 Open Access Article
Open Access ArticleSynthesis of sp3-rich scaffolds for molecular libraries through complexity-generating cascade reactions†
T.
Flagstad
ab,
G.
Min
ab,
K.
Bonnet
c,
R.
Morgentin
c,
D.
Roche
c,
M. H.
Clausen
*ab and
T. E.
Nielsen
*ad
aDepartment of Chemistry, Technical University of Denmark, DK-2800 Kgs. Lyngby, Denmark. E-mail: ten@kemi.dtu.dk; mhc@kemi.dtu.dk
bCenter for Nanomedicine and Theranostics, Technical University of Denmark, DK-2800 Kgs. Lyngby, Denmark
cEDELRIS, 115 Avenue, Lacassagne, F-69003, France
dSingapore Centre on Environmental Life Sciences Engineering, Nanyang Technological University, Singapore 637551, Singapore
First published on 10th May 2016
Abstract
An efficient strategy for the synthesis of complex small molecules from simple building blocks is presented. Key steps of the strategy include tandem Petasis and Diels–Alder reactions, and divergent complexity-generating cyclization cascades from a key dialdehyde intermediate. The methodology is validated through the synthesis of a representative compound set, which has been used in the production of 1617 molecules for the European Lead Factory.
Introduction
Small organic molecules are essential for the treatment of many diseases and constitute most medicines marketed today. The development of high-throughput screening (HTS) has enabled the extremely rapid biological evaluation of large collections of small organic molecules. However, despite advances in HTS technologies and general access to large molecular libraries, the annual number of approved small-molecule drugs has been declining for years.1 Growing evidence now suggests that many existing compound collections are inadequate in the search for new molecular entities capable of interacting with complex biological targets.2 Traditional compound collections are typically composed of flat molecules with a high degree of sp2-hybridization and only few stereocenters.3 These compound collections are commonly synthesized using classical combinatorial chemistry methods, powered by the development of effective sp2–sp2 coupling reactions, which have been widely exploited in the last decades. The routine synthesis of complex sp3-rich molecules with control of stereochemistry remains elusive and poses a substantial challenge for library production.4,5 Here, we report a strategy towards a screening library containing molecules with a relatively high degree of sp3 hybridization. In general, contributions to the European Lead Factory from the public consortium have a significantly higher fraction of sp3 compared to commercial compound collections.4Results and discussion
In this work, we wish to present a strategy for the efficient formation of two novel scaffolds, each containing four stereogenic centers, including a quaternary center, applicable to molecular library production. The approach starts with a Petasis 3-component reaction6 of salicylic aldehyde, allyl amine and 2-furyl boronic acid derivatives to form an aminophenol, which upon exposure to elevated temperatures undergoes an intramolecular Diels–Alder reaction7,8 (Fig. 1). The Diels–Alder product may then undergo oxidative cleavage to form a key dialdehyde intermediate, which can be reduced and used in a Mitsunobu9 cascade reaction (library A), or participate in a reductive cyclisation with amines (library B).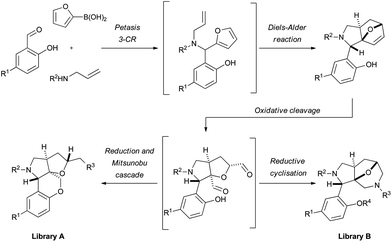 | ||
| Fig. 1 Synthesis strategies for the generation of two distinct and complex sp3-rich scaffolds (A and B), based on complexity-generating reactions from readily available starting materials. | ||
For library development, it was decided to use N-allylbenzylamine as the amine component to enable late stage functionalization of the resulting scaffolds through benzyl deprotection and appendage modification of the resulting secondary amine. Two 2-hydroxy benzaldehydes were used in the tandem Petasis/Diels–Alder sequence, which provided the desired products 1a and 1b as single diastereoisomers in high yield on a 90 gram scale (85% and 93%, respectively, Scheme 1).
The Diels–Alder products 1a and 1b were oxidatively cleaved using catalytic K2OsO4 and NMO as oxidant,10 followed by treatment with NaIO4. The intermediate dialdehyde was then either reduced to give the diols 2a and 2b in excellent yields (88% and 82%, respectively over three steps), or used in a reductive cyclisation with variable primary amines to give the compounds 3a–3d in high yields (80–95%, over three steps).
With the two diols 2a and 2b in hand, conditions for the Mitsunobu cascade sequence were investigated. By applying di-tert-butyl azodicarboxylate (DBAD) and PPh3 in CH2Cl2 at 10 °C, full conversion to the cyclized product was observed after 15 min, and the product 4 was isolated in near quantitative yield (>95%) (Scheme 2). DBAD was used as the azo-reagent to facilitate easy removal of the hydrazine by-products via acid treatment, improving the application for large-scale production of screening libraries.11
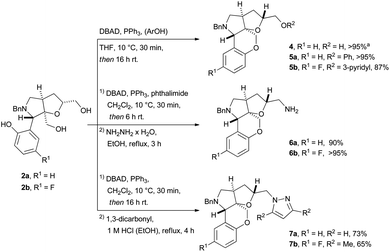 | ||
Scheme 2 Tandem Mitsunobu reactions for the synthesis of cyclic ethers (5a–b), primary amines (6a–b), and pyrazoles (7a–b). a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Reaction performed in CH2Cl2. Reaction performed in CH2Cl2. | ||
Having established an efficient protocol for the intramolecular Mitsunobu reaction, we wanted to investigate the possibility of a tandem intramolecular–intermolecular Mitsunobu cascade, which would enable simultaneous creation of the core scaffold and functionalization of the remaining alcohol. The reactions were conducted using either phthalimide or phenol along with three equivalents of DBAD and PPh3. The reactions with phthalimide as external nucleophile proceeded smoothly to give 6a and 6b in excellent yields (90% and >95%, respectively over two steps) after the deprotection with hydrazine. On the other hand, the use of phenol only gave 10% of the desired product, along with 90% of a di-Boc hydrazine side-product (from reaction with reduced DBAD).
The low selectivity of the aromatic alcohol addition was solved by changing the solvent to THF and employing an excess of the nucleophile (3 equiv.), which gave the desired compounds 5a and 5b in excellent yields (>95% and 87%, respectively) using either phenol or 3-hydroxy pyridine as nucleophile (Scheme 2).
Having observed the efficient addition of di-Boc hydrazine in the Mitsunobu reaction, we investigated if this could be exploited as an entry to create more structural diversity in the resulting library. When the reaction was conducted in CH2Cl2, notably without the addition of an external nucleophile, full conversion to the hydrazine product was observed. The crude reaction mixture was subsequently treated with 1 M HCl in EtOH and a 1,3-dicarbonyl electrophile, thus giving 1,2-diazole substituted compounds 7a and 7b in good yields (73% and 65%, respectively over two steps).
The two scaffolds, containing either a free phenol (3b–3d) or a primary amine (6a–6b) were subsequently functionalized. The phenols 3b–3d were alkylated with various alkyl halides in DMF using KOH as base to give ethers 8a–8c in high yields (75–94%) (Scheme 3). The primary amines 6a and 6b were functionalized using either a sulphonyl chloride, acid chloride or isocyanate to give derivatives 9a–9c in high yields (73–85%).12
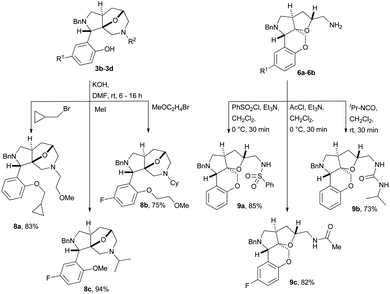 | ||
| Scheme 3 Alkylation of phenols 3b–3d and functionalization of amines 6a and 6b with sulfonyl chloride, acid chloride, and an isocyanate. | ||
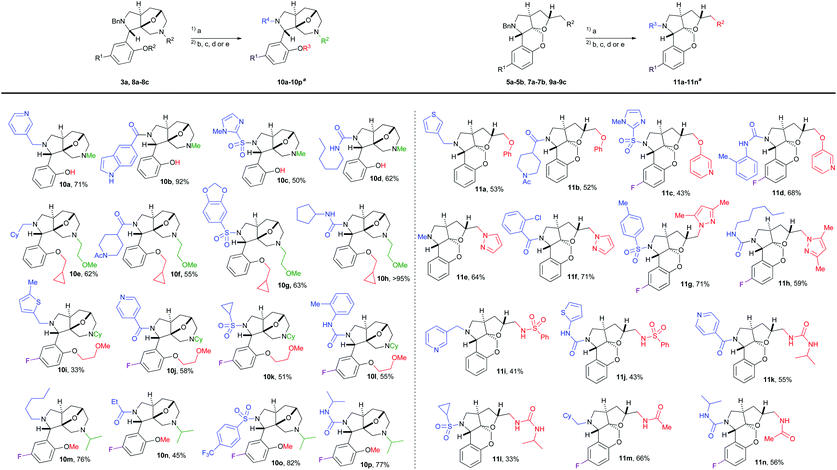
By using the benzyl protected building blocks, a small library was synthesized to validate the strategy for library production. Rewardingly, the benzyl group was smoothly removed using catalytic Pd/C with HCOONH4 as the hydrogen source. The resulting secondary amines were subsequently decorated with a variety of appendage functionalities using either reductive amination, amide couplings, sulfonylation or urea formation (Table 1). This process readily provided 30 compounds from the two scaffolds based on appendages using the 3–4 diversity points. The compounds were purified using preparative HPLC to simulate a large library production, and the compounds were generally isolated in good yields (33–95%, over two steps).
| a All library compounds were purified by preparative HPLC to simulate large library production. Reagents and conditions. (a) 2.5–10 mol% Pd/C, HCOONH4, MeOH or EtOH, reflux, 1–6 h. (b) RCHO, NaBH(OAc)3, DMF, rt, 8–16 h. (c) RCOOH, TBTU, DIPEA, DMF, rt, 1–16 h. (d) RSO2Cl, DIPEA, DMF, 1–16 h. (e) RNCO, DMF, 1–16 h. |
|---|
|
The validated chemistry was subsequently used to produce a total of 1617 screening compounds for the European Lead Factory. Library A (826 compounds) and library B (791 compounds) were both produced with a success rate of 90% in the final functionalization step. All compounds were purified by mass-directed preparative HPLC and obtained in purities exceeding 95%.
Conclusions
In conclusion, we have developed an efficient strategy for the synthesis of two complex and sp3-rich scaffolds (Fsp3 = 0.57), with excellent potential for appendage diversification reactions at three or four reactive sites. The benzyl protected building blocks for library production were obtained in 3–4 synthetic steps in high-yielding reactions amenable to large-scale synthesis. The chemistry was validated, first through production of thirty screening compounds (Table 1), and subsequently through large-scale production of 1617 compounds for the European Lead Factory.Acknowledgements
The research leading to these results has received support from the Innovative Medicines Initiative Joint Undertaking under grant agreement no. 115489, resources of which are composed of financial contribution from the European Union's Seventh Framework Programme (FP7/2007–2013) and EFPIA companies’ in-kind contribution. We also thank Caroline Gurcel, Luciane Adeikalam and Guillaume Ranty at Edelris for assistance in the purification of the final library compounds.References
- B. Munos, Nat. Rev. Drug Discovery, 2009, 8, 9591 CrossRef CAS PubMed; R. Macarron, M. N. Banks, D. Bojanic, D. J. Burns, D. A. Cirovic, T. Garyantes, D. V. S. Green, R. P. Hertzberg, W. P. Janzen, J. W. Paslay, U. Schopfer and G. S. Sittampalam, Nat. Rev. Drug Discovery, 2011, 10, 188 CrossRef PubMed; L. M. Mayr and P. Fuerst, J. Biomol. Screening, 2008, 13, 443 CrossRef PubMed.
- D. J. Payne, M. N. Gwynn, D. J. Holmes and D. L. Pompliano, Nat. Rev. Drug Discovery, 2007, 6, 29 CrossRef CAS PubMed; S. Dandapani and L. A. Marcaurelle, Nat. Chem. Biol., 2010, 6, 861 CrossRef PubMed.
- F. Lovering, J. Bikker and C. J. Humblet, Med. Chem., 2009, 52, 6752 CrossRef CAS PubMed; F. Lovering, Med. Chem. Commun., 2013, 4, 515 RSC; A. Chuprina, O. Lukin, R. Demoiseaux, A. Buzko and A. Shivanyuk, J. Chem. Inf. Model., 2010, 50, 470 CrossRef PubMed; A. Nadin, C. Hattotuwagama and I. Churcher, Angew. Chem., Int. Ed., 2012, 51, 1114 CrossRef PubMed.
- S. L. Schreiber, Science, 2000, 287, 1964 CrossRef CAS PubMed; D. R. Spring, Org. Biomol. Chem., 2003, 1, 3867 Search PubMed; D. S. Tan, Nat. Chem. Biol., 2005, 1, 74 CrossRef PubMed; T. E. Nielsen and S. L. Schreiber, Angew. Chem., Int. Ed., 2008, 47, 48 CrossRef PubMed; A. Karawajczyk, F. Giordanetto, J. Benningshof, D. Hamza, T. Kalliokoski, K. Pouwer, R. Morgentin, A. Nelson, G. Müller, A. Piechot and D. Tzalis, Drug Discovery Today, 2015, 20, 1310 CrossRef PubMed; H. G. Laverty, K. M. Orrling, F. Giordanetto, M. Poinot, E. Ottow, T. W. Rijnders, D. Tzalis and S. Jaroch, J. Med. Dev. Sci., 2015, 1, 20 Search PubMed.
- For recent examples of libraries with a high degree of sp3 character, see: M. J. Rawling, T. E. Storr, W. A. Bawazir, S. J. Cully, W. Lewis, M. S. I. T. Makki, I. R. Strutt, G. Jones, D. Hamza and R. A. Stockman, Chem. Commun., 2015, 51, 12867 RSC; I. Churcher, R. Doveston, D. Foley, S. Marsden and A. Nelson, Chem. Commun., 2015, 51, 11174 RSC; A. Nelson and D. Roche, Bioorg. Med. Chem., 2015, 23, 2607 CrossRef CAS PubMed.
- For early accounts, see: N. A. Petasis and I. Akritopoulou, Tetrahedron Lett., 1993, 34, 583 CrossRef CAS; G. Dyker, Angew. Chem., Int. Ed. Engl., 1997, 36, 1700 CrossRef . For reviews on the Petasis 3-CR, see: N. A. Petasis, in Multicomponent Reactions, ed. J. Zhu and H. Bienaymé, Wiley-VCH, Weinheim, Germany, 2005, pp. 199–223 CrossRef PubMed; R. A. Batey, in Boronic Acids, ed. D. G. Hall., Wiley-VCH, Weinheim, Germany, 2005, pp. 279–304 CrossRef PubMed; N. R. Candeias, F. Montalbano, P. M. S. D. Cal and P. M. P. Gois, Chem. Rev., 2010, 110, 6169 CrossRef PubMed; N. Kumagai, G. Muncipinto and S. L. Schreiber, Angew. Chem., Int. Ed., 2006, 45, 3635 CrossRef PubMed.
- For review on IMDA, see: K. Takao, R. Munakata and K. Tadano, Chem. Rev., 2005, 105, 4779 CrossRef CAS PubMed.
- T. Flagstad, M. R. Hansen, S. T. Le Quement, M. Givskov and T. E. Nielsen, ACS Comb. Sci., 2015, 17, 19 CrossRef CAS PubMed; A. Cannillo, S. Norsikian, P. Retailleau, M. T. H. Dau, B. I. Iorga and J. Beau, Chem. – Eur. J., 2013, 19, 9127 CrossRef PubMed; A. Cannillo, S. Norsikian, M. T. H. Dau, P. Retailleau, B. I. Iorga and J. Beau, Chem. – Eur. J., 2014, 20, 12133 CrossRef PubMed.
- For early accounts, see: O. Mitsunobu and Y. Yamada, Bull. Chem. Soc. Jpn., 1967, 40, 2380 CrossRef CAS; O. Mitsunobu and O. Eguchi, Bull. Chem. Soc. Jpn., 1971, 44, 3427 CrossRef . For reviews on the Mitsunobu reaction see: K. C. K. Swamy, N. N. B. Kumar, E. Balaraman and K. V. P. P. Kumar, Chem. Rev., 2009, 109, 2551 CrossRef PubMed; S. Fletcher, Org. Chem. Front., 2015, 2, 739 RSC.
- V. Vanrheenen, R. C. Kelly and D. Y. Cha, Tetrahedron Lett., 1976, 17, 1973 CrossRef.
- M. Kiankarimi, R. Lowe, J. R. McCarthy and J. P. Whitten, Tetrahedron Lett., 1999, 40, 4497 CrossRef CAS.
- T. Kalliokoski, ACS Comb. Sci., 2015, 17, 600 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ob00961a |
| This journal is © The Royal Society of Chemistry 2016 |

