Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A quantum chemical study of HOCl-induced transformations of carbamazepine†
Tana
Tandarić
 a,
Valerije
Vrček
a,
Valerije
Vrček
 *b and
Davor
Šakić
*b and
Davor
Šakić
 *b
*b
aRuđer Bošković Institute, Bijenička cesta 54, 10000 Zagreb, Croatia
bFaculty of Pharmacy and Biochemistry, University of Zagreb, Ante Kovačića 1, 10000 Zagreb, Croatia. E-mail: dsakic@pharma.hr; vvrcek@pharma.hr
First published on 28th October 2016
Abstract
The antiepileptic drug carbamazepine (CBZ) is one of the most persistent pharmaceuticals in the environment. Its chemical fate is influenced by the type of wastewater treatment. This study sets out to determine the degradation mechanism and products in the reaction between CBZ and hypochlorous acid (HOCl), which is the main chlorinating species in water. In the search for the most feasible pathways of HOCl-induced transformations of CBZ, a quantum chemical approach was employed. Chlorination and epoxidation of CBZ are two initial, competitive processes that result in two key intermediates: N-chloramide and 10,11-epoxide. The calculated free energy barriers (ΔG‡293) for these reactions are 105.7 and 95.7 kJ mol−1 resp., which is in agreement with the experimental energy barrier of 98.2 kJ mol−1. All transformation products detected in chlorination experiments were located by computational models, and the reaction mechanism underlying their formation was described in detail. Different computational methods (density functional and ab initio theory) were applied, and the double hybrid B2-PLYPD functional was found to be superior in terms of efficiency and accuracy. Of special interest are oxoiminostilbene and formylacridine, which are the final products in the degradation cascade. Their exceptional thermodynamic stability, as predicted by quantum chemical methods, suggests that these structures should be considered as recalcitrants in chlorinated waters. Fruitful interplay between computational models and experimental data proves that the quantum chemical approach can be used as a predictive tool in environmental degradation studies.
Introduction
Carbamazepine (CBZ) is a pharmaceutical compound that is commonly used as an anticonvulsant and mood stabilizing drug.1CBZ is one of the most frequently detected pharmaceuticals in the aquatic environment.2,3 It is present in surface waters and treated wastewaters at concentrations of up to 6 μg L−1.4–7 Although there are only preliminary data indicating that CBZ has a negative impact on the environment,8–10 its recalcitrance is worrisome especially as its usage has seen an upward trend in recent years.11–13CBZ is persistent in the environment and is negligibly degraded via biotic mechanisms.14–18 Beyond the biological treatments, a variety of other processes have been probed to eliminate CBZ from the environment. Physicochemical processes such as coagulation–flocculation and flotation did not give better results,19,20 but advanced oxidation processes and different photolytic processes resulted in higher percentages of CBZ degradation.21–25 However, the main drawback is the formation of a wide range of undesirable and toxic by-products.26,27
As chlorination remains to be a primary method for wastewater effluent disinfection, it is imperative that further studies be performed to examine the potential reactions between CBZ and free chlorine. The performance of chlorination treatment is somewhat controversial, with earlier studies describing the chlorination as an inefficient procedure for CBZ elimination,28,29 whereas recent results indicate that the reaction of CBZ and chlorinating agents is a feasible process.30–32
In this work we focus our investigation on the transformation reactions of CBZ induced exclusively with hypochlorous acid (HOCl), which is the main chlorinating agent in a neutral or slightly acidic aqueous environment (pH = 6–7). Quantum chemical models (density functional and ab initio theory) have been extensively used to identify major intermediates and reaction products. To assess the performance of selected computational models, all relevant structures located by the theory were compared with the products detected in chlorination experiments. Two recent experimental studies suggested the formation of 8 different products in the reaction between CBZ and HOCl (Chart 1).30,32 However, the final structures and reaction mechanisms proposed in these two reports do not match, although experiments are carried out under similar reaction conditions. Here we show that the use of quantum-chemical models is suitable to explain and interpret the discrepancy between the two sets of experimental data. The use of quantum chemical methods in solving the issues of ecological concern appears only rarely in the literature.33–36 We propose that an approach which combines computational techniques with available experimental data has to be employed more often when addressing environmental problems, e.g. to identify the major pathways of degradation, and to assist in experimental assignments of transformation products.
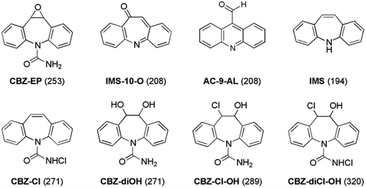 | ||
| Chart 1 Proposed structures of products, formed in the reaction between CBZ and HOCl, assigned to the respective m/z signals (in parentheses) from experimental mass spectra (ref. 30 and 32). | ||
Computational details
Quantum chemical calculations were performed using the Gaussian09 suite of programs.37 All structures were fully optimized with the B3LYP functional.38,39 The standard split valence and polarized 6-31G(d) basis set was used for geometry optimizations and frequency calculations. All energies are reported at 293.15 K in order to compare the calculated and experimental results. Thermal corrections to Gibbs free energies have been calculated at the same level using the rigid rotor/harmonic oscillator model. Improved energetics have been calculated using the double-hybrid methods B2K-PLYP40 and B2-PLYPD41 in combination with the 6-311+G(3df,2p) basis set. The former DFT method shows the best overall performance for calculating barrier heights for water-catalyzed proton-transfer reactions,42 whereas the latter has “high accuracy and extended applicability”.43 Both DFT models accurately reproduce the results obtained by high-level reference procedures (e.g. G3B3),31,44 with B2-PLYPD values being discussed throughout this text. In addition, single point energies have been calculated using second-order Møller–Plesset perturbation theory (MP2)45 with 6-311+G(3df,2p) and G3MP2Large basis sets. Energies calculated at the B2-PLYPD level agree well with the values obtained at the MP2 level of theory.Analytical vibrational analyses at the B3LYP level were performed to characterize each stationary point as a minimum (NImag = 0) or first-order saddle point (NImag = 1). Intrinsic reaction coordinate (IRC) calculations were performed to identify the minima connected through the transition state.46
Gibbs energies of solvation were determined using the CPCM continuum solvation model at the B3LYP/6-31G(d) level, with the UFF atomic radii and electrostatic scaling factor (alpha value) set to 1.1 for all atoms (default values in Gaussian09).47 The solvent relative permittivity of ε = 78.4 (water) was used. To correctly describe chemical systems in water the inclusion of bulk and specific solvent effects is mandatory. We have found that the addition of explicit water molecules substantially lowers the calculated free energy barriers for all the processes investigated. The number of explicit water molecules is varied in order to identify the most stable structure.31 In this work three explicit water molecules were included in all calculations throughout the text. For clarity, explicit water molecules are not included in Schemes 1–3.
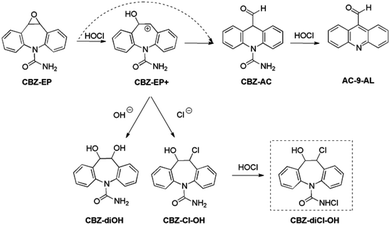 | ||
| Scheme 3 Rearrangement of CBZ-EP to chlorohydrin, dihydroxy, and acridine derivatives mediated by HOCl. Dashed arrow describes the process in which no carbocationic intermediate CBZ-EP+ exists. | ||
The most stable forms of water-complexed species are located by placing water molecules in a variety of locations to sample the different arrays of interaction networks available between the reactants and water. Initial configurations were created using a locally modified version of the stochastic search method.48,49 According to Pliego,50 the reactant and the explicit water molecules form a rigid cluster that constitutes a distinct chemical species. Therefore, the relative energy of reactants complexed with an optimal number of water molecules is set to zero. All other structures, intermediates and products, reported throughout the text also include extra water molecules.
In order to account for the entropic effect of the presence of solvent molecules around a solute, the cell model presented by Ardura et al. was used.51 This model is proposed in order to explicitly evaluate the effect of the loss of translation degrees of freedom in solution on the Gibbs activation energy in bimolecular (or higher order of molecularity) reaction.31
Results and discussion
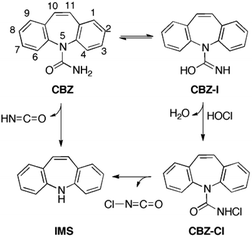
According to LC-MS analysis, four structures were detected and proposed as products of the reaction between CBZ and HOCl (Chart 1): carbamazepine 10,11-epoxide (CBZ-EP, m/z = 253), acridine-9-carboxaldehyde (AC-9-AL, m/z = 208), 10-oxoiminostilbene (IMS-10-O, m/z = 208), and iminostilbene (IMS, m/z = 194).32 The two latter signals were also observed by Soufan et al. together with three other peaks (Chart 1) corresponding to carbamazepine 10,11-chlorohydrin (CBZ-Cl-OH, m/z = 289), carbamazepine 10,11-diol (CBZ-diOH, m/z = 271), and carbamazepine N-chloramide (CBZ-Cl, m/z = 271).30 In addition, one signal having 320 m/z was termed “unknown”.In this work, our goal is to verify if the calculated potential energy landscape for the reaction between CBZ and HOCl involves the same set of structures as predicted by the two chlorination experiments. The computational search for energetically viable transformations of CBZ in chlorinated water (HOCl-induced reactions) revealed the two reaction pathways as the most feasible: chlorination and epoxidation of CBZ. In the first reaction pathway the N-chloramide CBZ-Cl is formed, whereas the second parallel reaction results in the 10,11-epoxide CBZ-EP. Once formed, both CBZ-Cl and CBZ-EP can undergo rearrangements that result in a series of additional transformation products.
In the following sections, we will discuss the mechanism of the initial reactions (chlorination and epoxidation of CBZ) responsible for carbamazepine loss, and then we will explain the formation of additional transformation products observed in the chlorination/oxidation experiments.
N-Chlorination of CBZ
Contrary to older reports,28,29 it has been found that CBZ can be chlorinated with HOCl.32 This is in agreement with a recent study by Soufan et al.,30 and also with our high-level computational results.31 In our detailed description of the reaction mechanism, the N-chloramide product CBZ-Cl is formed in a two-step manner (Scheme 1).31 In the first step, carbamazepine undergoes tautomerization in which the iminol intermediate CBZ-I is formed. The less stable (Table 1) but more reactive iminol reacts with HOCl in the second step CBZ-I → CBZ-Cl. The latter reaction is the rate-determining step and the calculated Gibbs free energy of activation (see below) is within the barrier limits set by experimental measurements.| Entry | B3LYPc | B2K-PLYPd | B2-PLYPDe | MP2f | MP2g |
|---|---|---|---|---|---|
| a Gibbs free energy of reactants (CBZ complexed with two water molecules and HOCl complexed with one water molecule) set to zero; intermediates and products are complexed with two water molecules. b CPCM(UFF, α = 1.1)//B3LYP/6-31G(d) level (ε = 78.4). c B3LYP/6-31G(d). d B2K-PLYP/6-311+G(3df,2p)//B3LYP/6-31G(d). e B2PLYP-D/6-311+G(3df,2p). f MP2/6-311+G(3df,2p)//B3LYP/6-31G(d). g MP2/G3MP2Large//B3LYP/6-31G(d). | |||||
| CBZ + HOCl(H2O) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| CBZ-I + HOCl(H2O) | 62.6 | 60.2 | 58.9 | 56.9 | 56.2 |
| CBZ-Cl + (H2O)2 | −33.0 | −44.0 | −48.5 | −67.8 | −64.0 |
| CBZ-EP + HCl(H2O) | −93.7 | −94.4 | −93.3 | −114.0 | −108.0 |
| IMS HNCO + HOCl(H2O) | 31.9 | 33.7 | 26.4 | 41.5 | 39.8 |
| IMS + ClNCO + (H2O)2 | 4.7 | −3.7 | −6.6 | −2.2 | −2.3 |
| CBZ-TS-I + HOCl(H2O) | 62.2 | 70.3 | 64.8 | 64.4 | 62.2 |
| CBZ-TS-Cl | 107.4 | 115.6 | 105.7 | 99.6 | 100.0 |
| CBZ-TS-EP | 88.9 | 125.9 | 95.7 | 95.5 | 96.3 |
| CBZ-TS-IMS + HOCl(H2O) | 147.8 | 165.8 | 127.8 | 122.2 | 122.0 |
| CBZ-Cl-TS-IMS + (H2O)2 | 79.2 | 32.2 | −1.6 | −1.1 | 0.8 |
It has been estimated recently that the rate constant for an elementary reaction between CBZ and HOCl at 20 (±2) °C is <1.0 × 10−4 M−1 s−1, which corresponds to the Gibbs free energy of activation slightly higher than 94 kJ mol−1.30 In an earlier study,32 the experimental Gibbs free energy of activation (ΔG‡293) for the reaction between CBZ and HOCl is 98.2 kJ mol−1, which is in agreement with the estimation of Soufan et al. (ΔG‡293 > 94 kJ mol−1).30
A similar free energy barrier has been obtained by quantum-chemical methods (Table 1). The calculated Gibbs free energy of activation for chlorination of CBZ ranges from 99.6 kJ mol−1 (MP2 level) to 115.6 kJ mol−1 (B2K-PLYP level), which proves that selected theoretical models can successfully reproduce the experimental results. Gibbs free energies of activation, determined from the difference in energy between the transition state structure CBZ-TS-Cl and reactants (Table 1), govern the reaction rate according to the transition state theory.52 The structure CBZ-TS-Cl in which three water molecules assist the chlorination of the iminol form CBZ-I is presented in Fig. 1. This structure is essential to explain the experimental observation that the N-chloramide CBZ-Cl is one of the major transformation intermediates of carbamazepine.30 It is known that chloramides are reactive species,53,54 and can undergo fast rearrangements. Their detection, therefore, depends on the reaction conditions employed. In the experiment reported by Soufan et al. the N-chloramide CBZ-Cl corresponds to 271 m/z in the mass spectrum.30
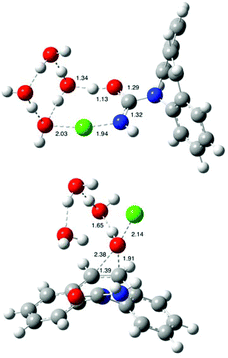 | ||
| Fig. 1 B3LYP/6-31G(d) optimized structures of transition states for N-chlorination (CBZ-TS-Cl) and epoxidation (CBZ-TS-EP) of carbamazepine by HOCl. Bond distances are in angstroms. | ||
10,11-Epoxidation of CBZ
Among 30 metabolites of CBZ isolated in biological systems, carbamazepine 10,11-epoxide (CBZ-EP) is the most important from a clinical point of view. In addition, this metabolite is a transformation product of CBZ frequently detected in the aqueous and soil environment,55 as well in experiments performed under different reaction conditions (aerobic degradation in soil or a bioreactor, UV-radiation, UV/H2O2 treatment, oxidation with Cl2O or Cl2…).24,30,56 Therefore, various reactants can mediate the epoxidation of CBZ, including HOCl, which is predominately used in water treatment procedures.We set out to investigate computationally the epoxidation of CBZ, which is a process competitive to the N-chlorination of CBZ (see above) in chlorinated water. The mechanism of carbamazepine epoxidation by HOCl follows a similar reaction mechanism reported earlier for the parent system, i.e. ethylene.57 Oxygen is transferred onto the C10–C11 double bond of CBZ (atom numbering in Scheme 1) via a transition state structure CBZ-TS-EP (Fig. 1), in which the three atoms (C10, C11, and O) form vertices of a scalene triangle (C10–C11: 1.39 Å, C10–O: 2.38 Å and C11–O: 1.91 Å).
This process is assisted by three explicit water molecules which facilitate proton transfer between O and Cl atoms in HOCl. Oxygen is transferred onto the C10–C11 double bond of CBZ (atom numbering in Scheme 1) via a transition state structure CBZ-TS-EP (Fig. 1), in which the three atoms (C10, C11, and O) form vertices of a scalene triangle (C10–C11: 1.39 Å, C10–O: 2.38 Å and C11–O: 1.91 Å). The calculated free energy barrier for CBZ epoxidation is 95.7 kJ mol−1 (Table 1), which is very similar to the corresponding barrier for ethylene epoxidation (ΔG‡ = 94.6 kJ mol−1) (see the ESI†). To compare the epoxidation power of HOCl, four typical epoxidizing agents have been selected: peroxynitrous acid, performic acid, oxaziridine, and peroxide.58 In the reaction between CBZ and each of the oxidants the product CBZ-EP has been obtained.
It comes out that HOCl is less effective than peroxynitrous acid, but comparable to performic acid, and more effective than oxaziridine and peroxide (for details, see Table S4 in the ESI†). Therefore, the computational results on the epoxidation reactivity of HOCl support experimental observation that epoxidation of CBZ (ΔG‡293 = 95.7 kJ mol−1) occurs competitively with the N-chlorination process (ΔG‡293 = 105.7 kJ mol−1) in chlorinated water. The latter process is somewhat slower due to a higher free energy barrier, and is thermodynamically less feasible. Chlorination products (CBZ-Cl and H2O) have been calculated to be 48.5 kJ mol−1 more stable than reactants (CBZ and HOCl), whereas epoxidation products (CBZ-EP and HCl) are 93.3 kJ mol−1 more stable than the starting reactants (Table 1).
In conclusion, the two parallel reactions, epoxidation and N-chlorination, contribute the most to the HOCl-induced degradation of carbamazepine. The calculated free energy barriers (95.7 and 105.7 kJ mol−1, resp.) for these reaction pathways are in agreement with the experimental value of 98.2 kJ mol−1. The similar barrier heights (for example, ΔΔG‡293 at MP2 levels is only 4 kJ mol−1) suggest that the rates for the formation of the respective products (CBZ-EP and CBZ-Cl) are comparable. Their structures have been proposed and the respective peaks at m/z 253 and 271, resp., have been detected in the MS spectra.
Reaction cascade from N-chloramide CBZ-Cl
In this study we set out to rationalize the two different mechanisms underlying the formation of iminostilbene from (i) carbamazepine or (ii) N-chlorinated carbamazepine. In the first reaction, iminostilbene could have resulted from the cleavage of the HNCO fragment (deamidation). In the second reaction, the elimination of the Cl–NCO fragment from CBZ is a HOCl-mediated process. The first reaction could take place under metabolic conditions or as a by-product of the ion source at high temperatures, whereas the second process could undergo in the chlorinated aqueous environment. Several possible pathways for the elimination of the isocyanate fragment from CBZ and CBZ-Cl have been considered (see the ESI†), but only the most plausible reaction channels are presented in each case (Table 1 and Scheme 1).
The calculated free energy barrier for elimination of the isocyanate moiety from CBZ is 205.9 kJ mol−1. If two explicit water molecules are included in the calculation, the free energy barrier is lowered to 127.8 kJ mol−1 (Table 1). It is still a high barrier to overcome, suggesting that either strong (bio)catalysis is involved or elevated temperature is needed for reaction CBZ → IMS to occur (e.g. in the GC liner). The transition state structure CBZ-TS-IMS for this process has one imaginary frequency (979i cm−1) which corresponds to the proton transfer from the NH2 to N5 atom simultaneously with N5–C bond cleavage (Fig. S1 in the ESI†). The analogous mechanism has been established for water-assisted decomposition of urea,62,63 which is the parent compound of carbamazepine.
In the case of N-chlorinated carbamazepine CBZ-Cl, the calculated free energy barrier for elimination of N-chloro-isocyanate is only 46.9 kJ mol−1 (energy difference between the corresponding transition states CBZ-Cl-TS-IMS and CBZ-Cl in Table 1), which is 81 kJ mol−1 lower than the barrier for the corresponding process (isocyanate elimination) in CBZ. This suggests that the formation of iminostilbene from carbamazepine is kinetically strongly favored if carbamazepine exists in its N-chlorinated form CBZ-Cl. The evidence for the intermediacy of CBZ-Cl in the degradation of carbamazepine has been provided earlier.30
To summarize this part, the occurrence of IMS is not limited only to metabolic degradation or high-temperature induced side reactions, but is also related to HOCl-mediated degradation of CBZ in the aqueous environment. During water treatment the chlorination of CBZ gives rise to N-chloramide CBZ-Cl, which may easily undergo ClNCO cleavage forming IMS.
| Entry | B3LYPc | B2K-PLYPd | B2-PLYPDe | MP2f | MP2g |
|---|---|---|---|---|---|
| a Gibbs free energy of reactants (IMS complexed with two water molecules and HOCl complexed with one water molecule) set to zero; intermediates and products are complexed with two water molecules. b CPCM(UFF, α = 1.1)//B3LYP/6-31G(d) model (ε = 78.4). c B3LYP/6-31G(d). d B2K-PLYP/6-311+G(3df,2p)//B3LYP/6-31G(d). e B2PLYP-D/6-311+G(3df,2p). f MP2/6-311+G(3df,2p)//B3LYP/6-31G(d). g MP2/G3MP2Large//B3LYP/6-31G(d). h Relative to IMS + 2(HOCl(H2O)). | |||||
| IMS + HOCl(H2O) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| IMS-10-Cl + (H2O)2 | −82.1 | −143.3 | −134.8 | −161.2 | −160.0 |
| IMS-10-OH + HCl(H2O) | −57.2 | −48.2 | −97.6 | −116.5 | −105.4 |
| IMS-TS-10-Cl | 69.5 | 72.2 | 41.8 | 48.8 | 50.2 |
| IMS-TS-10-OH | 80.9 | 90.1 | 84.7 | 97.9 | 92.8 |
| IMS-10-OH-TS-O | −5.3 | −22.9 | −43.9 | −40.1 | −49.3 |
| IMS-10-O + HCl(H2O)h | −336.4 | −371.7 | −353.3 | −360.5 | −354.8 |
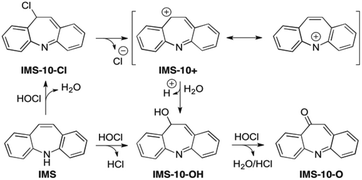
For comparison, the calculated barriers for epoxidations at the C10–C11 and C2–C3 positions (Scheme S2 in the ESI†) are more than 50 kJ mol−1 higher in energy, and therefore less favorable than the two electrophilic reactions at the C-10 position (IMS → IMS-10-Cl and/or IMS-10-OH).
The transient species IMS-10-OH was detected earlier by Li et al.55 They recorded a signal of m/z 210 [M + H] and assigned it to IMS-10-OH. On the contrary, the existence of the chlorinated intermediate IMS-10-Cl has never been confirmed. It is kinetically very unstable, considering that the ionization of the C10–Cl bond occurs rapidly through the aromatic carbocation intermediate IMS-10+ (presented with two resonance structures in Scheme 2), which in the aqueous environment undergoes fast addition of water to give IMS-10-OH.64 The 10-hydroxyiminostilbene is by 97.6 kJ mol−1 (Table 2) more stable than the starting IMS, but represents a short-lived intermediate that can easily be oxidized by HOCl to 10-oxoiminostilbene (IMS-10-O). It is known that HOCl is an effective oxidant for conversion of a secondary alcohol to the corresponding ketone.65 The oxidation step IMS-10-OH → IMS-10-O is both kinetically and thermodynamically driven: the calculated free energy barrier is 53.7 kJ mol−1 (the energy difference between IMS-10-OH and the transition state IMS-10-OH-TS-O, Table 2), and the final product IMS-10-O is 353.3 kJ mol−1 more stable than IMS. This large change in energy is due to the resonance energy, since IMS-10-O is an aromatic structure, unlike its parent compound IMS. Similar oxidation has been reported in the course of the transformation of 2-hydroxyiminostilbene to 2-oxoiminostilbene. However, this process is relevant only for hepatic metabolism of IMS mediated by cytochrome P450.66,67
The experimental (MS signal at m/z = 208) and our computational results suggest that the formation of 10-oxoiminostilbene (IMS-10-O) is an important process for the chemical fate of iminostilbene in chlorinated water.
Reaction cascade from epoxide CBZ-EP
The opening of the three-membered ring in epoxide CBZ-EP (Scheme 3) has the lowest free energy barrier (ΔG‡293 = 128.7 kJ mol−1, at the B3LYP level, Table 3). This process is mediated by HOCl (or H2O), and results in the formation of the carbocation intermediate CBZ-EP+.
| B3LYPc | B2K-PLYPd | B2-PLYPDe | MP2f | MP2g | |
|---|---|---|---|---|---|
| a Gibbs free energy of reactants (CBZ-EP complexed with two water molecules and HOCl complexed with one water molecule) set to zero; intermediates and products are complexed with two water molecules. b CPCM(UFF,α = 1.1)//B3LYP/6-31G(d) model (ε = 78.4). c B3LYP/6-31G(d). d B2K-PLYP/6-311+G(3df,2p)//B3LYP/6-31G(d). e B2PLYP-D/6-311+G(3df,2p). f MP2/6-311+G(3df,2p)//B3LYP/6-31G(d). g MP2/G3MP2Large//B3LYP/6-31G(d). | |||||
| CBZ-EP + HOCl(H2O) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| CBZ-EP+ + OCl−(H2O)2 | 44.7 | 59.4 | 58.9 | 62.0 | 62.2 |
| CBZ-AC + HOCl(H2O) | −81.2 | −91.8 | −90.5 | −89.5 | −89.2 |
| CBZ-EP-TS-EP+ | 128.7 | 137.6 | 134.9 | 129.9 | 131.4 |
| CBZ-EP+-TS-AC | 65.8 | 71.3 | 49.7 | 55.0 | 51.2 |
| CBZ-EP-TS-AC | 136.8 | 150.4 | 158.2 | 172.6 | 171.2 |
| CBZ-diOH | −26.7 | −35.2 | −32.3 | −41.2 | −39.3 |
| CBZ-Cl-OH | −27.6 | −48.7 | −46.1 | −54.8 | −52.6 |
| CBZ-Cl-OH-I | 35.1 | 20.7 | 22.8 | 12.3 | 13.8 |
| CBZ-diCl-OH | −38.8 | −70.3 | −62.9 | −91.8 | −85.0 |
| CBZ-EP-TS-diOH | 171.4 | 195.3 | 192.7 | 181.3 | 182.0 |
| CBZ-EP+-TS-diOH | 142.7 | 127.3 | 156.3 | 149.3 | 153.1 |
| CBZ-EP-TS-Cl-OH | 64.5 | 82.3 | 71.4 | 106.1 | 102.4 |
| CBZ-EP+-TS-Cl-OH | 51.4 | 62.3 | 53.6 | 80.2 | 79.9 |
| CBZ-Cl-OH-TS-Cl-OH-I | 50.9 | 47.1 | 45.4 | 36.0 | 36.0 |
| CBZ-Cl-OH-I-TS-diCl-OH | 90.8 | 66.7 | 68.1 | 52.8 | 56.7 |
| AC-9-AL + HCl(H2O) | −307.1 | −352.9 | -339.8 | −344.0 | −339.4 |
| CBZ-AC-TS-AC-9-AL | 32.7 | 15.1 | 38.5 | 43.1 | 42.3 |
The formed carbocation can easily react with the hydroxide or chloride ion producing 10,11-diol CBZ-diOH or 10,11-chlorohydrin CBZ-Cl-OH, respectively. Both structures were detected in mass spectrometry as respective signals at m/z = 271 and m/z = 289. The latter intermediate can undergo additional N-chlorination resulting in the proposed product CBZ-diCl-OH (structure in the dashed rectangle in Scheme 3) which can be assigned to the unknown compound detected in the MS spectrum (peak at m/z = 320).
Interestingly, no ring contraction process in carbamazepine metabolites has been observed earlier in the chlorination experiment.30 This is probably due to the different reaction conditions employed (e.g. use of carbonate instead of phosphate buffer, change in acidic medium, or variation in the level of chloride ions in the free chlorine stock solution).
On the other hand, CBZ-AC has been proposed as a tentative intermediate in photodegradation,23,68 and advanced oxidation processes.69 We have found that CBZ-AC can undergo oxidation, mediated by HOCl, to give 9-formylacridine (AC-9-AL). This process is thermodynamically driven and can be explained by the tendency of CBZ-AC to yield an aromatic structure. The final product AC-9-AL has been calculated as the most stable intermediate on the corresponding potential energy surface. It is 249.3 and 339.8 kJ mol−1 more stable than CBZ-AC and starting reactants (CBZ and HOCl), respectively. The exceptional stability of AC-9-AL suggests that this structure should be considered as a recalcitrant metabolite formed in chlorinated water.
As IMS-10-O and AC-9-AL are both aromatic, and have the same mass, it is hard to differentiate between those two products. According to our calculations, AC-9-AL has been calculated almost 100 kJ mol−1 more stable than the former. It is clear that the acridine moiety in AC-9-AL provides much better stabilization than the tricyclic system with the azepine ring in IMS-10-O. The MS peak corresponding to the mass of either IMS-10-O or AC-9-AL has been detected earlier in the chlorination experiment,30 but neither 10-oxoiminostilbene nor acridine-9-carboxaldehyde has been proposed as a possible carbamazepine degradation intermediate. On the contrary, in a number of other analytical or biochemical studies, acridine-9-carboxaldehyde has been discussed as an important product of carbamazepine transformations in both aqueous58,63 and physiological environments.62,70
Conclusions
Quantum chemical investigation of HOCl-mediated transformation of CBZ was accomplished. Five different theoretical levels (DFT and MP2 methods) were tested, and B2-PLYPD was selected as superior in terms of better precision and lower computational cost. The experimental Gibbs free energy of activation for the reaction between HOCl and CBZ (ΔG‡293 = 98.2 kJ mol−1) was successfully reproduced by the B2-PLYPD functional. The two parallel reactions contribute to the overall barrier: C10,C11-epoxidation (ΔG‡293 = 95.7 kJ mol−1) and N-chlorination (ΔG‡293 = 105.7 kJ mol−1) of carbamazepine.These processes give rise to epoxide CBZ-EP and chloramide CBZ-Cl, respectively, which undergo a series of fast rearrangements resulting in an additional set of intermediates. The two chlorination experiments30,32 revealed 8 degradation products, with some structures tentatively assigned or unknown. Full assignment of signals in mass spectra was performed by assistance of computational models. In addition to epoxide CBZ-EP and chloramide CBZ-Cl, the suggested structures include iminostilbene (IMS), carbamazepine 10,11-diol (CBZ-diOH), carbamazepine 10,11-chlorohydrin (CBZ-Cl-OH), and its N-chlorinated derivative (CBZ-diCl-OH), oxoiminostilbene (IMS-10-OH), and formylacridine (AC-9-AL). The two latter are of special importance due to their exceptional thermodynamic stability (326.9 kJ mol−1 and 433.1 kJ mol−1, respectively, more stable than CBZ). Unlike other intermediates, IMS-10-O and AC-9-AL have planar aromatic structures which can be associated with their (bio)recalcitrant properties.
In this work the fruitful interplay between computational models and available experimental data was implemented in assessing the environmental fate of carbamazepine. The measured kinetic parameters for reactions (ΔG‡) and signals observed in mass spectra were used as a guide for calculations of reaction mechanisms underlying the HOCl-induced transformations of carbamazepine.
Acknowledgements
We thank the Computing Centre SRCE of the University of Zagreb for allocating the computer time on the Isabella cluster. We thank Prof. Peter J. Vikesland, Virginia Polytechnic Institute, for a fruitful discussion.Notes and references
- E. J. Fertig and R. H. Mattson, in Epilepsy: A Comprehensive Textbook, ed. J. Engel and T. A. Pedley, Lippincott Williams & Wilkins, Philadelphia, Pa, 2007, p. 1543 Search PubMed.
- R. Loos, G. Locoro, S. Comero, S. Contini, D. Schwesig, F. Werres, P. Balsaa, O. Gans, S. Weiss, L. Blaha, M. Bolchi and B. M. Gawlik, Water Res., 2010, 44, 4115 CrossRef CAS PubMed.
- H. D. Zhou, C. Y. Wu, X. Huang, M. J. Gao, X. H. Wen, H. Tsuno and H. Tanaka, Water Environ. Res., 2010, 82, 2239 CrossRef CAS PubMed.
- S. Kern, K. Fenner, H. P. Singer, R. P. Schwarzenbach and J. Hollender, Environ. Sci. Technol., 2009, 43, 7039 CrossRef CAS PubMed.
- X. S. Miao, J. J. Yang and C. D. Metcalfe, Environ. Sci. Technol., 2005, 39, 7469 CrossRef CAS PubMed.
- S. D. Richardson and T. A. Ternes, Anal. Chem., 2011, 83, 4614 CrossRef CAS PubMed.
- A. Bahlmann, W. Brack, R. J. Schneider and M. Krauss, Water Res., 2014, 57, 104 CrossRef CAS PubMed.
- B. Ferrari, N. Paxéus, R. Lo Giudice, A. Pollio and J. Garric, Ecotoxicol. Environ. Saf., 2003, 55, 359 CrossRef CAS PubMed.
- A. Almeida, V. Calisto, V. I. Esteves, R. J. Schneider, A. M. V. M. Soares, E. Figueira and R. Freitas, Aquat. Toxicol., 2014, 156, 74 CrossRef CAS PubMed.
- R. Andreozzi, R. Marotta, G. Pinto and A. Pollio, Water Res., 2002, 36, 2869 CrossRef CAS PubMed.
- R. Mojtabai and M. Olfson, Arch. Gen. Psychiatry, 2010, 67, 26 CrossRef PubMed.
- J. M. Nicholas, L. Ridsdale, M. P. Richardson, M. Ashworth and M. C. Gulliford, Seizure, 2012, 21, 466 CrossRef PubMed.
- C. J. Landmark, H. Fossmark, P. G. Larsson, E. Rytter and S. I. Johannessen, Epilepsy Res., 2011, 95, 51 CrossRef PubMed.
- M. Bernhard, J. Muller and T. P. Knepper, Water Res., 2006, 40, 3419 CrossRef CAS PubMed.
- Y. Zhang and S. U. Geiben, J. Hazard. Mater., 2010, 176, 1089 CrossRef CAS PubMed.
- M. Clara, B. Strenn and N. Kreuzinger, Water Res., 2004, 38, 947 CrossRef CAS PubMed.
- M. Carballa, F. Omil, T. Ternes and J. M. Lema, Water Res., 2007, 41, 2139 CrossRef CAS PubMed.
- M. D. Celiz, S. Perez, D. Barcelo and D. S. Aga, J. Chromatogr. Sci., 2009, 47, 19 CAS.
- S. Suarez, M. Carballa, F. Omil and J. M. Lema, Rev. Environ. Sci. Biotechnol., 2008, 7, 125 CrossRef CAS.
- M. Carballa, F. Omil, J. M. Lema, M. Llompart, C. Garcıa-Jares, I. Rodrıguez, M. Gomez and T. Ternes, Water Res., 2004, 38, 2918 CrossRef CAS PubMed.
- S. Esplugas, D. M. Bila, L. G. T. Krause and M. Dezotti, J. Hazard. Mater., 2007, 149, 631 CrossRef CAS PubMed.
- T. A. Ternes, M. Meisenheimer, D. McDowell, F. Sacher, H. J. R. Brauch, B. Haist-Gulde, G. Preuss, U. Wilme and N. Zulei-Seibert, Environ. Sci. Technol., 2002, 36, 3855 CrossRef CAS PubMed.
- E. De Laurentiis, S. Chiron, S. Kouras-Hadef, C. Richard, M. Minella, V. Maurino, C. Minero and D. Vione, Environ. Sci. Technol., 2012, 46, 8164 CrossRef CAS PubMed.
- D. Vogna, R. Marotta, R. Andreozzi, A. Napolitano and M. D'Ischia, Chemosphere, 2004, 54, 497 CrossRef CAS PubMed.
- D. C. McDowell, M. M. Huber, M. Wagner, U. von Gunten and T. A. Ternes, Environ. Sci. Technol., 2005, 39, 8014 CrossRef CAS PubMed.
- M. Petrović and D. Barcelo, Trends Anal. Chem., 2007, 26, 486 CrossRef.
- S. Chiron, C. Minero and D. Vione, Environ. Sci. Technol., 2006, 40, 5977 CrossRef CAS PubMed.
- T. Okuda, Y. Kobayashi, R. Nagao, N. Yamashita, H. Tanaka, S. Tanaka, S. Fujii, C. Konishi and I. Houwa, Water Sci. Technol., 2008, 57, 65 CrossRef CAS PubMed.
- J. Gibs, P. E. Stackelberg, E. T. Furlong, M. Meyer, S. D. Zaugg and R. L. Lippincott, Sci. Total Environ., 2007, 373, 240 CrossRef CAS PubMed.
- M. Soufan, M. Deborde, A. Delmont and B. Legube, Water Res., 2013, 47, 5076 CrossRef CAS PubMed.
- D. Šakić, P. Šonjić, T. Tandarić and V. Vrček, J. Phys. Chem. A, 2014, 118, 2367 CrossRef PubMed.
- K. Kotcharaksa, In the Thesis: The Mechanisms, Products, and Kinetic of Carbamazepine-Free Chlorine Reactions, Virginia Polytechnic Institute, 2008. Accessed on September 1, 2016 at: http://theses.lib.vt.edu/theses/available/etd-12232008-141520/ Search PubMed.
- J. Blotevogel, A. N. Mayeno, T. C. Sale and T. Borch, Environ. Sci. Technol., 2011, 45, 2236 CrossRef CAS PubMed.
- W. J. Barr, T. Yi, D. Aga, O. Acevedo and W. F. Harper Jr., Environ. Sci. Technol., 2012, 46, 760 CrossRef CAS PubMed.
- H. Zhang, H. Xie, J. Chen and S. Zhang, Environ. Sci. Technol., 2015, 49, 1552 CrossRef CAS PubMed.
- D. Šakić, F. Achrainer, V. Vrček and H. Zipse, Org. Biomol. Chem., 2013, 11, 4232 Search PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, et al., Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford, CT, 2009 Search PubMed.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter, 1988, 37, 785 CrossRef CAS.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648 CrossRef CAS.
- A. Tarnopolsky, A. Karton, R. Sertchook, D. Vuzman and J. M. L. Martin, J. Phys. Chem. A, 2008, 112, 3 CrossRef CAS PubMed.
- S. Grimme, J. Chem. Phys., 2006, 124, 034108 CrossRef PubMed.
- A. Karton, R. J. O'Reilly and L. Radom, J. Phys. Chem. A, 2012, 116, 4211 CrossRef CAS PubMed.
- T. Schwabe and S. Grimme, Phys. Chem. Chem. Phys., 2007, 9, 3397 RSC.
- D. Šakić, H. Zipse and V. Vrček, Org. Biomol. Chem., 2011, 9, 4336 Search PubMed.
- M. J. Frisch, M. Head-Gordon and J. A. Pople, Chem. Phys. Lett., 1990, 166, 275 CrossRef CAS.
- H. P. Hratchian and H. B. Schlegel, J. Chem. Phys., 2004, 120, 9918 CrossRef CAS PubMed.
- V. Barone and M. Cossi, J. Phys. Chem. A, 1998, 102, 1995 CrossRef CAS; M. Cossi, G. Scalmani, N. Rega and V. Barone, J. Chem. Phys., 2002, 117, 43 CrossRef.
- D. Šakić and V. Vrček, J. Phys. Chem. A, 2012, 116, 1298 CrossRef PubMed.
- V. Vrček, O. Kronja and M. Saunders, J. Chem. Theory Comput., 2007, 3, 1223 CrossRef PubMed.
- J. R. Pliego Jr., Chem. Phys., 2004, 306, 273 CrossRef.
- D. Ardura, R. López and T. L. Sordo, J. Phys. Chem. B, 2005, 109, 23618 CrossRef CAS PubMed.
- H. P. Hratchian and H. B. Schlegel, Finding minima, transition states, and following reaction pathways on ab initio potential energy surfaces, in Theory and Applications of Computational Chemistry: The First Forty years, ed. C. Dykstra, G. Frenking, K. Kim and G. Scuseria, Elsevier, Amsterdam, 2005, p. 195 Search PubMed.
- M. D. Rees, C. L. Hawkins and M. J. Davies, J. Am. Chem. Soc., 2003, 125, 13719 CrossRef CAS PubMed.
- C. L. Hawkins and M. J. Davies, Free Radical Biol. Med., 1998, 24, 1396 CrossRef CAS PubMed.
- J. Li, L. Dodgen, Q. Ye and J. Gan, Environ. Sci. Technol., 2013, 47, 3678 CrossRef CAS PubMed.
- A. Jelic, C. Cruz-Morató, E. Marco-Urrea, M. Sarrà, S. Perez, T. Vicent, M. Petrović and D. Barcelo, Water Res., 2012, 46, 955 CrossRef CAS PubMed.
- M. Srnec, M. Ončak and R. Zahradnik, J. Phys. Chem. A, 2008, 112, 3631 CrossRef CAS PubMed.
- K. N. Houk, J. Liu, N. C. DeMello and K. R. Condroski, J. Am. Chem. Soc., 1997, 119, 10147 CrossRef CAS.
- J. Csetenyi, K. M. Baker, A. Frigerio and P. L. Morselli, J. Pharm. Pharmacol., 1973, 25, 340 CrossRef CAS PubMed.
- H. Breton, M. Cociglio, F. Bressolle, H. Peyriere, J. P. Blayac and D. Hillaire-Buys, J. Chromatogr., B: Biomed. Appl., 2005, 828, 80 CrossRef CAS PubMed.
- T. Kosjek, H. R. Andersen, B. Kompare, A. Ledin and E. Heath, Environ. Sci. Technol., 2009, 43, 6256 CrossRef CAS PubMed.
- A. N. Alexandrova and W. L. Jorgensen, J. Phys. Chem. B, 2007, 111, 720 CrossRef CAS PubMed.
- G. Estiu and K. M. Merz Jr., J. Phys. Chem. B, 2007, 111, 6507 CrossRef CAS PubMed.
- J. P. Richard, T. L. Amyes and M. M. Toteva, Acc. Chem. Res., 2001, 34, 981 CrossRef CAS PubMed.
- R. V. Stevens, K. T. Chapman and H. N. Weller, J. Org. Chem., 1980, 45, 2030 CrossRef CAS.
- R. E. Pearce, J. P. Uetrecht and J. S. Leeder, Drug Metab. Dispos., 2005, 33, 1819 CAS.
- C. Ju and J. P. Uetrecht, J. Pharmacol. Exp. Ther., 1999, 288, 51 CAS.
- P. Calza, C. Medana, E. Padovano, V. Giancotti and C. Baiocchi, Rapid Commun. Mass Spectrom., 2012, 26, 1687 CrossRef CAS PubMed.
- O. S. Keen, S. Baik, K. G. Linden, D. S. Aga and N. G. Love, Environ. Sci. Technol., 2012, 46, 6222 CrossRef CAS PubMed.
- S. M. Furst and J. P. Uetrecht, Biochem. Pharmacol., 1993, 45, 1267 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Coordinates for all the calculated structures and corresponding energies. See DOI: 10.1039/c6ob02166b |
| This journal is © The Royal Society of Chemistry 2016 |