Direct aqueous synthesis of cyanomethyl thioglycosides from reducing sugars; ready access to reagents for protein glycosylation†
Abstract
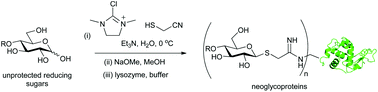
Unprotected carbohydrates can be directly converted into cyanomethyl thioglycosides in a completely stereoselective manner by reaction with 2-chloro-1,3-dimethylimidazolinium chloride (DMC) and mercaptoacetonitrile in aqueous solution in the presence of triethylamine. Reaction with methoxide provides 2-imino-2-methoxyethyl (IME) thioglycosides, which may be used directly for the glycosylation of surface protein lysine residues.

 Please wait while we load your content...
Please wait while we load your content...