Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Photooxygenation of alkanes by dioxygen with p-benzoquinone derivatives with high quantum yields†
Kei
Ohkubo
*abc,
Kensaku
Hirose
b and
Shunichi
Fukuzumi
*cd
aDepartment of Applied Chemistry, Graduate School of Engineering, Osaka University, Suita, Osaka 565-0871, Japan. E-mail: ookubo@chem.eng.osaka-u.ac.jp
bDepartment of Material and Life Science, Graduate School of Engineering, Osaka University, ALCA and SENTAN, Japan Science and Technology Agency (JST), Suita, Osaka 565-0871, Japan
cDepartment of Chemistry and Nano Science, Ewha Womans University, Seoul 120-750, Korea. E-mail: fukuzumi@chem.eng.osaka-u.ac.jp
dFaculty of Science and Technology, Meijo University, ALCA and SENTAN, Japan Science and Technology Agency (JST), Nagoya, Aichi 468-8502, Japan
First published on 29th April 2016
Abstract
Alkanes were oxygenated by dioxygen with p-benzoquinone derivatives such as p-xyloquinone in alkanes which are used as solvents to yield the corresponding alkyl hydroperoxides, alcohols and ketones under visible light irradiation with high quantum yields (Φ = 1000, 1600%). The photooxygenation is started by hydrogen atom abstraction from alkanes by the triplet excited states of p-benzoquinone derivatives as revealed by laser-induced transient absorption spectral measurements.
Oxidation of alkanes, which are saturated hydrocarbons, to produce basic raw materials in chemical industry is quite difficult and requires harsh conditions. For example, the conventional industrial process for oxidation of cyclohexane to a mixture of cyclohexanol and cyclohexanone known as KA-oil, which are important intermediates for the manufacture of nylon-6 and nylon-6,6, requires the use of soluble cobalt or manganese salts as homogeneous catalysts at 140–180 °C and 0.8–2 MPa (7.9–19.8 atm) in air.1,2 So far extensive efforts have been devoted to develop catalytic oxidation of cyclohexane using hydrogen peroxide (H2O2),3–6tert-butyl hydroperoxide (TBHP),6,7 iodosylbenzene (PhIO),8 ozone (O3),9 and dioxygen (O2)3,10–12 as oxidants. Among various oxidants, O2 is an ideal oxidant because it is ubiquitous and the reduced product (H2O or H2O2) is safe. Oxygenation of cyclohexane by O2 was made possible by using the 9-mesityl-10-methylacridinium ion as an organic photocatalyst combined with HCl in O2-saturated acetonitrile (MeCN) at room temperature under visible light irradiation.13 The photochemical oxygenation of cyclohexane is initiated by electron transfer from Cl− to the mesitylene radical cation moiety of the electron-transfer state of the 9-mesityl-10-methylacridinium ion to produce Cl˙ that abstracts a hydrogen atom from cyclohexane to generate a cyclohexyl radical, which is readily oxygenated by O2.13 There has been no report on the photochemical oxygenation of cyclohexane with an organic photooxidant and without an inorganic compound such as HCl.
We report herein inorganic-free oxygenation of cyclohexane by O2 using p-benzoquinone derivatives as the only organic photocatalyst in O2-saturated cyclohexane under visible light irradiation. Other alkanes were also oxygenated by O2 with p-benzoquinone derivatives in O2-saturated alkanes under visible light irradiation. The photooxygenation mechanism is clarified by laser-induced transient absorption measurements.
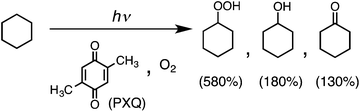
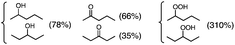
Photoirradiation of an O2-saturated cyclohexane solution (3.0 cm3) containing p-xyloquinone (PXQ, 2.0 mM) for 26 h with a xenon lamp (λ > 390 nm) resulted in the production of oxygenated products (Fig. 1), which were detected by GC-MS (Fig. S1 in the ESI†).14 The yields based on PXQ were 580% for cyclohexyl hydroperoxide, 180% for cyclohexanol and 130% for cyclohexanone (Scheme 1). Cyclohexyl hydroperoxide was also detected by the titrations of sodium iodide15 and triphenylphosphine12 (see the Experimental section in the ESI†). The conversion of neat cyclohexane was 22%. The quantum yield of the photooxygenation was determined to be as high as 100% from the initial rate using monochromatised light (λ = 420 nm) (see the Experimental section in the ESI†).
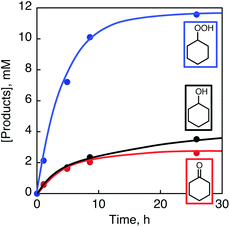 | ||
| Fig. 1 Reaction time profiles for the formation of cyclohexyl hydroperoxide (blue), cyclohexanol (black) and cyclohexanone (red) in the photochemical oxygenation of an oxygen-saturated cyclohexane solution containing PXQ (2.0 mM) under photoirradiation by the use of a xenon lamp (500 W; λ > 390 nm) at 298 K. The GC charts for the determination of the yields are shown in Fig. S1 (ESI†). | ||
When PXQ was replaced by p-benzoquinone (Q), the yields were decreased to 16% for cyclohexanol and 14% for cyclohexanone (Fig. S2a in the ESI†). In the case of tetramethyl-p-benzoquinone, no oxygenated product was formed under the otherwise same experimental conditions. No photoreactivity of tetramethyl-p-benzoquinone may result from the steric effect of the methyl group.
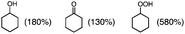
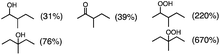
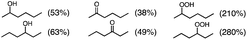
Photooxygenation of branched alkanes such as 3-methylpentane with PXQ occurred to produce the corresponding alcohol and ketone products (Table 1, entry 2) with the total yield of 1000% based on the initial concentration of PXQ (2.0 mM). In the case of normal-chain alkanes such as n-hexane and n-pentane, the efficient catalytic oxygenation also took place with the high quantum yields (510% for n-hexane and 140% for n-pentane) as shown in Table 1 (GC data are shown in Fig. S3–S5 in the ESI†).
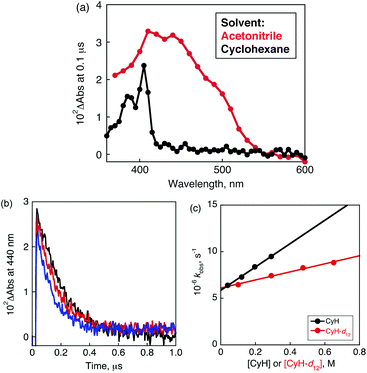
Nanosecond laser flash photolysis was employed to observe the hydrogen-transfer dynamics of the triplet excited state of PXQ (3PXQ*: * denotes the excited state) in cyclohexane. The transient absorption spectra were recorded at 0.1 μs after nanosecond pulse irradiation at 355 nm of a deaerated cyclohexane solution (spectrum in black) and acetonitrile solution (red) containing PXQ as shown in Fig. 2a. The transient absorption bands appearing at 440 nm and 405 nm are assigned to 3PXQ*![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 and PXQH˙,17 respectively. The decay at 440 nm obeyed pseudo-fist-order kinetics as shown in Fig. 2b. The decay rate constants (kobs) of 3PXQ* increased with increasing concentrations of cyclohexane (Fig. 2c). The rate constant of hydrogen transfer from cyclohexane to 3PXQ* (kH) was determined to be 1.3 × 107 M−1 s−1 from the slope of the plot of kobsvs. [cyclohexane] (Fig. 2c). The value in the case of cyclohexane-d12 (kD) was also determined to be 4.3 × 106 M−1 s−1 (Fig. 2c; red plot). The deuterium kinetic isotope effect (KIE = kH/kD) was determined to be 3.0. Such a large KIE value indicates that the rate-determining step of the photochemical oxygenation is hydrogen-atom transfer from cyclohexane to the triplet excited state of PXQ (3PXQ*), which produces a cyclohexyl radical and PXQH˙. The hydrogen-atom abstraction from various substrates by the triplet excited states of ketones has been known for a long time.18–21 However, the hydrogen-atom abstraction from cyclohexane has been difficult, because cyclohexane has been used as an inert solvent for transient absorption measurements of the triplet excited states of ketones.22
16 and PXQH˙,17 respectively. The decay at 440 nm obeyed pseudo-fist-order kinetics as shown in Fig. 2b. The decay rate constants (kobs) of 3PXQ* increased with increasing concentrations of cyclohexane (Fig. 2c). The rate constant of hydrogen transfer from cyclohexane to 3PXQ* (kH) was determined to be 1.3 × 107 M−1 s−1 from the slope of the plot of kobsvs. [cyclohexane] (Fig. 2c). The value in the case of cyclohexane-d12 (kD) was also determined to be 4.3 × 106 M−1 s−1 (Fig. 2c; red plot). The deuterium kinetic isotope effect (KIE = kH/kD) was determined to be 3.0. Such a large KIE value indicates that the rate-determining step of the photochemical oxygenation is hydrogen-atom transfer from cyclohexane to the triplet excited state of PXQ (3PXQ*), which produces a cyclohexyl radical and PXQH˙. The hydrogen-atom abstraction from various substrates by the triplet excited states of ketones has been known for a long time.18–21 However, the hydrogen-atom abstraction from cyclohexane has been difficult, because cyclohexane has been used as an inert solvent for transient absorption measurements of the triplet excited states of ketones.22
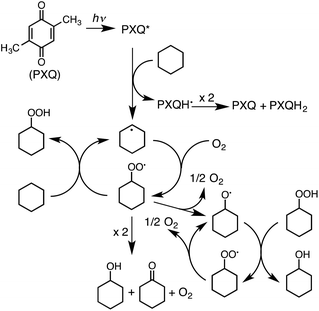
The photooxygenation of cyclohexane (CyH) is initiated by hydrogen abstraction from cyclohexane by 3PXQ* to form the radical pair of a cyclohexyl radical (Cy˙) and a PXQ semiquinone radical (PXQH˙) (Scheme 2). The addition of O2 to Cy˙ affords a cyclohexylperoxyl radical (CyOO˙), which abstracts a hydrogen atom from cyclohexane to produce the cyclohexyl radical (Cy˙) and cyclohexyl hydroperoxide (CyOOH). Cyclohexanol (CyOH) and cyclohexanone (Cy![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) as final oxygenated products are formed via the disproportionation of CyOO˙.13,23 PXQH˙ also disproportionates to PXQ and PXQH2. However, the yield of CyOH (180%) is higher than that of Cy
O) as final oxygenated products are formed via the disproportionation of CyOO˙.13,23 PXQH˙ also disproportionates to PXQ and PXQH2. However, the yield of CyOH (180%) is higher than that of Cy![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (130%) (Table 1, entry 1). The additional CyOH may be formed by decomposition of CyOOH to CyOH via the radical chain reactions, where the cyclohexyloxyl radical (CyO˙) produced by disproportionation of CyOO˙ abstracts hydrogen from CyOOH to produce CyOH, accompanied by regeneration of CyOO˙.
O (130%) (Table 1, entry 1). The additional CyOH may be formed by decomposition of CyOOH to CyOH via the radical chain reactions, where the cyclohexyloxyl radical (CyO˙) produced by disproportionation of CyOO˙ abstracts hydrogen from CyOOH to produce CyOH, accompanied by regeneration of CyOO˙.
The extremely high quantum yields of the photooxygenation such as 1600% for 3-methylpentane and 1000% for cyclohexane result from these autoxidation radical chain processes.
In conclusion, efficient oxygenation reactions of alkanes are initiated by hydrogen-atom transfer from alkanes to the triplet excited state of p-xyloquinone acting as a photocatalyst under the solvent-free and ambient conditions, where molecular oxygen acts as an oxidant. Radical chain autoxidation processes with high concentrations of substrates (solvent) have enabled us to obtain the high quantum yields. Thus, the present study provides an environmentally benign way for photooxygenation of alkanes by O2 with unusually high quantum yields.
This work was supported by Grants-in-Aid (no. 16K13964, 26620154 and 26288037 to K. O.) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT); ALCA and SENTAN projects from JST, Japan (to S. F.).
Notes and references
- I. Hermans, E. S. Spier, U. Neunschwanedr, N. Turrá and A. Baiker, Selective oxidation catalysis: opportunities and challenges, Top. Catal., 2009, 52, 1162–1174 CrossRef CAS.
- M. T. Musser, Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005 Search PubMed.
- M. Bordeaux, A. Galarneau and J. Drone, Catalytic, mild, and selective oxyfunctionalization of linear alkanes: current challenges, Angew. Chem., Int. Ed., 2012, 51, 10712–10723 CrossRef CAS PubMed.
- K. Kamata, K. Yonehara, Y. Nakagawa, K. Uehara and N. Mizuno, Efficient stereo- and regioselective hydroxylation of alkanes catalysed by a bulky polyoxometalate, Nat. Chem., 2010, 2, 478–483 CrossRef CAS PubMed.
- S. S. Acharyya, S. Ghosh and R. Bal, Nanoclusters of Cu(II) supported on nanocrystalline W(VI) oxide: a potential catalyst for single-step conversion of cyclohexane to adipic acid, Green Chem., 2015, 17, 3490–3499 RSC.
- A. C. Lindhorst, S. Haslinger and F. E. Kühn, Molecular iron complexes as catalysts for selective C–H bond oxygenation reactions, Chem. Commun., 2015, 51, 17193–17212 RSC.
- C. Wang, L. Chen and Z. Qi, One-pot synthesis of gold nanoparticles embedded in silica for cyclohexane oxidation, Catal. Sci. Technol., 2013, 3, 1123–1128 CAS.
- M. H. Xie, X. L. Yang, Y. He, J. Zhang, B. Chen and C. D. Wo, Highly efficient C–H oxidative activation by a porous MnIII–porphyrin metal–organic framework under mild conditions, Chem. – Eur. J., 2013, 19, 14316–14321 CrossRef CAS PubMed.
- K. C. Hwang and A. Sagadevan, One-pot room-temperature conversion of cyclohexane to adipic acid by ozone and UV light, Science, 2014, 346, 1495–1498 CrossRef CAS PubMed.
- N. Imanaka, T. Masui and K. Jyoko, Selective liquid phase oxidation of cyclohexane over Pt/CeO2-ZrO2-SnO2/SiO2 catalysts with molecular oxygen, J. Adv. Ceram., 2015, 4, 111–117 CrossRef CAS.
- J. Hao, B. Liu, H. Cheng, Q. Wang, J. Wang, C. Cai and F. Zhao, Cyclohexane oxidation on a novel Ti70Zr10Co20 catalyst containing quasicrystal, Chem. Commun., 2009, 3460–3462 RSC.
- B. P. C. Hereijgers and B. M. Weckhuysen, Aerobic oxidation of cyclohexane by gold-based catalysts: new mechanistic insight by thorough product analysis, J. Catal., 2010, 270, 16–25 CrossRef CAS.
- K. Ohkubo, A. Fujimoto and S. Fukuzumi, Metal-free oxygenation of cyclohexane with oxygen catalyzed by 9-mesityl-10-methylacridinium and hydrogen chloride under visible light irradiation, Chem. Commun., 2011, 47, 8515–8517 RSC.
- Photooxidation of cyclohexane by O2 is known to occur under UV light irradiation. Under visible light irradiation, no photooxidation of cyclohexane by O2 occurred. See: N. Kulevsky, P. V. Sneeringer, L. D. Grina and V. I. Stenberg, Photochemical oxidations—IV. The photooxidation of cyclohexane with oxygen, Photochem. Photobiol., 1970, 12, 395–403 CrossRef CAS.
- S. Fukuzumi, S. Kuroda and T. Tanaka, Flavin analogue-metal ion complexes acting as efficient photocatalysts in the oxidation of p-methylbenzyl alcohol by oxygen under irradiation with visible light, J. Am. Chem. Soc., 1985, 107, 3020–3027 CrossRef CAS.
- J.-C. Ronfard-Haret, R. V. Bensasson and E. Amouyal, Assignment of transient species observed on laser flash photolysis of p-benzoquinone and methylated p-benzoquinones in aqueous solution, J. Chem. Soc., Faraday Trans. 1, 1980, 76, 2432–2436 RSC.
- Y. Tanimoto and M. Itoh, Nanosecond laser photolysis study of the magnetic field effect on p-xylosemiquinone radical in sodium dodecylsulfate micelle, Chem. Phys. Lett., 1981, 83, 626–629 CrossRef CAS.
- M. V. Ensinas and J. C. Scaiano, Reaction of benzophenone triplets with allylic hydrogens. Laser flash photolysis study, J. Am. Chem. Soc., 1981, 103, 6393–6397 CrossRef.
- J. C. Scaiano, Intermolecular photoreductions of ketones, J. Photochem., 1973/1974, 2, 81–118 Search PubMed.
- K. Okada, M. Yamaji and H. Shizuka, Laser photolysis investigation of induced quenching in photoreduction of benzophenone by alkylbenzenes and anisoles, J. Chem. Soc., Faraday Trans., 1998, 94, 861–866 RSC.
- C. Coenjarts and J. C. Scaiano, Reaction pathways involved in the quenching of the photoactivated aromatic ketones xanthone and 1-azaxanthone by polyalkylbenzenes, J. Am. Chem. Soc., 2000, 122, 3635–3641 CrossRef CAS.
- D. Jornet, R. Tormos and M. A. Miranda, Photobehavior of mixed nπ*/ππ* triplets: simultaneous detection of the two transients, solvent-dependent hydrogen abstraction, and reequilibration upon protein binding, J. Phys. Chem. B, 2011, 115, 10768–10774 CrossRef CAS PubMed.
- I. Hermans, P. A. Jacobs and J. Peeters, To the core of autocatalysis in cyclohexane autoxidation, Chem. – Eur. J., 2006, 12, 4229–4240 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and GC data for the product analyses. See DOI: 10.1039/c6pp00102e |
| This journal is © The Royal Society of Chemistry and Owner Societies 2016 |