A new luminescent lanthanide supramolecular network possessing free Lewis base sites for highly selective and sensitive Cu2+ sensing†
Tianshu
Chu
a,
Yunsong
Hu
a,
Jinlun
Wu
a,
Chenghui
Zeng
ab,
Yangyi
Yang
*a and
Seik Weng
Ng
c
aMOE Key Laboratory of Bioinorganic and Synthetic Chemistry, KLGHEI of Environment and Energy Chemistry, School of Chemistry and Chemical Engineering, Sun Yat-Sen, University, Guangzhou, 510275, P. R. China. E-mail: cesyyy@mail.sysu.edu.cn
bCollege of Chemistry and Chemical Engineering, Jiangxi Normal University, Nanchang, 330022, P. R. China
cChemistry Department, Faculty of Science, King Abdulaziz University, PO Box 80203, Jeddah, Saudi Arabia
First published on 26th April 2016
Abstract
A series of new lanthanide complexes, formulated as [Ln2(DCSAL)3(H2O)11]·3DCSAL·4H2O [Ln = Eu (1), Gd (2) and Tb (3); DCSAL = 3,5-dichlorosalicylate], have been synthesized and characterized by single crystal X-ray analysis. They are dinuclear clusters and form a 3D supramolecular network via π–π stacking and halogen bonding interactions. 3 exhibits strong Tb characteristic emission, whose quantum yield is as high as 38%. Due to binding with Cu2+ ions via its Lewis acid–base interactions, 3 displayed a high selectivity and sensitivity for Cu2+ detection based on Tb3+ emission quenching. The possible quenching mechanism was further proved to be a static quenching mechanism by Stern–Volmer plots and UV-vis spectrum. More importantly, the binding constant between 3 and Cu2+ is also calculated by the Benesi–Hildebrand method, which is helpful for quantitative analysis.
Introduction
Lanthanide complexes have already attracted broad attention in recent decades because of the combination of inorganic lanthanide ions and organic fragments which may generate various structures. So far, lanthanide complexes have been studied in many fields, such as catalysis,1,2 gas storage and separation,3,4 temperature sensing5–7 and molecular recognition.8–11 In particular, lanthanide complexes exhibit unique optical properties owing to the abundant 4f orbitals of lanthanide ions. As a result, lanthanide complexes are intriguing and remarkably suitable as luminescent sensors for chemical species.12Copper is one of the essential elements for humans. It can promote iron uptake and utilization and plays an important role in the formation of hemoglobin. Anemia, genu valgum and paratrichosis are typical symptoms of cuprum deficiency. However, excess cuprum can also do great harm to health, causing adverse effects such as nausea and vomiting, liver and kidney failure, and neurological disorders.13,14 On the other hand, copper is widely used in the electrical and electronic industry (e.g. wires, motors and printed circuit boards), the defense industry (e.g. bullets and guns), the construction industry (e.g. pipes and decorations), and so on. Therefore, the determination of copper ion is of great importance because of this wide variety of applications.
The traditional methods of analyzing copper include atomic absorption spectroscopy (AAS),15,16 inductively coupled plasma-mass spectroscopy (ICP-MS)17 and inductively coupled plasma-atomic emission spectrometry (ICP-AES).18 These methods offer low detection limits but require relatively complicated sample pretreatment and costly analytical instruments. In recent years, various fluorescence probes have been reported for Cu2+ owing to the low costs of spectrophotometric methods.19–23
Compared to conventional fluorophores, lanthanide complexes have highly sensitive detection because of their unique optical properties, and the “Antenna effect” can overcome the drawbacks of lanthanide ions with low absorption coefficients. In addition, the narrow emission bands and relatively long luminescence lifetime can avoid various background signals from organic compounds.24,25
Our group have been working on luminescent chemosensors for a number of years,26,27 especially for detecting heavy metal ions. Recently, we reported a novel luminescent terbium-succinate thin film for sensing Cu2+.27 However, the film is not particularly sensitive (Ksv is about 6000 M−1), perhaps because of the following two reasons: (1) the ligand succinate does not contain a large conjugate system, leading to a low absorption coefficient; (2) the ligand succinate does not have specific recognition sites and the luminescent quenching is primarily due to ion-exchange. In view of these problems, we would prefer to select ligands containing large conjugate systems, such as aromatic nuclei, and typical Lewis base sites, such as oxygen, nitrogen and sulfur.
In our previous work, we have synthesized a number of lanthanide complexes based on salicylic ligands and found that the coordination mode of salicylic ligands depends on the pH value of the reaction system.28 At low pH, the phenolic hydroxyl groups of salicylic ligands are uncoordinated. These bare hydroxyl groups are potential Lewis base sites which may act as probes for Lewis acid, exactly what we hope for.
Based on the above reasons, we selected the ligand 3,5-dichlorosalicylic acid (HDCSAL) as the Cu2+ receptor. In this article, we present the synthesis of a series of lanthanide supramolecular networks, formulated as [Ln2(DCSAL)3(H2O)11]·3DCSAL·4H2O [Ln = Eu (1), Gd (2) and Tb (3); DCSAL = 3,5-dichlorosalicylate], and their crystal structures were determined by X-ray diffraction analysis. It is worth noting that 3 presents high selectivity and sensitivity for Cu2+ ion detection. The quenching mechanism was further proved to be a static quenching mechanism based on Lewis acid–base interactions. We used the Benesi–Hildebrand method to confirm a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding between 3 and Cu2+ and calculate its binding constant. Moreover, we determined the LOD (limit of detection) for sensing Cu2+. The LOD is as low as 340 nM, suggesting that 3 is a good candidate as a luminescence probe and may be applied in environmental and biological systems.
1 binding between 3 and Cu2+ and calculate its binding constant. Moreover, we determined the LOD (limit of detection) for sensing Cu2+. The LOD is as low as 340 nM, suggesting that 3 is a good candidate as a luminescence probe and may be applied in environmental and biological systems.
Experimental
Materials
All reagents and solvents were obtained commercially and used without further purification.Single crystal structure determination
Single crystal X-ray diffraction data were collected at 100 K on a Bruker Smart 1000 CCD diffractometer, using graphite monochromatized Mo Kα radiation (λ = 0.71073 Å). The structure was solved by direct methods and refined with the full matrix least-squares method against Fo2 using SHELXTL-97. All non-hydrogen atoms were refined with anisotropic displacement parameters.IR and PXRD
IR spectra were recorded with a Nicolet 330 FT-IR spectrometer using KBr pellets in the wavenumber range of 4000–400 cm−1. The powder X-ray diffraction patterns were recorded on a Rigaku D/MAX 2200VPC diffractometer with Cu Kα radiation (λ = 1.5409 Å) at a scanning rate of 5° min−1 with 2θ ranging from 5 to 50°.UV–Vis absorption and luminescence spectroscopy
UV–vis absorption spectra were recorded on a Jena SPECORD 50 PLUS UV/vis spectrophotometer. Phosphorescence spectra were measured using a Hitachi F-7000 spectrofluorophotometer equipped with a xenon lamp, 1.0 cm quartz cells, and 5.0/10.0 nm slits. For all measurements, the excitation wavelength was chosen as 380 nm and all spectra were recorded at 25 °C. The luminescence lifetime was determined on an Edinburgh FLS920 time-correlated pulsed single-photon-counting instrument. Luminescence quantum yields at room temperature were measured using the technique for powdered samples described by Bril et al., through the following expressionwhere rx and rst represent the diffuse reflectance (with respect to a fixed wavelength) of the complex and of the standard phosphor, respectively, and Φst is the quantum yield of the standard phosphor. The terms Ax and Ast represent the areas under the complex spectra and the standard emission spectra, respectively. To acquire absolute intensity values, BaSO4 was used as a reflecting standard. Three measurements were made for each sample, and the reported Φoverall value corresponds to the arithmetic mean value of the three values.
Synthesis of [Eu2(DCSAL)3(H2O)11]·3DCSAL·4H2O (1)
A mixture of Eu(NO3)3·6H2O (90 mg, 0.2 mmol), HDCSAL (124 mg, 0.6 mmol), NaOH aqueous solution (0.5 mL, 1 M), isopropanol (15 mL) and deionized water (15 mL) was added to a 50 mL beaker and stirred for 10 min. The resulting solution was left at ambient temperature to crystallize. A block of colorless crystals of 1 was obtained after the solution had evaporated for 1 week. Yield: 55% based on HDCSAL. Anal. Calcd for 1: C, 27.84%; H, 2.652%. Found: C, 28.12%, H 2.567%. IR (KBr, cm−1): 3417 (m), 1560 (s), 1488 (s), 1371 (s), 1254 (m), 1237 (w), 879 (w), 767 (m), 569 (w), 511 (w), 447 (w).Synthesis of [Gd2(DCSAL)3(H2O)11]·3DCSAL·4H2O (2)
This compound was synthesized following the same synthetic procedure as that for 1 except that Eu(NO3)3·6H2O was replaced by Gd(NO3)3·6H2O. A block of colorless crystals of 2 was obtained with a yield of 51% based on HDCSAL. Anal. Calcd for 2: C, 27.68%; H, 2.636%. Found: C, 27.97%, H 2.552%. IR (KBr, cm−1): 3415 (m), 1553 (s), 1487 (s), 1372 (s), 1250 (m), 1229 (w), 886 (w), 754 (m), 580 (w), 501 (w), 424 (w).Synthesis of [Tb2(DCSAL)3(H2O)11]·3DCSAL·4H2O (3)
This compound was synthesized following the same synthetic procedure as that for 1 except that Eu(NO3)3·6H2O was replaced by Tb(NO3)3·6H2O. A block of colorless crystals of 3 was obtained with a yield of 51% based on HDCSAL. Anal. Calcd for 3: C, 27.63%; H, 2.632%. Found: C, 27.77%, H 2.590%. IR (KBr, cm−1): 3413 (m), 1564 (s), 1492 (s), 1375 (s), 1258 (m), 1237 (w), 882 (w), 770 (m), 585 (w), 508 (w).Synthesis of CuDCSAL
A mixture of Cu(NO3)2·3H2O (49 mg, 0.2 mmol), HDCSAL (83 mg, 0.4 mmol), NaOH aqueous solution (0.5 mL, 1 M), isopropanol (15 mL) and deionized water (15 mL) was added to a 50 mL beaker and stirred for 10 min. The resulting solution was left at ambient temperature to crystallize. Blue microcrystals of CuDCSAL were obtained after the solution had evaporated. Unfortunately, we did not obtain a suitable single crystal of this complex for structure analysis.Results and discussion
Crystal structure of complexes 1–3
Single-crystal X-ray analysis revealed that complexes 1–3 are isostructural dinuclear derivatives and crystallize in the monoclinic space group P21/c. Thus, only the structure of 3 will be described in detail here. As shown in Fig. 1a, two Tb(III) ions and three ligands construct a discrete dinuclear unit. There are two kinds of coordination environment of Tb. Tb1 is coordinated by three O atoms from the carboxyl groups of three ligands and five O atoms from the terminal coordinated water molecules. Its coordination environment can be described as a distorted dodecahedron (Fig. S1a†). On the other hand, Tb2 is surrounded by two O atoms from the carboxyl groups of two ligands and six O atoms from the terminal coordinated water molecules. The environment of Tb2 can also be described as a distorted bicapped trigonal prism (Fig. S1b†). The Tb–O bond lengths are in the range of 2.392(3)–2.568(3) Å, comparable to the bond distances reported in a previous study.29 It should be noted that there are also three uncoordinated ligands and these adopt a face-to-face π–π stacking with the three coordinated ligands, respectively, which play a crucial role in the 3D supramolecular net formation. | ||
| Fig. 1 (a) ORTEP view (30% thermal ellipsoids) of the dinuclear structure of 3. (b) One-dimensional (1D) chain along c axis, (c) 2D layer in ac plane of 3 and (d) 3D supramolecular network of 3. | ||
Further study into the structure of 3 showed that the dinuclear unit is linked by two of the three uncoordinated ligands via π–π stacking interactions, generating the 1D chain along the c axis (Fig. 1b). There are also abundant H-bonds between coordinated water and free water to reinforce this structure. The 1D chains are further linked by the third uncoordinated ligand via π–π stacking and give rise to the 2D layer in the ac plane (Fig. 1c). In addition, the chlorine atoms of the ligand are exposed on the surface of the 2D layer. As a result, the layers are further connected together by Cl⋯Cl interactions giving rise to a supramolecular 3D network (Fig. 1d).
Photochemistry properties
The excitation and emission spectra of 1 and 3 in the solid state were recorded on a fluorescence spectrophotometer at room temperature. The excitation spectra of complexes 1 and 3 were recorded by monitoring the intensity of the 5D0 → 7F2 transition of Eu3+ ion and the 5D4 → 7F5 transition of Tb3+ ion, respectively. As revealed in Fig. 2, the excitation spectra of 3 shows a broad band in the range of 330–380 nm, which can be ascribed to energy transfer from the ligand to the metal. As is known, ligand-to-metal energy-transfer becomes available providing there is sufficient overlap of excited states of the ligands with excited 4f states of the lanthanides, which confirms that luminescence sensitization through excitation of the ligand is much more efficient than the direct excitation of the Tb3+ ion absorption level. Upon excitation at 368 nm, the emission spectra displayed the typical pattern associated with terbium-centered 5D4 → 4FJ (J = 6–3) transitions, with sharp emissions centered at 495, 545, 589 and 624 nm. The emission band of the ligand can also be observed at 438 nm, which may be ascribed to uncoordinated ligands. In comparison, for complex 1, neither the characteristic emission of Eu(III) nor the fluorescence of ligands can be observed under the same conditions due to the poor match between the triplet energy level of ligands and the emitting state of Eu(III) ions.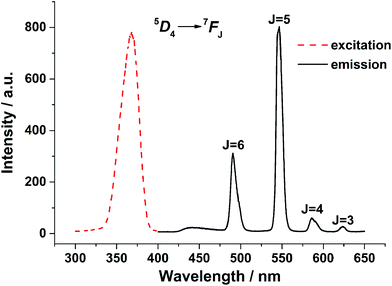 | ||
| Fig. 2 Excitation (λem = 545 nm) and emission (λex = 352 nm) spectra of 3 in solid state recorded at room temperature. | ||
Apart from the emission spectra, the luminescent lifetimes and absolute quantum yields of 3 were also measured at room temperature. To effectively estimate the lifetime values of the excited state 5D4 (Tb3+), the luminescence decay curves were measured by monitoring the most intense emission line of Tb3+ (545 nm, 5D4 → 7F5) upon excitation at 368 nm. As shown in Fig. S2,† the decay curve can well be fitted to a double-exponential function with a good approximation and yields a lifetime of 0.45 ms. The double-exponential decay process means the presence of two emissive terbium centers in the solid sample, which is consistent with the crystal structure analysis mentioned above. The absolute quantum yield of 3, determined by an integrating sphere in the solid state, is found to be 0.38 suggesting an efficient ligand-to-metal energy-transfer process in this complex.
Cation sensing
Taking advantage of the excellent luminescent properties of 3, a further study for sensing cations in solution was investigated. To verify that 3 did not decompose in solution, the infrared spectra of 3 in the solid state and in acetonitrile solution were measured and compared. As shown in Fig. S3,† the spectra of complex 3 in the solid state display two strong absorption bands at 1622 and 1375 cm−1, which are due to the characteristic νas(COO–) and νs(COO–) stretching modes of carboxylic groups. The value of Δν (νas(COO–) − νs(COO–), 247 cm−1) is determined by the coordination mode of carboxylic groups. According to the value of Δν, we can infer whether the coordination mode changes. In our experiment, we found that the value of Δν in solution (250 cm−1, νas(COO–) = 1624 cm−1νs(COO–) = 1374 cm−1) was exactly the same as that in the solid state (Fig. S3†). Therefore, we speculate that 3 can maintain its structure in acetonitrile solution.The luminescence intensity of 3 (100 μM in acetonitrile) was found to decrease rapidly with the addition of Cu2+ ions. The effect of the addition of various other cations (Na+, K+, Ca2+, Mg2+, Cr3+, Zn2+, Ni2+, Cd2+, Hg2+, Ag+) was also investigated to evaluate the selectivity for Cu2+ ions. As shown in Fig. 3 and S4,† all of these metal ions have little or no effect on the luminescence intensity of 3. On the other hand, it should be noted that Cu2+ can quench the emission of 3 completely. The color change under 254 nm laboratory UV light is clearly discriminated with the naked eye, showing that 3 features highly selectivity and sensitivity for Cu2+.
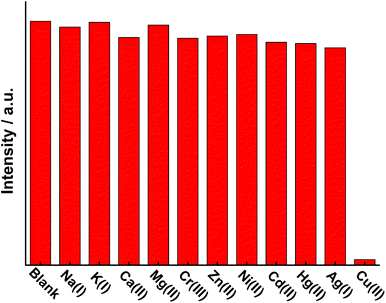 | ||
| Fig. 3 The 5D4 → 7F5 transition (545 nm) intensities of 3 in acetonitrile containing 0.0005 mol L−1 different metal ions. The concentration of 3 was 0.0001 mol L−1. | ||
One challenge for the chemosensor is to obtain a unique detection system for Cu2+ over a wide range of potentially competing ions because the system might show cross-sensitivity toward other metal ions. The interferences by other metal ions were further assessed through competitive experiments. Moreover, competition experiments also confirmed that background metal ions resulted in no interference with regard to the detection of Cu2+ in acetonitrile solvent, based on the luminescence spectra. As shown in Fig. 4, no significant variation in luminescence (λmax 545 nm) was observed during the comparison with the same amounts of Cu2+ solution in the presence and absence of other metal ions. These results indicate that the recognition of Cu2+ by 3 is hardly influenced by other coexisting metal ions; therefore, 3 exhibits a high selectivity toward Cu2+.
The quantified value of the quenching effect was obtained using the Stern–Volmer equation. A good linear relationship was found between the luminescence intensity and Cu2+ ion concentration in the range from 3 μM to 50 μM with a correlation coefficient of 0.9938 as demonstrated in Fig. 5. The detection limit was calculated to be 0.17 μM according to the 3σ/m criterion, where m is the slope for the range of the linearity used and σ is the standard deviation of blank (n = 10). Compared to the developed Cu2+ detection methods, the sensitivity of our complex was quite competitive.30–32
 | ||
| Fig. 5 Comparison of intensity-based (triangles) and lifetime-based (diamonds) Stern–Volmer plots in the presence of copper. Static quenching is predominant. | ||
Mechanism of the quenching effect
The predominant quenching mechanism (dynamic or static) of Cu2+ can also be studied with the Stern–Volmer equation. For dynamic quenching, equal slopes for I0/I and τ0/τ are found using I0/I = τ0/τ = 1 + Ksv[Q]. In contrast, only changes in I0/I can be observed for static quenching. As shown in Fig. 5, the lifetime-based Stern–Volmer plot gave τ0/τ = 1.08 and Ksv = 806 M−1. The intensity-based plot gave I0/I = 3.36 and Ksv = 4.80 × 104 M−1. Therefore, we speculated that a static quenching process dominates over dynamic quenching since intensity-based Ksv is about 60-fold higher than the lifetime-based Ksv. Indeed, the phenolic hydroxyl groups of the ligands in 3 are uncoordinated, providing Lewis base sites which may bind with Lewis acids such as metal ions. It is reasonable to suggest that Cu2+ may bind with the ligands and hinder the energy transfer from the ligand to Tb3+. The quenching mechanism is similar to that proposed in an earlier study.33To further verify our claims, the ultraviolet-visible spectrum of 3 in the absence and presence of Cu2+ in acetonitrile was measured. The absorption spectrum shows maxima at 320 nm which can be attributed to the organic linker's π–π* transitions (Fig. S5†). After the addition of Cu2+, a shoulder peak at 365 nm was observed. To explore the attribution of the shoulder peak, we also synthesized the complex Cu-DSCAL. The ultraviolet absorption of Cu-DSCAL is at 342 nm which is quite different from the shoulder peak. Therefore, we speculate that no ion exchange reaction occurred. Instead, there is probably some interaction between 3 and Cu2+.
It is worth mentioning that the binding mechanism is reversible. To check whether the binding mechanism was reversible, we added appropriate amounts of Na2S solution (Na2S + HCl, pH 7) to the Cu2+ quenched 3 solution. As shown in Fig. 6, the emission intensity of 3 gradually recovered with the addition of Na2S solution. Therefore, we can infer that the binding mechanism is reversible.
Binding constant
To calculate the binding constant, a luminescence titration experiment was used. Upon gradual addition of Cu2+, the intensity of the absorption maximum at 545 nm decreased. A large decrease in the intensity was observed up to the addition of 1 equivalent, which slowly leveled off when excess amounts of Cu2+ were added (Fig. 7a). This behavior unambiguously pointed to the formation of a compound with a dinuclear to Cu2+ ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,34 where I0 and I are the luminescence intensities before and after the addition of the Cu2+. The binding constant Ks was determined from the Benesi–Hildebrand plot of I0/(I0 − I) against [Cu2+]−1. As shown in Fig. 7b, a good linear relationship was obtained, characteristic of 1
1,34 where I0 and I are the luminescence intensities before and after the addition of the Cu2+. The binding constant Ks was determined from the Benesi–Hildebrand plot of I0/(I0 − I) against [Cu2+]−1. As shown in Fig. 7b, a good linear relationship was obtained, characteristic of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding. The binding constant can be calculated from the ratio of intercept/slope,35 and its value is 1.58 × 103 M−1.
1 binding. The binding constant can be calculated from the ratio of intercept/slope,35 and its value is 1.58 × 103 M−1.
 | ||
| Fig. 7 (a) Changes in the 5D4 → 7F5 transition (545 nm) intensities of 3 in acetonitrile with the addition of Cu2+. (b) Benesi–Hildebrand plot against [Cu2+]−1. | ||
Conclusions
In summary, a series of novel lanthanide supramolecular networks (1–3) has been synthesized using deprotonated 3,5-dichlorosalicylic acid. They have a discrete dinuclear structure and further assembled into a 3D supramolecular network via aromatic π–π stacking and halogen bonding interactions. 3 exhibits strong green luminescence emission and its quantum yield is as high as 38%. Based on the quenching of Tb3+ emission, 3 displays highly selectivity and sensitivity for Cu2+ ions. Through Stern–Volmer plots and UV-vis spectra, the quenching mechanism proved to be static quenching, which may be related to the blocked energy transfer from the ligands to Tb3+ ions caused by binding with Cu2+. Furthermore, we used the Benesi–Hildebrand method to confirm 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding and calculate the binding constant of 3 with Cu2+. Owing to its excellent luminescent properties, this material may be a good candidate for sensing Cu2+ in environmental and biological systems.
1 binding and calculate the binding constant of 3 with Cu2+. Owing to its excellent luminescent properties, this material may be a good candidate for sensing Cu2+ in environmental and biological systems.
Acknowledgements
The authors acknowledge financial support from the National Natural Science Foundation of China (51472275, 20973203 and 91022012) and Guangdong Natural Science Foundation (2014A030313207).References
- M. Shibasaki and N. Yoshikawa, Lanthanide Complexes in Multifunctional Asymmetric Catalysis, Chem. Rev., 2002, 102, 2187–2210 CrossRef CAS PubMed.
- S. Hong and T. J. Marks, Oranolanthanide-Catalyzed Hydroamination, Acc. Chem. Res., 2004, 37, 673–686 CrossRef CAS PubMed.
- M. Dincǎ, A. F. Yu and J. R. Long, Microporous Metal–Organic Frameworks Incorporating 1,4-Benzeneditetrazolate: Syntheses, Structures, and Hydrogen Storage Properties, J. Am. Chem. Soc., 2006, 128, 8904–8913 CrossRef PubMed.
- H.-L. Jiang, N. Tsumori and Q. Xu, A Series of (6,6)-Connected Porous Lanthanide−Organic Framework Enantiomers with High Thermostability and Exposed Metal Sites: Scalable Syntheses, Structures, and Sorption Properties, Inorg. Chem., 2010, 49, 10001–10006 CrossRef CAS PubMed.
- K. Miyata, Y. Konno, T. Nakanishi, A. Kobayashi, M. Kato, K. Fushimi and Y. Hasegawa, Chameleon Luminophore for Sensing Temperatures: Control of Metal-to-Metal and Energy Back Transfer in Lanthanide Coordination Polymers, Angew. Chem., Int. Ed., 2013, 52, 6413–6416 CrossRef CAS PubMed.
- Y. Cui, R. Song, J. Yu, M. Liu, Z. Wang, C. Wu, Y. Yang, Z. Wang, B. Chen and G. Qian, Dual-Emitting MOF⊃Dye Composite for Ratiometric Temperature Sensing, Adv. Mater., 2015, 27, 1420–1425 CrossRef CAS PubMed.
- Y. Zhou and B. Yan, Ratiometric detection of temperature using responsive dual-emissive MOF hybrids, J. Mater. Chem. C, 2015, 3, 9353–9358 RSC.
- K. L. Wong, G. L. Law, Y. Y. Yang and W. T. Wong, A Highly Porous Luminescent Terbium–Organic Framework for Reversible Anion Sensing, Adv. Mater., 2006, 18, 1051–1054 CrossRef CAS.
- K. Binnemans, Lanthanide-Based Luminescent Hybrid Materials, Chem. Rev., 2009, 109, 4283–4374 CrossRef CAS PubMed.
- C. F. Chow, M. H. Lam and W. Y. Wong, Design and Synthesis of Heterobimetallic Ru(II)–Ln(III) Complexes as Chemodosimetric Ensembles for the Detection of Biogenic Amine Odorants, Anal. Chem., 2013, 85, 8246–8253 CrossRef CAS PubMed.
- J.-N. Hao and B. Yan, Recyclable lanthanide-functionalized MOF hybrids to determine hippuric acid in urine as a biological index of toluene exposure, Chem. Commun., 2015, 51, 14509–14512 RSC.
- Y.-W. Yip, H. Wen, W.-T. Wong, P. A. Tanner and K.-L. Wong, Increased Antenna Effect of the Lanthanide Complexes by Control of a Number of Terdentate N-Donor Pyridine Ligands, Inorg. Chem., 2012, 51, 7013–7015 CrossRef CAS PubMed.
- P. Fox, The copper-iron chronicles: The story of an intimate relationship, BioMetals, 2003, 16, 9–40 CrossRef CAS PubMed.
- M. Turel, A. Duerkop, A. Yegorova, Y. Scripinets, A. Lobnik and N. Samec, Detection of nanomolar concentrations of copper(II) with a Tb-quinoline-2-one probe using luminescence quenching or luminescence decay time, Anal. Chim. Acta, 2009, 644, 53–60 CrossRef CAS PubMed.
- M. Miró, J. M. Estela and V. c. Cerdà, Application of flowing stream techniques to water analysis: Part III. Metal ions: alkaline and alkaline-earth metals, elemental and harmful transition metals, and multielemental analysis, Talanta, 2004, 63, 201–223 CrossRef PubMed.
- B. Wagner, E. Bulska, T. Meisel and W. Wegscheider, Use of atomic spectrometry for the investigationof ancient manuscripts, J. Anal. At. Spectrom., 2001, 16, 417–420 RSC.
- J. S. Becker, M. V. Zoriy, C. Pickhardt, N. Palomero-Gallagher and K. Zilles, Imaging of Copper, Zinc, and Other Elements in Thin Section of Human Brain Samples (Hippocampus) by Laser Ablation Inductively Coupled Plasma Mass Spectrometry, Anal. Chem., 2005, 77, 3208–3216 CrossRef CAS PubMed.
- J. Otero-Romaní, A. Moreda-Piñeiro, A. Bermejo-Barrera and P. Bermejo-Barrera, Evaluation of commercial C18 cartridges for trace elements solid phase extraction from seawater followed by inductively coupled plasma-optical emission spectrometry determination, Anal. Chim. Acta, 2005, 536, 213–218 CrossRef.
- R. S. Shetty, S. K. Deo, Y. Liu and S. Daunert, Fluorescence-based sensing system for copper using genetically engineered living yeast cells, Biotechnol. Bioeng., 2004, 88, 664–670 CrossRef CAS PubMed.
- K. Mariappan, M. Alaparthi, G. Caple, V. Balasubramanian, M. M. Hoffman, M. Hudspeth and A. G. Sykes, Selective Fluorescence Sensing of Copper(II) and Water via Competing Imine Hydrolysis and Alcohol Oxidation Pathways Sensitive to Water Content in Aqueous Acetonitrile Mixtures, Inorg. Chem., 2014, 53, 2953–2962 CrossRef CAS PubMed.
- J. Cody and C. J. Fahrni, Fluorescence sensing based on cation-induced conformational switching: copper-selective modulation of the photoinduced intramolecular charge transfer of a donor–acceptor biphenyl fluorophore, Tetrahedron, 2004, 60, 11099–11107 CrossRef CAS.
- Y. Xiao, Y. Cui, Q. Zheng, S. Xiang, G. Qian and B. Chen, A microporous luminescent metal–organic framework for highly selective and sensitive sensing of Cu2+ in aqueous solution, Chem. Commun., 2010, 46, 5503–5505 RSC.
- Z. Liang, T.-H. Tsoi, C.-F. Chan, L. Dai, Y. Wu, G. Du, L. Zhu, C.-S. Lee, W.-T. Wong, G.-L. Law and K.-L. Wong, A smart “off–on” gate for the in situ detection of hydrogen sulphide with Cu(II)-assisted europium emission, Chem. Sci., 2016, 7, 2151–2156 RSC.
- W.-K. Wong, X. Zhu and W.-Y. Wong, Synthesis, structure, reactivity and photoluminescence of lanthanide(III) monoporphyrinate complexes, Coord. Chem. Rev., 2007, 251, 2386–2399 CrossRef CAS.
- J. Roosen and K. Binnemans, Adsorption and chromatographic separation of rare earths with EDTA- and DTPA-functionalized chitosan biopolymers, J. Mater. Chem. A, 2014, 2, 1530–1540 CAS.
- Y.-M. Zhu, C.-H. Zeng, T.-S. Chu, H.-M. Wang, Y.-Y. Yang, Y.-X. Tong, C.-Y. Su and W.-T. Wong, A novel highly luminescent LnMOF film: a convenient sensor for Hg2+ detecting, J. Mater. Chem. A, 2013, 1, 11312–11319 CAS.
- Z. Wang, H. Liu, S. Wang, Z. Rao and Y. Yang, A luminescent Terbium-Succinate MOF thin film fabricated by electrodeposition for sensing of Cu2+ in aqueous environment, Sens. Actuators, B, 2015, 220, 779–787 CrossRef CAS.
- C.-H. Zeng, F.-L. Zhao, Y.-Y. Yang, M.-Y. Xie, X.-M. Ding, D.-J. Hou and S. W. Ng, Unusual method for phenolic hydroxyl bridged lanthanide CPs: Syntheses, characterization, one and two photon luminescence, Dalton Trans., 2013, 42, 2052–2061 RSC.
- J.-Y. Wu, T.-T. Yeh, Y.-S. Wen, J. Twu and K.-L. Lu, Unusual Robust Luminescent Porous Frameworks Self-Assembled from Lanthanide Ions and 2,2′-Bipyridine-4,4′-dicarboxylate, Cryst. Growth Des., 2006, 6, 467–473 CAS.
- B. Chen, L. Wang, Y. Xiao, F. R. Fronczek, M. Xue, Y. Cui and G. Qian, A Luminescent Metal–Organic Framework with Lewis Basic Pyridyl Sites for the Sensing of Metal Ions, Angew. Chem., Int. Ed., 2009, 48, 500–503 CrossRef CAS PubMed.
- C. Li, J. Liu, S. Alonso, F. Li and Y. Zhang, Upconversion nanoparticles for sensitive and in-depth detection of Cu2+ ions, Nanoscale, 2012, 4, 6065–6071 RSC.
- R. An, D. Zhang, Y. Chen and Y.-Z. Cui, A “turn-on” fluorescent and colorimetric sensor for selective detection of Cu2+ in aqueous media and living cells, Sens. Actuators, B, 2016, 222, 48–54 CrossRef CAS.
- W. T. Xu, Y. F. Zhou, D. C. Huang, M. Y. Su, K. Wang, M. Xiang and M. C. Hong, Luminescent sensing profiles based on anion-responsive lanthanide(III) quinolinecarboxylate materials: solid-state structures, photophysical properties, and anionic species recognition, J. Mater. Chem. C, 2015, 3, 2003–2015 RSC.
- T. Liu, A. Nonat, M. Beyler, M. Regueiro-Figueroa, K. Nchimi Nono, O. Jeannin, F. Camerel, F. Debaene, S. Cianférani-Sanglier, R. Tripier, C. Platas-Iglesias and L. J. Charbonnière, Supramolecular Luminescent Lanthanide Dimers for Fluoride Sequestering and Sensing, Angew. Chem., Int. Ed., 2014, 53, 7259–7263 CrossRef CAS PubMed.
- E. Arunkumar, A. Ajayaghosh and J. Daub, Selective Calcium Ion Sensing with a Bichromophoric Squaraine Foldamer, J. Am. Chem. Soc., 2005, 127, 3156–3164 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Coordination environment of lanthanide ions; luminescence spectra of 3 after adding different metal ions; UV-vis spectra of 3; decay time of 3 (0.0001 M) in the presence and absence of Cu2+; infrared spectra of 3 in solid state and in acetonitrile solution; selected bond lengths of complexes 1–3. CCDC 1022845–1022847 for 1–3. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6pp00059b |
| This journal is © The Royal Society of Chemistry and Owner Societies 2016 |



