Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Azogabazine; a photochromic antagonist of the GABAA receptor†
Rosemary
Huckvale
a,
Martin
Mortensen
b,
David
Pryde
c,
Trevor G.
Smart
*b and
James R.
Baker
*a
aDepartment of Chemistry, University College London, 20 Gordon Street, London, WC1H 0AJ, UK. E-mail: j.r.baker@ucl.ac.uk
bDepartment of Neuroscience, Physiology & Pharmacology, University College London, Gower Street, London, WC1E 6BT, UK. E-mail: t.smart@ucl.ac.uk
cPfizer Worldwide Medicinal Chemistry, Neuroscience and Pain Research Unit, Portway Building, Granta Park, Great Abington, Cambridgeshire, CB21 6GS, UK
First published on 21st June 2016
Abstract
The design and synthesis of azogabazine is described, which represents a highly potent (IC50 = 23 nM) photoswitchable antagonist of the GABAA receptor. An azologization strategy is adopted, in which a benzyl phenyl ether in a high affinity gabazine analogue is replaced by an azobenzene, with resultant retention of antagonist potency. We show that cycling from blue to UV light, switching between trans and cis isomeric forms, leads to photochemically controlled antagonism of the GABA ion channel.
GABAA receptors are ligand-gated chloride ion channels,1 which are activated by γ-aminobutyric acid (GABA); the major inhibitory neurotransmitter present in the central nervous system.2 GABAA receptors are also modulated by an array of compounds including neurosteroids, benzodiazepines and barbiturates.3 A number of small molecule antagonists are known for the GABAA receptor,4 of which gabazine (Fig. 1, also called SR-95531)4a,5 is one of the most widely used.4e Gabazine is a competitive antagonist (IC50 = 300 nM), and we recently described the development of enhanced potency analogues including Gz-i1 (IC50 = 13 nM).6 Furthermore, by incorporation of a benzophenone, we constructed a photoaffinity labelled version (GZ-B1, IC50 = 153 nM) which upon irradiation could be employed to irreversibly block populations of native neuronal GABAA receptors, facilitating the study of receptor trafficking.7 We envisaged that reversible light mediated control of activity would offer further opportunities to probe this important class of ion channels.
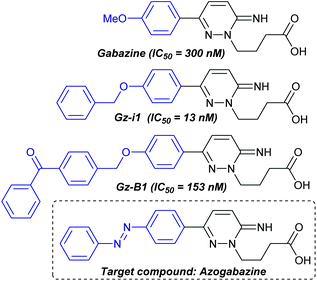 | ||
| Fig. 1 Gabazine, potent analogues and the targeted azogabazine as a photochromic antagonist of the GABAA receptor. | ||
Chemical photoswitches are photochromic compounds which can be activated and deactivated in cycles.8 These can be used to reversibly control the function of a biological system with light.9 By far the most well-known and widely-used photoswitches are azobenzenes, which upon irradiation with UV light form a cis-enriched photostationary state, which converts to the more stable trans isomer upon visible light irradiation or thermal relaxation. Two main strategies have been developed to exploit the use of photoswitches to infer photochemical control over ion channel activity. The first involves covalent attachment of ligands onto the channel, such that the ligand can bind in its active isomeric form, but upon irradiation is expelled from the binding site. This approach is known as photoswitchable tethered ligands (PTL) and essentially serves to provide a constant high local concentration of the ligand.9 The second strategy involves the use of freely diffusible ligands, which are only able to bind effectively in one of the isomeric forms. Such compounds are known as photochromic ligands (PCL), and preclude the requirement for mutagenesis to incorporate reactive handles for covalent attachment.
Examples of PTLs and PCLs for GABAA receptors have been described recently in the literature. Propofol analogues have been designed as photochromic potentiators of GABAA receptors.10 Lin et al. have also described the use of an engineered GABAA receptor to enable the tethering of the agonist muscimol. Rather than the expected agonism they observed antagonism using this PTL, which was also observed using guanidinium analogues of GABA.10c,d
Having previously established that elaboration of the gabazine molecule to incorporate benzyl substituents leads to enhanced binding to the GABAA receptor (i.e. Gz-i1), we envisaged that this strategy could be harnessed for the design of potent antagonists as PCLs. Following the ‘azologization’ approach pioneered by Trauner and co-workers11 we identified the benzyl ether in Gz-i1 (Fig. 1) as being an ideal structural motif to be replaced by an azobenzene photoswitch. Thus we targeted ‘azogabazine’ as a prospective new tool for this important class of ion channels.
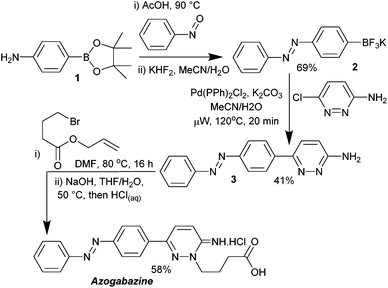
The synthesis of azogabazine was accomplished in just five synthetic steps (Scheme 1). Condensation of aniline 1 with nitrosobenzene was followed by conversion of the boronate ester into the trifluoroborate salt 2. Suzuki reaction with 3-amino-6-chloropyridazine12 afforded the triaryl intermediate 3, which underwent N(2) alkylation and subsequent deallylation to afford azogabazine. The overall yield for the sequence was 16%.
Azogabazine was tested for potency on α1β2γ2 GABAA receptors expressed in HEK293 cells, using whole-cell patch-clamp recording, and proved to be one of the most potent GABA antagonists known (Fig. 2). An IC50 of 23 nM was determined, representing a 13-fold improvement over gabazine. This enhanced potency is similar to that observed with Gz-i1, suggesting that the benzyl phenyl ether and azobenzene represented ideal bioisosteres in this application.
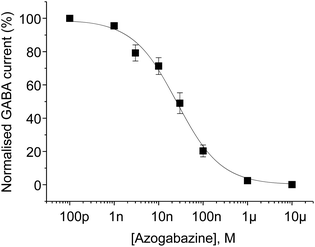 | ||
| Fig. 2 Concentration inhibition curve for azogabazine. Azogabazine was applied in combination with 10 μM GABA. pIC50: 7.646 ± 0.141, n = 6 (IC50: 22.6 nM). | ||
UV/Vis absorbance spectra were taken to confirm the retention of azobenzene absorbance characteristics. The initial spectrum was consistent with the thermodynamically favoured trans-azogabazine, showing a characteristic large absorbance with λmax of 342 nm representing a π → π* transition. After 30 s of irradiation with a hand-held torch containing LEDs of 365 nm a further UV spectrum was taken (Fig. 3). This was now characteristic of a cis azobenzene-containing compound, with an increase in absorbance at 435 nm and a concomitant decrease at lower wavelengths.
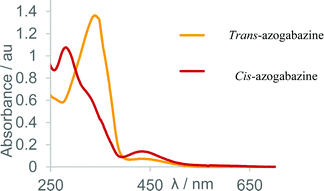 | ||
| Fig. 3 Overlaid UV/Vis absorbance spectra for (i) sample of azogabazine (orange) representing the trans isomer and (ii) sample of UV irradiated azogabazine (red) representing the cis isomer. | ||
1H-NMR studies confirmed this photoswitching behaviour and allowed quantification of the ratios at photostationary states. Irradiation with a UV light (a 365 nm LED) afforded the cis-enriched photostationary state in a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. By contrast irradiation with blue light (a 470 nm LED) afforded predominantly the trans product in a 3
1 ratio. By contrast irradiation with blue light (a 470 nm LED) afforded predominantly the trans product in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. No further changes were seen after 60 s irradiation in each case, showing the interconversions to form the photostationary states to be rapid. Once formed, the cis isomer was found to show slow thermal conversion to the more stable trans isomer, requiring 20 days in the dark to achieve full conversion; thus representing a high bistability system.
1 ratio. No further changes were seen after 60 s irradiation in each case, showing the interconversions to form the photostationary states to be rapid. Once formed, the cis isomer was found to show slow thermal conversion to the more stable trans isomer, requiring 20 days in the dark to achieve full conversion; thus representing a high bistability system.
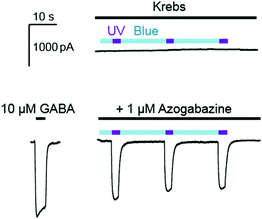
Azogabazine was then constantly perfused onto GABAA receptor expressing HEK cells, along with 10 μM GABA, followed by alternating application of blue (a 470 nm LED) and UV light (a 365 nm LED; Fig. 4). Each cycle of blue light led to reduced currents, confirming that the predominating trans isomer was serving as the most potent antagonist. In contrast, UV light produced an inward current, as the cis azogabazine was generated and no longer served as an effective antagonist. Thus after presumed ejection from the GABA binding sites GABA-mediated activation was taking place.
Conclusions
We have described the design and convenient synthesis of azogabazine, a potent photochromic antagonist of the GABAA receptor. This study represents a powerful demonstration of the ‘azologization’ strategy for the design of photochromic ligands. The identification of a benzyl phenyl ether as an ‘azostere’ guided our strategy for the introduction of the azobenzene motif, and resulted in an antagonist which was 13 times more potent than gabazine itself. Repeated cycling from blue-to-UV light showed robust photo-control of GABAA receptor channel activity making azogabazine a useful new research tool for studying GABAA receptors.Acknowledgements
The authors gratefully acknowledge BBSRC, Pfizer, the EPSRC UK National Mass Spectrometry Facility (NMSF, Swansea), MRC and the Leverhulme Trust for supporting this research.Notes and references
- N. G. Bowery and T. G. Smart, Br. J. Pharmacol., 2006, 147, S109–S119 CrossRef CAS PubMed.
- B. Luscher and C. A. Keller, Pharmacol. Ther., 2004, 102, 195–221 CrossRef CAS PubMed.
- G. A. R. Johnston, Pharmacol. Ther., 1996, 69, 173–198 CrossRef CAS PubMed.
- (a) J. P. Chambon, P. Feltz, M. Heaulme, S. Restle, R. Schlichter, K. Biziere and C. G. Wermuth, Proc. Natl. Acad. Sci. U. S. A., 1985, 82, 1832–1836 CrossRef CAS PubMed; (b) G. Tunnicliff and T. T. Ngo, J. Neurochem., 1982, 39, 998–1000 CrossRef CAS PubMed; (c) W. Zhang, S. Xia, J. J. Ye, Y. Tang, Z. Li, W. P. Zhu and J. G. Cheng, Med. Chem. Res., 2013, 22, 5961–5972 CrossRef CAS; (d) B. H. Gahwiler, R. Maurer and H. J. Wuthrich, Neurosci. Lett., 1984, 45, 311–316 CrossRef CAS PubMed; (e) G. A. R. Johnston, Br. J. Pharmacol., 2013, 169, 328–336 CrossRef CAS PubMed; (f) B. Frølund, L. S. Jensen, S. I. Storustovu, T. B. Stensbøl, B. Ebert, J. Kehler, P. Krogsgaard-Larsen and T. Liljefors, J. Med. Chem., 2007, 50, 1988–1992 CrossRef PubMed; (g) B. Frølund, A. T. Jørgensen, L. Tagmose, T. B. Stensbøl, H. T. Vestergaard, C. Engblom, U. Kristiansen, C. Sanchez, P. Krogsgaard-Larsen and T. Liljefors, J. Med. Chem., 2002, 45, 2454–2468 CrossRef.
- (a) M. Heaulme, J. P. Chambon, R. Leyris, J. C. Molimard, C. G. Wermuth and K. Biziere, Brain Res., 1986, 384, 224–231 CrossRef CAS PubMed; (b) C. G. Wermuth, J. J. Bourguignon, G. Schlewer, J. P. Gies, A. Schoenfelder, A. Melikian, M. J. Bouchet, D. Chantreux, J. C. Molimard, M. Heaulme, J. P. Chambon and K. Biziere, J. Med. Chem., 1987, 30, 239–249 CrossRef CAS PubMed.
- F. Iqbal, R. Ellwood, M. Mortensen, T. G. Smart and J. R. Baker, Bioorg. Med. Chem. Lett., 2011, 21, 4252–4254 CrossRef CAS PubMed.
- M. Mortensen, F. Iqbal, A. P. Pandurangan, S. Hannan, R. Huckvale, M. Topf, J. R. Baker and T. G. Smart, Nat. Commun., 2014, 5, 4454 CAS.
- J. Broichhagen, J. A. Frank and D. Trauner, Acc. Chem. Res., 2015, 48, 1947–1960 CrossRef CAS PubMed.
- W. Szymanski, J. M. Beierle, H. A. V. Kistemaker, W. A. Velema and B. L. Feringa, Chem. Rev., 2013, 113, 6114–6178 CrossRef CAS PubMed.
- (a) M. Stein, S. I. Middendorp, V. Carta, E. Pejo, D. E. Raines, S. A. Forman, E. Sigel and D. Trauner, Angew. Chem., Int. Ed., 2012, 51, 10500–10504 CrossRef CAS PubMed; (b) L. Yue, M. Pawlowski, S. S. Dellal, A. Xie, F. Feng, T. S. Otis, K. S. Bruzik, H. H. Qian and D. R. Pepperberg, Nat. Commun., 2012, 3, 12 Search PubMed; (c) W.-C. Lin, C. M. Davenport, A. Mourot, D. Vytla, C. M. Smith, K. A. Medeiros, J. J. Chambers and R. H. Kramer, ACS Chem. Biol., 2014, 9, 1414–1419 CrossRef CAS PubMed; (d) W.-C. Lin, M.-C. Tsai, C. M. Davenport, C. M. Smith, J. Veit, N. M. Wilson, H. Adesnik and R. H. Kramer, Neuron, 2015, 88, 879–891 CrossRef CAS PubMed.
- M. Schoenberger, A. Damijonaitis, Z. Zhang, D. Nagel and D. Trauner, ACS Chem. Neurosci., 2014, 5, 514–518 CrossRef CAS PubMed.
- B. U. W. Maes, G. L. F. Lemiere, R. Dommisse, K. Augustyns and A. Haemers, Tetrahedron, 2000, 56, 1777–1781 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ob01101b |
| This journal is © The Royal Society of Chemistry 2016 |