORR is desirable. Recently, we proposed15 a mechanism for the ORR activity in non-doped carbon materials based on the introduction of defect regions in a sp2 carbon matrix. These materials are synthesized by the high temperature treatment. For example, N-containing PAF40 with the carbonization process promoting removal of heteroatoms such as nitrogen. It was found that the resulting carbon materials arising from this process possess high catalytic activities and the types of nitrogen such as pyridinic, pyrrolic, graphitic and pyridine-N-oxides have no effect on the ORR activity. This observation is in contrast to the reported mechanism for N-doped materials9 and is evidenced by the smallest N containing sample (0.21 at%) exhibiting the highest catalytic activity. From these data the defect mechanism for ORR of carbon-based materials was presented.15 However, as there are trace amounts of nitrogen remaining in the samples some ambiguity on the role of nitrogen in our initial data could be inferred. To further support our hypothesis regarding the role of defects in ORR processes, we report the carbonization and subsequent catalytic activity of a non-nitrogen containing material, IRMOF-8 (a Zn metal–organic framework (MOF)). Carbonization of IRMOF-8 at 950 °C in a similar fashion to processes reported previously16 (see ESI†) yielded PC-I8-950 as a black soot-like material.
To characterize the pore structure of PC-I8-950, the nitrogen adsorption and desorption measurements were carried out at 77 K as shown in Fig. 1(a). At low pressure (P/P0 < 0.01), a sharp increase in the quantity of adsorbate describes the permeation of gas into the micro pores of the carbon framework. A second rapid increase is observed within the high pressure range (P/P0 > 0.8) and the observed hysteresis of the adsorption and desorption isotherms reflect the effect of capillary tube coacervation in large pores. The adsorption and desorption isotherms of PC-I8-950 reflect a Type-IV sorption isotherm. Brunauer–Emmett–Teller (BET) analysis reveals a surface area of 836 m2 g−1 and the pore distribution curve (Fig. 1(b)) indicates that the average pore size of the derived PC material is approximately 1.5 nm. When compared to the starting MOF precursor, the narrower pores in the carbonized product may reflect collapse and subsequent constriction of the porous framework during carbonization process.17 The total pore volume (Vt), evaluated at a relative pressure of 0.96, and micropore volume (Vm) calculated by the t-plot method (the data are listed in ESI Table S1†) affords a Vt/Vm ratio that is consistent with the majority of the pores being nanoporous in dimension. The porous framework evident in PC-I8-950 from the BET data, can also observed in transmission electron microscopy (TEM) and HRTEM images (Fig. 1(c and d)), the latter revealing the disorder structure and the multilayer/graphitic stacked domains in the carbon framework.
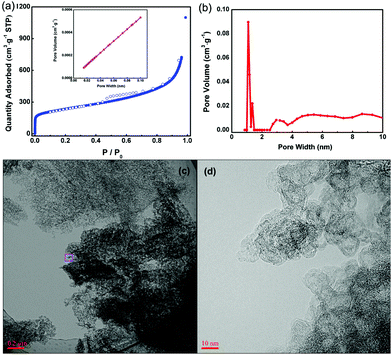 | ||
| Fig. 1 (a) Nitrogen adsorption and desorption isotherms, (b) pore distribution curve, (c) TEM and (d) HRTEM images of PC-Zn-950 sample. | ||
X-ray diffraction (XRD) and Raman analysis of PC-Zn-950 exhibit the typical features of the defects and graphitic layers in the carbon framework. XRD analysis performed at room temperature reveal peaks at 2θ = 24° and 44°, which can be assigned to the typical interlayer signals arising from graphitic carbon (002) and (101) diffractions (Fig. 2(a)). No other peaks were observed in the XRD spectra, indicating that no impurity was contained in the carbon products but the lack of a signal at 2θ = 13°, (assigned to the in-plane structural diffraction typical graphitic carbon), suggested that the structure of the layers were not fully graphitic.18–20
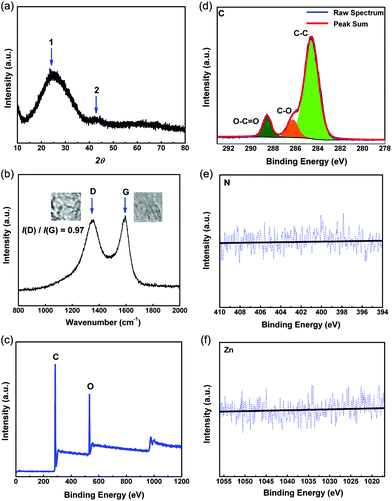 | ||
| Fig. 2 (a) XRD pattern, (b) Raman spectrum, (c) XPS survey spectrum, and high-resolution XPS spectra ((d) C 1s, (e) N 1s and (f) Zn 2p) of PC-I8-950. | ||
The Raman spectrum of PC-I8-950 is shown in Fig. 2(b). The spectrum exhibits typical D and G bands at 1350 and 1588 cm−1, respectively. In general, the D band is attributed to the defects and disorders in the carbon materials and the G band represents the vibrational mode of the movement in opposite directions of two carbon atoms in a graphite sheet.19–21 The high intensity of the D band is consistent with the formation of numerous defects, edge effects or disorders within the material.21 Base on XRD and Raman measurement, the degree of the disorder in PC-I8-950 is higher than a majority of other porous carbon materials, which were reported in previous literatures, such as carbon nanotube/nanocage, mesoporous carbon and porous graphene.11b,h,22
X-ray photoelectron spectroscopy (XPS) reveals (Fig. 2(c)) the presence of C 1s and O 1s emissions only. The high-resolution XPS spectra (Fig. 2(d)) of the C 1s region can be deconvolved into three peaks corresponding to distinctive C 1s binding energies; the peak at 284.6 eV is attributed to the non-oxygenated graphite carbon (C–C), whilst the peaks at 286.2 eV and 288.6 eV are assigned to the oxygen-containing carbon arising from C–O and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups, respectively.23,24 Of interest is that none of the high-resolution XPS spectra reveal the presence of either nitrogen or zinc confirming the literature report that, when Zn-based MOF materials were carbonized at a high temperature, the organic ligands were converted to porous carbon frameworks, and that residual zinc can be removed when the samples were heated at over 900 °C for >50 min.16,18,25
O groups, respectively.23,24 Of interest is that none of the high-resolution XPS spectra reveal the presence of either nitrogen or zinc confirming the literature report that, when Zn-based MOF materials were carbonized at a high temperature, the organic ligands were converted to porous carbon frameworks, and that residual zinc can be removed when the samples were heated at over 900 °C for >50 min.16,18,25
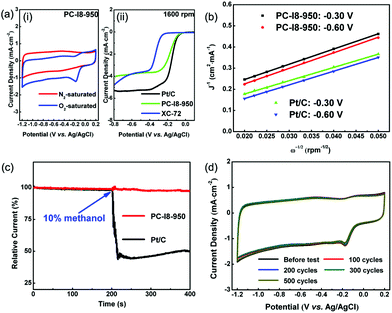
The electrochemical performance of PC-I8-950 was compared to an existing Pt/C catalyst reference sample for the evaluation of ORR activities. Both materials were investigated using a three-electrode system namely a glassy carbon (GC) working electrode, and a Pt wire and Ag/AgCl electrode as counter and reference electrodes, respectively. Cyclic voltammetry (CV) was measured in 0.1 M KOH aqueous solution at a scan rate of 50 mV s−1. The measurements were completed in O2-saturated and O2-free KOH aqueous solutions respectively and the results were compared with a Pt/C reference catalyst (20 wt% Pt/C). The corresponding CV curves are shown in Fig. 3(a) and the data reproduced in ESI Fig. S1.† Across a potential range from −1.20–0.20 V (vs. Ag/AgCl), both PC-I8-950 and Pt/C catalysts display featureless CV curves in the O2 free electrolyte (PC-I8-950, red). In contrast, for the electrolyte solution saturated with O2, the distinct oxygen reduction is observed for PC-I8-950 at a potential of −0.06 V and the corresponding reduction peak of ORR observed at −0.18 V (blue). The result compares favourably with the Pt/C catalyst reference with onset potential at −0.06 V and the reduction peak of ORR at −0.16 V (black line in ESI Fig. S1†).
Linear sweep voltammograms (LSV) of rotating disk electrode (RDE) measurements were carried out with a scanned rate of 10 mV s−1, and recorded at various rotating speeds between 400 to 2500 rpm. As shown in Fig. S2,† the LSV curves of PC-I8-950 are similar to Pt/C supporting the four-electron process for direct reduction of O2 to OH−. Contrasting the LSV curves of PC-I8-950 (at 1600 rpm) with the Pt/C reference catalyst (Fig. 3(a)) the onset potentials of both materials commence at −0.06 V at a current density of −0.1 mA cm−2. This potential is more positive than any other reported carbon catalysts.
As the control sample, a commercial carbon material, XC-72, was measured the electrochemical performance for ORR by CV and LSV (Fig. S11 and S12†). XC-72 is composed of carbon and oxygen elements without nitrogen element, and its pore size and carbon content are similar to PC-I8-950 (Fig. S7–S9 and Table S2†). As shown in Fig. 3(a), the onset potential of XC-72 (−0.25 V at −0.1 mA cm−2) is more negative than that of PC-I8-950. Through LSV measurement (Fig. S12†), a two-electron transferred process is observed obviously at the lower potential (e.g. −0.30 to −0.40 V). Oppositely, PC-I8-950 displays a four-electron transferred reaction from −0.30 to −0.60 V.
The LSV curves were subsequently analyzed using Koutecky–Levich (K–L) plots (J−1vs. ω−1/2) and the K–L equation at various electrode potentials from −0.30 to −0.60 V (vs. Ag/AgCl) (relative details of the calculation are provided in the ESI.† By using the K–L equation, the number of electron transfers (n) per O2 molecule for ORR are calculated and listed in the ESI (Table S3†). The n value of PC-I8-950 was calculated to be 3.6 at −0.30 V, confirming a four-electron transfer reaction. The number of electron transfers for PC-I8-950 is approximately constant over the range of −0.30 V to −0.60 V, which is analogous to the Pt/C reference. Likewise, the parallel status of the K-L plots at −0.30 V and −0.60 V indicates first-order reaction kinetics toward O2 reduction within the potential range of −0.30 V to −0.60 V.
The presence of methanol in the reaction medium is a challenge for metal-based cathodic electrodes in fuel cells because methanol can pass through proton exchange membrane and cause deterioration of the catalyst.26 Therefore, the activity of PC-I8-950 and the Pt/C reference material were further investigated after the addition of methanol to the 0.1 M KOH aqueous working solution (Fig. 3(c)). The current-time (i–t) chronoamperometric response was acquired after addition of the methanol (volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) into the reaction cell (arrow indicated). For the Pt/C reference catalysts a sharp decrease in relative current was observed, consistent with the initiation of the competing methanol oxidation reaction. In contrast, however, the PC-I8-950 catalyst remained very stable and maintained a strong current response consistent with the ORR. The result demonstrates clearly that PC-I8-950 possesses the better electrocatalytic selectivity.
10) into the reaction cell (arrow indicated). For the Pt/C reference catalysts a sharp decrease in relative current was observed, consistent with the initiation of the competing methanol oxidation reaction. In contrast, however, the PC-I8-950 catalyst remained very stable and maintained a strong current response consistent with the ORR. The result demonstrates clearly that PC-I8-950 possesses the better electrocatalytic selectivity.
The durability of any electrochemical catalysts in the working electrolyte is another key indicator for success in fuel cell applications. Accordingly, the PC-I8-950 material was subjected to continual CVs for 500 cycles at a scan rate of 50 mV s−1, over which time the material exhibited excellent long-term performance for the ORR in alkaline media (Fig. 3(d)). No noticeable changes were observed for the onset potential and the reduction peak between the CV curves for the duration of the experiment.
As indicated in characterization, the derived PC-I8-950 contains only C and O, without any other elements (especially the so-called “active” elements such as N, P, B, or S for ORR). The high activity of PC-I8-950 is, therefore, not from the heteroatom doping, but should be functional by other mechanism. In other previous paper,15 we reported a defect mechanism for ORR, in which the removal of N will create the desirable defects such as G585 that are responsible for the ORR activity. In the present research, we propose the similar process to create the defects, but using Zn removal, in order to avoiding attachment of any “active” elements. The fact that derived PC-I8-950 is with high ORR activity strongly supports the defect mechanism, which is the aim of current research because it excludes any effect of heteroatom doping. Although we do not directly observe the tomography of defects, the theoretical calculations suggested the defects with some typical tomography are functional for ORR. The removal of elements from sp2 carbon definitely hurts its integrity and creates defects/disorders (supported by the Raman spectrum). Of course, as a new theory, defect mechanism needs more investigations, for which the direct observations of defects that created from the removal of N or Zn may be realized in the future research.
In summary, we have synthesised a porous carbon material, PC-Zn-950, by carbonization of a Zn MOF. The material is composed of only carbon and oxygen atoms supporting the hypothesis that it may be defect points within the sp2 carbon matrix that are responsible for the catalytic activity of the material and not the incorporation of heteroatoms such as nitrogen, boron or sulphur as has been reported previously. Analysis of this material reveals a porous carbon material that not only displays excellent electrocatalytic activities but a material that is comparable in stability to commercial Pt/C catalysts. Moreover the derived material demonstrates improved molecular selectivity against contaminants when compared to more traditional Pt/C-based fuel cell technologies.
Acknowledgements
The financial support from Australian Research Council (DP130104759) is appreciated.Notes and references
- (a) Y. Guo, J. Hu and L. Wan, Adv. Mater., 2008, 20, 2878 CrossRef CAS; (b) A. Chroneos, B. Yildiz, A. Tarancon, D. Parfitta and J. A. Kilner, Energy Environ. Sci., 2011, 4, 2774 RSC; (c) R. Marom, S. F. Amalraj, N. Leifer, D. Jacob and D. Aurbach, J. Mater. Chem., 2011, 21, 9938 RSC; (d) N. Choi, Z. Chen, S. A. Freunberger, X. Ji, Y. Sun, K. Amine, G. Yushin, L. F. Nazar, J. Cho and P. G. Bruce, Angew. Chem., Int. Ed., 2012, 51, 9994 CrossRef CAS PubMed; (e) S. Chu and A. Majumdar, Nature, 2012, 488, 294 CrossRef CAS PubMed; (f) N. G. Sahoo, Y. Pan, L. Li and S. H. Chan, Adv. Mater., 2012, 24, 4203 CrossRef CAS PubMed; (g) A. L. M. Reddy, S. R. Gowda, M. M. Shaijumon and P. M. Ajayan, Adv. Mater., 2012, 24, 5045 CrossRef CAS PubMed; (h) H. Wang and H. Dai, Chem. Soc. Rev., 2013, 42, 3088 RSC; (i) N. Mahmood, C. Zhang, H. Yin and Y. Hou, J. Mater. Chem. A, 2014, 2, 15 RSC; (j) H. Huang and X. Wang, J. Mater. Chem. A, 2014, 2, 6266 RSC; (k) C. Yuan, H. Wu, Y. Xie and X. Lou, Angew. Chem., Int. Ed., 2014, 53, 1488 CrossRef CAS PubMed; (l) A. Zahoor, M. Christy, Y. J. Hwang, Y. R. Lim, P. Kim and K. S. Nahm, Appl. Catal., B, 2014, 147, 633 CrossRef CAS.
- M. K. Debe, Nature, 2012, 486, 43 CrossRef CAS PubMed.
- (a) Y. Shao, J. Sui, G. Yin and Y. Gao, Appl. Catal., B, 2008, 79, 89 CrossRef CAS; (b) C. W. B. Bezerra, L. Zhang, K. Lee, H. Liu, A. L. B. Marques, E. P. Marques, H. Wang and J. Zhang, Electrochim. Acta, 2008, 53, 4937 CrossRef CAS.
- N. Daems, X. Sheng, I. F. J. Vankelecom and P. P. Pescarmona, J. Mater. Chem. A, 2014, 2, 4085 CAS.
- A. Morozan, B. Jousselme and S. Palacin, Energy Environ. Sci., 2011, 4, 1238 CAS.
- I. Katsounaros, S. Cherevko, A. R. Zeradjanin and K. J. J. Mayrhofer, Angew. Chem., Int. Ed., 2014, 53, 102 CrossRef CAS PubMed.
- (a) F. Jaouen, E. Proietti, M. Lefèvre, R. Chenitz, J. P. Dodelet, G. Wu, H. T. Chung, C. M. Johnston and P. Zelenay, Energy Environ. Sci., 2011, 4, 114 RSC; (b) Z. Chen, D. Higgins, A. Yu, L. Zhang and J. Zhang, Energy Environ. Sci., 2011, 4, 3167 RSC; (c) L. Dai, Y. Xue, L. Qu, H. Choi and J. B. Baek, Chem. Rev., 2015, 113, 4823 CrossRef PubMed.
- (a) J. Zhang, K. Sasaki, E. Sutter and R. Adzic, Science, 2007, 315, 220 CrossRef CAS PubMed; (b) R. Silva, D. Voiry, M. Chhowalla and T. Asef, J. Am. Chem. Soc., 2013, 135, 7823 CrossRef CAS PubMed.
- Y. Zhao, L. Yang, S. Chen, X. Wang, Y. Ma, Q. Wu, Y. Jiang, W. Qian and Z. Hu, J. Am. Chem. Soc., 2013, 135, 1201 CrossRef CAS PubMed.
- (a) K. P. Gong, F. Du, Z. H. Xia, M. Durstock and L. Dai, Science, 2009, 323, 760 CrossRef CAS PubMed; (b) L. Qu, Y. Liu, J. Baek and L. Dai, ACS Nano, 2010, 4, 1321 CrossRef CAS PubMed; (c) M. Zhang and L. Dai, Nano Energy, 2012, 1, 514 CrossRef CAS; (d) Y. Zheng, Y. Jiao, M. Jaroniec, Y. Jin and S. Qiao, Small, 2012, 8, 3550 CrossRef CAS PubMed; (e) R. Cao, J. Lee, M. Liu and J. Cho, Adv. Energy Mater., 2012, 2, 816 CrossRef CAS.
- (a) R. Liu, D. Wu, X. Feng and K. Mullen, Angew. Chem., Int. Ed., 2010, 49, 2565 CrossRef CAS PubMed; (b) L. Yang, S. Jiang, Y. Zhao, L. Zhu, S. Chen, X. Wang, Q. Wu, J. Ma, Y. Ma and Z. Hu, Angew. Chem., Int. Ed., 2011, 50, 7132 CrossRef CAS PubMed; (c) W. Yang, T. P. Fellinger and M. Antonietti, J. Am. Chem. Soc., 2010, 133, 206 CrossRef PubMed; (d) S. Yang, X. Feng, X. Wang and K. Mullen, Angew. Chem., Int. Ed., 2011, 123, 5451 CrossRef; (e) Z. Wang, R. Jia, J. Zheng, J. Zhao, L. Li, J. Song and Z. Zhu, ACS Nano, 2011, 2, 1677 CrossRef PubMed; (f) Y. Zhao, C. Hu, Y. Hu, H. Cheng, G. Shi and L. Qu, Angew. Chem., Int. Ed., 2012, 51, 11371 CrossRef CAS PubMed; (g) J. Liang, Y. Zheng, J. Chen, J. Liu, D. Hulicova-Jurcakova, M. Jaroniec and S. Qiao, Angew. Chem., Int. Ed., 2012, 51, 3892 CrossRef CAS PubMed; (h) J. Liang, Y. Jiao, M. Jaroniec and S. Qiao, Angew. Chem., Int. Ed., 2012, 51, 11496 CrossRef CAS PubMed; (i) H. Liang, W. Wei, Z. Wu, X. Feng and K. Mullen, J. Am. Chem. Soc., 2013, 135, 16002 CrossRef CAS PubMed.
- (a) G. G. Wildgoose, C. E. Banks and R. G. Compton, Small, 2006, 2, 182 CrossRef CAS PubMed; (b) Y. Yang, K. Chiang and N. Burke, Catal. Today, 2011, 178, 197 CrossRef CAS; (c) M. Inagakia, H. Konnoa and O. Tanaike, J. Power Sources, 2011, 195, 7880 CrossRef.
- (a) K. Waki, R. Wong, H. Oktaviano, T. Fujio, T. Nagai, K. Kimoto and K. Yamada, Energy Environ. Sci., 2014, 7, 1950 RSC; (b) S. Jiang, Z. Li, H. Wang, L. Meng and S. Song, Nanoscale, 2014, 6, 14262 RSC.
- (a) L. Wang, A. Ambrosi and M. Pumera, Angew. Chem., Int. Ed., 2013, 52, 13818 CrossRef CAS PubMed; (b) J. Masa, A. Zhao, W. Xia, Z. Sun, B. Mei, M. Muhler and W. Schuhmann, Electrochem. Commun., 2013, 34, 113 CrossRef CAS.
- H. Zhao, C. Sun, Z. Jin, D. Wang, X. Yan, Z. Chen, G. Zhu and X. Yao, J. Mater. Chem. A, 2015, 3, 11736 CAS.
- (a) B. Liu, H. Shioyama, H. Jiang, X. Zhang and Q. Xu, Carbon, 2010, 48, 456 CrossRef CAS; (b) S. Yang, T. Kim, J. Im, Y. Kim, K. Lee, H. Jung and C. Park, Chem. Mater., 2012, 24, 464 CrossRef CAS; (c) A. Aijaz, N. Fujiwara and Q. Xu, J. Am. Chem. Soc., 2014, 136, 6790 CrossRef CAS PubMed; (d) J. Li, S. Li, Y. Tang, K. Li, L. Zhou, N. Kong, Y. Lan, J. Bao and Z. Dai, Sci. Rep., 2014, 4, 5130 CAS; (e) J. Li, Y. Chen, Y. Tang, S. Li, H. Dong, K. Li, M. Han, Y. Lan, J. Ba and Z. Dai, J. Mater. Chem. A, 2014, 2, 6316 RSC; (f) W. Xia, A. Mahmood, R. Zou and Q. Xu, Energy Environ. Sci., 2015, 8, 1837 RSC; (g) Y. Liu, X. Xu, M. Wang, T. Lu, Z. Sun and L. Pan, Chem. Commun., 2015, 51, 12020 RSC.
- M. Eddaoudi, J. Kim, N. Rosi, D. Vodak, J. Wachter, M. O'Keeffe and O. M. Yaghi, Science, 2002, 295, 469 CrossRef CAS PubMed.
- P. Pachfule, B. Biswal and R. Banerjee, Chem. – Eur. J., 2012, 18, 11399 CrossRef CAS PubMed.
- (a) K. Saenger, J. Tsang, A. Bol, J. Chu and A. Grill, Appl. Phys. Lett., 2010, 96, 153105 CrossRef; (b) M. Hu, J. Reboul, S. Furukawa, N. L. Torad, Q. Ji, P. Srinivasu, K. Ariga, S. Kitagawa and Y. Yamauchi, J. Am. Chem. Soc., 2012, 134, 2864 CrossRef CAS PubMed.
- N. Bozovic, I. Bozovi and J. Misewich, Nano Lett., 2008, 8, 4477 CrossRef CAS PubMed.
- D. Graf, F. Molitor, K. Ensslin, C. Stampfer, A. Jungen, C. Hierold and L. Wirtz, Nano Lett., 2007, 7, 238 CrossRef CAS PubMed.
- (a) T. Chen, Z. Cai, Z. Yang, L. Li, X. Sun, T. Huang, A. Yu, H. Kia and H. Peng, Adv. Mater., 2011, 23, 4620 CrossRef CAS PubMed; (b) Y. Shao, S. Zhang, M. Engelhard, G. Li, G. Shao, Y. Wang, J. Liu, I. A. Aksayc and Y. Lin, J. Mater. Chem., 2010, 20, 7491 RSC; (c) Y. Ma, L. Sun, W. Huang, L. Zhang, J. Zhao, Q. Fan and W. Huang, J. Phys. Chem. C, 2011, 115, 24592 CrossRef CAS; (d) S. Wang, D. Yu and L. Dai, J. Am. Chem. Soc., 2011, 133, 5182 CrossRef CAS PubMed; (e) X. Bo and L. Guo, Phys. Chem. Chem. Phys., 2013, 15, 2459 RSC.
- O. Akhavan, ACS Nano, 2010, 4, 4174 CrossRef CAS PubMed.
- (a) Z. R. Yue, W. Jiang, L. Wang, S. D. Gardner Jr. and C. U. Pittman, Carbon, 1999, 37, 1785 CrossRef CAS; (b) G. Liu, X. Li, P. Ganesan and B. N. Popov, Electrochim. Acta, 2010, 55, 2853 CrossRef CAS; (c) J. Gao, F. Liu, Y. Liu, N. Ma, Z. Wang and X. Zhang, Chem. Mater., 2010, 22, 2213 CrossRef CAS; (d) C. Zhang, X. Peng, Z. Guo, C. Cai, Z. Chen, D. Wexler, S. Li and H. Liu, Carbon, 2012, 50, 1897 CrossRef CAS.
- (a) W. Chaikittisilp, M. Hu, H. Wang, H. Huang, T. Fujita, K. Wu, L. Chen, Y. Yamauchi and K. Ariga, Chem. Commun., 2012, 48, 7259 RSC; (b) K. Xi, S. Cao, X. Peng, C. Ducati, R. V. Kumar and A. K. Cheetham, Chem. Commun., 2013, 49, 2192 RSC; (c) Q. Wang, W. Xia, W. Guo, L. An, D. Xia and R. Zou, Chem. – Asian J., 2013, 8, 1879 CrossRef CAS PubMed.
- (a) C. Zhang, R. Hao, H. Yin, F. Liu and Y. Hou, Nanoscale, 2012, 4, 7326 RSC; (b) Y. Tan, C. Xu, G. Chen, X. Fang, N. Zheng and Q. Xie, Adv. Funct. Mater., 2012, 22, 4584 CrossRef CAS; (c) D. Yang, D. Bhattacharjya, S. Inamdar, J. Park and J. Yu, J. Am. Chem. Soc., 2012, 134, 16127 CrossRef CAS PubMed; (d) C. Zhang, N. Mahmood, H. Yin, F. Liu and Y. Hou, Adv. Mater., 2013, 35, 4932 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details of Experimental section and electrochemical measurements, CV curves, linear sweep voltammograms, Koutecky–Levich plots and the data of N2 adsorption, XPS, ORR. See DOI: 10.1039/c5qi00236b |
| This journal is © the Partner Organisations 2016 |