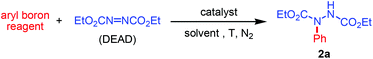Open Access Article
Open Access ArticleCp*Rh(III)-catalyzed electrophilic amination of arylboronic acids with azo compounds for synthesis of arylhydrazides†
Yan-Fung
Lau
,
Chun-Ming
Chan
,
Zhongyuan
Zhou
and
Wing-Yiu
Yu
*
The Hong Kong Polytechnic University, Hung Hom, Kowloon, Hong Kong, China. E-mail: wing-yiu.yu@polyu.edu.hk
First published on 16th June 2016
Abstract
A [Cp*Rh(III)]-catalyzed electrophilic amination of arylboronic acids with diethyl azodicarboxylate (DEAD) was developed, and arylhydrazides were produced in excellent yields and selectivity. The analogous amination with the arylazocarboxylates afforded the corresponding N,N-diarylhydrazides. The electrophilic amination of arylboronic acids with azocarboxylates proceeds readily under mild conditions with excellent functional group tolerance. Up to 99% yields were obtained. Preliminary mechanistic studies revealed that prior formation of an arylrhodium(III) intermediate for the azo coupling reaction can be ruled out.
Transition metal-catalyzed electrophilic (umpolung) aminations are attractive approaches for arylamine synthesis under mild conditions.1 Characterized by weak N–X (X = leaving group) σ-bonds, haloamines and hydroxyamine derivatives have been extensively investigated for electrophilic amination with organolithium and -magnesium reagents.2 Dialkyl azodicarboxylates are conceptually different classes of electrophilic amination reagents. Unlike the halo/hydroxyamine-type reagents, the azodicarboxylates react with carbanionic nucleophiles via N–N π-bond cleavage. While dialkyl azodicarboxylates are known to react with stoichiometric organometallic reagents for C–N bond coupling reactions,3 examples involving transition metal catalysis are sparse in the literature (Scheme 1). About a decade ago, Carreira and coworkers reported a Co- and Mn-catalyzed alkene hydrohydrazination using di-tert-butyl azodicarboxylate and triphenylsilane as reagents.3e–g Recently, Chatani and coworkers reported a Cu-catalyzed hydroarylation of azodicarboxylates.3h Muniz and coworkers reported a Pd-catalyzed coupling of arylboronic acids with diethyl azodicarboxylate (DEAD). A palladadiaziridine complex was structurally characterized and was shown to mediate the C–N bond coupling reaction.3i,j
Owing to an interest in developing transition metal catalyzed C–H bond aminations under mild conditions,4 we previously accomplished regioselective Pd-/Rh-catalyzed ortho-selective arene C–H amination with tosyloxycarbamates and N-chloroamines.4k–o The catalytic arene C–H amination should proceed by coupling of reactive arylpalladium(II) and -rhodium(III) complexes with the amination reagents. By virtue of the weak N–N π-bond, we envisioned that dialkyl azodicarboxylates would be effective coupling partners with arylmetal complexes for C–N bond formation. Here we describe [Cp*Rh(III)]-catalyzed (Cp* = 1,2,3,4,5-pentamethyl-cyclopentadienyl) cross coupling of arylboronic acids with azo compounds for the synthesis of arylhydrazides.
When phenylboronic acid (1a; 0.3 mmol) was treated with DEAD (0.2 mmol) and [Cp*Rh(OAc)2] (5 mol%) in THF at 80 °C under an N2 atmosphere for 4 h, phenylhydrazide (2a) was obtained in 85% yield (Table 1, entry 1). In this work, we found that employing phenylboronic acid pinacol ester and potassium phenyltrifluoroborate alone did not bring about effective C–N coupling reactions (entries 2 and 3). The boron reagents were fully recovered with substantial decomposition of the DEAD. Interestingly, when potassium phenyltrifluoroborate was employed together with B(OH)3 as additives and DMF as the solvent, 2a was formed in 70% yield (entry 4).
| Entry | Aryl boron reagent | Catalyst | Solvent | T (°C) | Yieldb (%) |
|---|---|---|---|---|---|
| a Conditions: aryl boron reagent (0.3 mmol), DEAD (0.2 mmol), catalyst (5 mol%), solvent (1 mL), 4 h in an N2 atmosphere. b Isolated yield. c n.d. = not detected. d B(OH)3 (0.3 mmol) was added. e [Cp*Rh(OAc)2] (2 mol%) was used. f Di-tert-butyl azodicarboxylate (0.2 mmol) was used instead. | |||||
| 1 | PhB(OH)2 (1a) | [Cp*Rh(OAc)2] | THF | 80 | 85 |
| 2 | PhB(pin) | [Cp*Rh(OAc)2] | THF | 80 | n.d.c |
| 3 | KPhBF3 | [Cp*Rh(OAc)2] | THF | 80 | n.d.c |
| 4d | KPhBF3 | [Cp*Rh(OAc)2] | THF | 80 | 70 |
| 5 | 1a | [Cp*RhCl2]2 | THF | 80 | 10 |
| 6 | 1a | [Rh(COD)Cl]2 | THF | 80 | 11 |
| 7 | 1a | [Rh(COD)(OH)]2 | THF | 80 | n.d.c |
| 8 | 1a | [Cp*IrCl2]2 | THF | 80 | n.d.c |
| 9 | 1a | [Cp*Rh(OAc)2] | t BuOH | 80 | 64 |
| 10 | 1a | [Cp*Rh(OAc)2] | MeCN | 80 | 3 |
| 11 | 1a | [Cp*Rh(OAc)2] | Dioxane | 80 | 50 |
| 12 | 1a | [Cp*Rh(OAc)2] | DCE | 80 | 31 |
| 13e | 1a | [Cp*Rh(OAc)2] | DMF | 40 | 99 |
| 14f | 1a | [Cp*Rh(OAc)2] | THF | 80 | 42 |
Other rhodium catalysts such as [Cp*RhCl2]2 are less effective catalysts (entry 5). According to the literature, rhodium(I) diene complexes such as [Rh(COD)X]2 (X = Cl, OH) are known to catalyze arylation of enones with arylboron reagents.5 However, these Rh(I)–diene complexes were found to be ineffective catalysts for the reaction of 1a with DEAD (entries 6 and 7). In this work, the related [Cp*IrCl2]2 complex exhibited negligible catalytic activities under our reaction conditions (entry 8).
Other solvents such as tBuOH, MeCN, dioxane and DCE gave inferior results compared to THF (entries 9–12). After several trials, we found that DMF gave the best result with 2a being formed in a nearly quantitative yield.6 Upon further refinement of several experimental parameters, an optimized reaction protocol was established: [Cp*Rh(OAc)2] (2 mol%), 1a (0.3 mmol), DEAD (0.2 mmol) in DMF at 40 °C (entry 13). It is noteworthy that the azo coupling reaction is sensitive to the ester substituents on the azocarboxylates. For instance, the amination of 1a with di-tert-butyl azodicarboxylate produced the corresponding arylhydrazides in only 42% yield (entry 14). The coupling with azobenzene was unsuccessful, and no C–N coupled products were obtained.6
With DEAD as the model substrate, the scope of the arylboronic acids was examined (Scheme 2). The reactions of arylboronic acids containing electron-donating and -withdrawing groups (e.g. OMe, Me and Br) afforded the corresponding hydrazides (2a–2d) in excellent yields. Other functionalized arylboronic acids bearing TMS, CHO, C(O)Me, CO2Et, NHC(O)Me and SO2Me were converted to 2e–2j in 83–98% yields. Fruitful results were achieved for the analogous amidation of 6-methoxy-1-naphthyl, 3-chloro and 3,5-bis(trifluoromethyl)phenylboronic acids with 2k–2m being formed in excellent yields. Likewise, effective transformations of styrylboronic acid and heteroaromatic boronic acids were also achieved to give the corresponding products (2n–2q) in good to moderate yields.
Diarylamines are prevalent scaffolds found in many natural products, pharmaceuticals and functional materials.7 The Pd- and Cu-catalyzed arylation of anilines with haloarenes are widely employed for diarylamine synthesis.8 Yet, examples of diarylamine synthesis via electrophilic amination are sparse.9 Lei and coworkers reported the synthesis of diarylamines by Cu-catalyzed arylation of N-chloroanilides with arylboronic acids.9e Recently, Chang and coworkers reported a reaction of aryl azides with aryliridium(III) complexes for diarylamine synthesis.9f–h In this work, we developed the catalytic arylation of arylazocarboxylates for the synthesis of N,N-diarylhydrazides.
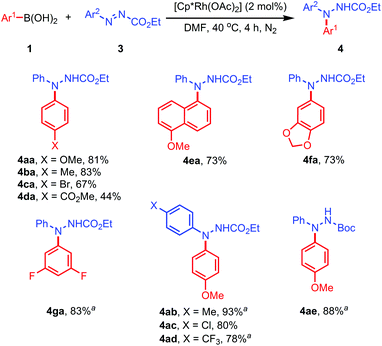
The arylazocarboxylate was prepared by reacting arylhydrazine with ethyl chloroformate, followed by NBS oxidation. When phenylazocarboxylate (3a) was treated with 4-methoxyphenylboronic acid (1b) and [Cp*Rh(OAc)2] (2 mol%) in DMF at 40 °C under an N2 atmosphere, N,N-diarylhydrazides (4aa) was isolated as a single regioisomer in 81% yield (Scheme 3). The molecular structure of 4aa has been established by single-crystal X-ray crystallography. Arylboronic acids containing electron-donating and -withdrawing substituents were well tolerated (see results for 4ba–4da). Similarly, amidation of 6-methoxy-1-naphthyl, 3,4-(methylenedioxy) and 3,5-ditrifluorophenylboronic acids furnished 4ea–4ga in excellent yields.
With 4-methoxyphenylboronic acid as the arylating reagent, the reactions of some substituted arylazocarboxylates were examined. Effective C–N coupling was observed in all cases, and the diarylhydrazides (4ab–4ae) were formed in 78–93% yields.
Arylrhodium(III) complexes are known to mediate catalytic C–N bond coupling reactions.4m,10 To examine the involvement of the arylrhodium(III) complexes, we prepared the well-defined [Cp*Rh(Ph)(Br)(PPh3)] complex 5a (71% yield) by reacting [Cp*RhCl2(PPh3)] with PhMgBr.11 The analogous [Cp*Rh(Ph)(Cl) (PPh3)] complex 5b (83% yield) was also prepared by employing phenylboronic acid as the aryl source (Scheme 4).12 The molecular structures of 5a and 5b have been confirmed by single-crystal X-ray crystallography.6
In this work, when [Cp*Rh(Ph)(Br)(PPh3)] (5a) (10 mol%) was treated with AgSbF6 (10 mol%) and phenylazocarboxylate (0.5 mmol) in DMF at 40 °C for 4 h, no N,N-diphenylhydrazide was formed. Notably, [Cp*Rh(Ph)2(PPh3)] was isolated in 30% yield, and 18% of the starting [Cp*Rh(Ph)(Br)(PPh3)] was recovered (Scheme 5). Notwithstanding, [Cp*RhCl2(PPh3)] was found to be an effective catalyst for the arylation reaction. For example, reacting [Cp*RhCl2(PPh3)] (5 mol%) with 4-methoxyphenylboronic acid (1b) and phenylazocarboxylate (3a) in DMF at 40 °C afforded 4aa in 99% yield. Based on the above findings, direct coupling of arylrhodium(III) with the azo reagent may not be a productive step for the arylation reaction.
 | ||
| Scheme 5 Investigation of the stoichiometric reaction of arylrhodium(III) complexes with phenylazocarboxylate. | ||
Previously, Muniz and coworkers reported the Pd-catalyzed arylation of DEAD by arylboronic acids, and palladadiaziridine complexes have been characterized as the key intermediate. However, the attempt to characterize well-defined rhodalladiaziridine complexes was unsuccessful. The preparation and characterization of some reactive metalladiaziridine complexes are currently in progress, and the results will be reported separately.
Conclusions
In conclusion, we developed a [Cp*Rh(III)]-catalyzed electrophilic amination of arylboronic acids by employing azo reagents. Effective coupling of DEAD and the aryl azocarboxylates with arylboronic acids afforded mono- and diarylhydrazides in good yields under mild conditions.Acknowledgements
We thank the Hong Kong Research Grants Council (PolyU5032/13P, C5023/14G) for financial support.Notes and references
- For transition metal-catalyzed electrophilic amination, see: (a) A. Ricci, Amino Group Chemistry: From Synthesis to the Life Sciences, Wiley-VCH, Weinheim, 2008 Search PubMed; (b) A. Ricci, Modern Amination Methods, Wiley-VCH, Weinheim, 2000 CrossRef.
- For a selected review on electrophilic amination of carbanions, see: (a) E. Erdik and M. Ay, Chem. Rev., 1989, 89, 1947 CrossRef CAS. For selected articles on the stoichiometric addition of organometallic reagents: for the use of organolithium reagents, see: (b) P. Beak and B. J. Kokko, J. Org. Chem., 1982, 47, 2823 CrossRef. For the use of Grignard reagents, see: (c) M. J. Campbell and J. S. Johnson, Org. Lett., 2007, 9, 1521 CrossRef CAS PubMed; (d) E. Erdik and S. Ates, Synth. Commun., 2006, 36, 2813 CrossRef CAS; (e) M. Kitamura, T. Suga, S. Chiba and K. Narasaka, Org. Lett., 2004, 6, 4619 CrossRef CAS PubMed. For the use of organozinc reagents, see: (f) A. M. Berman and J. S. Johnson, J. Org. Chem., 2006, 71, 219 CrossRef CAS PubMed; (g) A. M. Berman and J. S. Johnson, J. Org. Chem., 2005, 70, 364 CrossRef CAS PubMed; (h) E. Erdik and T. J. Daskapan, Chem. Soc., Perkin Trans. 1, 1999, 3139 RSC. For the use of cuprates, see: (i) P. Bernardi, P. Dembech, G. Fabbri, A. Ricci and G. Seconi, J. Org. Chem., 1999, 64, 641 CrossRef CAS; (j) A. Alberti, F. Cane, P. Dembech, D. Lazzari, A. Ricci and G. Seconi, J. Org. Chem., 1996, 61, 1677 CrossRef CAS PubMed; (k) A. Casarini, P. Dembech, D. Lazzari, E. Marini, G. Reginato, A. Ricci and G. Seconi, J. Org. Chem., 1993, 58, 5620 CrossRef CAS. For the use of organostannane reagents, see: (l) Z. Zhang, Y. Yu and L. S. Liebeskind, Org. Lett., 2008, 10, 3005 CrossRef CAS PubMed.
- For stoichiometric addition of organometallic reagents reacting with azo compounds: for the use of Grignard reagents, see: (a) I. Sapountzis and P. Knochel, Angew. Chem., Int. Ed., 2004, 43, 897 CrossRef CAS PubMed. For the use of organozinc reagents, see: (b) P. Sinha, C. C. Kofink and P. Knochel, Org. Lett., 2006, 8, 3741 CrossRef CAS PubMed; (c) H. Mitchell and Y. Leblanc, J. Org. Chem., 1994, 59, 682 CrossRef CAS. For the use of organotitanium reagents, see: (d) D. K. An, K. Hirakawa, S. Okamoto and F. Sato, Tetrahedron Lett., 1999, 40, 3737 CrossRef CAS. For transition metal catalyzed C–N bond formation employing azo compounds: for Co- and Mn-catalyzed alkene hydrohydrazination, see: (e) J. Waser, B. Gaspar, H. Nambu and E. M. Carreira, J. Am. Chem. Soc., 2006, 128, 11693 CrossRef CAS PubMed; (f) J. Waser, J. C. Gonzalez-Gomez, H. Nambu, P. Huber and E. M. Carreira, Org. Lett., 2005, 7, 4249 CrossRef CAS PubMed; (g) J. Waser and E. M. Carreira, J. Am. Chem. Soc., 2004, 126, 5676 CrossRef CAS PubMed. For Cu-mediated C–N bond coupling of arylboronic acid with azo compounds, see: (h) T. Uemura and N. Chatani, J. Org. Chem., 2005, 70, 8631 CrossRef CAS PubMed. For Pd-catalyzed C–N bond coupling of arylboronic acid with azo compounds, see: (i) K. Muniz and A. Iglesias, Angew. Chem., Int. Ed., 2007, 46, 6350 CrossRef CAS PubMed; (j) K. Muniz and M. Nieger, Angew. Chem., Int. Ed., 2006, 45, 2305 CrossRef CAS PubMed.
- Here our recent studies on catalytic C–H bond cross coupling reactions are depicted. For transition metal-catalyzed ortho-selective arene C–H bond carbenoid insertion, see: (a) H.-W. Lam, K.-Y. Man, W.-W. Chan, Z. Zhou and W.-Y. Yu, Org. Biomol. Chem., 2014, 12, 4112 RSC; (b) W.-W. Chan, S.-F. Lo, Z. Zhou and W.-Y. Yu, J. Am. Chem. Soc., 2012, 134, 13565 CrossRef CAS PubMed; (c) W.-W. Chan, T.-L. Kwong and W.-Y. Yu, Org. Biomol. Chem., 2012, 10, 3749 RSC; (d) W.-W. Chan, S.-H. Yeung, Z. Zhou, A. S. C. Chan and W.-Y. Yu, Org. Lett., 2010, 12, 604 CrossRef CAS PubMed. For transition metal-catalyzed C2-/ortho-selective arene C–H bond coupling with carboradical, see: (e) C.-W. Chan, P.-Y. Lee and W.-Y. Yu, Tetrahedron Lett., 2015, 56, 2559 CrossRef CAS; (f) W.-W. Chan, Z. Zhou and W.-Y. Yu, Chem. Commun., 2013, 49, 8214 RSC; (g) C.-W. Chan, Z. Zhou and W.-Y. Yu, Adv. Synth. Catal., 2011, 353, 2999 CrossRef CAS; (h) C.-W. Chan, Z. Zhou, A. S. C. Chan and W.-Y. Yu, Org. Lett., 2010, 12, 3926 CrossRef CAS PubMed; (i) W.-Y. Yu, W. N. Sit, Z. Zhou and A. S. C. Chan, Org. Lett., 2009, 11, 3174 CrossRef CAS PubMed; (j) W.-Y. Yu, W. N. Sit, K.-M. Lai, Z. Zhou and A. S. C. Chan, J. Am. Chem. Soc., 2008, 130, 3304 CrossRef CAS PubMed. For Pd-catalyzed ortho-selective arene C–H bond amination with tosyloxycarbamates, see: (k) K.-H. Ng, F.-N. Ng and W.-Y. Yu, Chem. Commun., 2012, 48, 11680 RSC; (l) K.-H. Ng, A. S. C. Chan and W.-Y. Yu, J. Am. Chem. Soc., 2010, 132, 12862 CrossRef CAS PubMed. For Rh-catalyzed ortho-selective arene C–H amination with N-chloramines, see: (m) F.-N. Ng, Z. Zhou and W.-Y. Yu, Chem. – Eur. J., 2014, 20, 4474 CrossRef CAS PubMed; (n) K.-H. Ng, Z. Zhou and W.-Y. Yu, Chem. Commun., 2013, 49, 7031 RSC; (o) K.-H. Ng, Z. Zhou and W.-Y. Yu, Org. Lett., 2012, 14, 272 CrossRef CAS PubMed.
- For selective examples of rhodium(I) diene complexes catalyzed arylation of enones, see: (a) S. Gosiewska, J. A. Raskatov, R. Shintani, T. Hayashi and J. M. Brown, Chem. – Eur. J., 2012, 18, 80 CrossRef CAS PubMed; (b) H. J. Edwards, J. D. Hargrave, S. D. Penrose and C. G. Frost, Chem. Soc. Rev., 2010, 39, 2093 RSC; (c) R. Shintani and T. Hayashi, Aldrichimica Acta, 2009, 42, 31 CAS; (d) T. Hayashi, K. Ueyama, N. Tokunaga and K. Yoshida, J. Am. Chem. Soc., 2003, 125, 11508 CrossRef CAS PubMed.
- Refer to the ESI† for detailed experimental data.
- (a) A. Kleeman, J. Engel, B. Kutscher and D. Reichert, Pharmaceutical Substances: Syntheses, Patents, Applications of the most relevant APIs, Thieme, Stuttgart, 5th edn, 2009 Search PubMed; (b) S. M. Wilhelm, L. Adnane, P. Newell, A. Villanueva, J. M. Llovet and M. Lynch, Mol. Cancer Ther., 2008, 7, 3129 CrossRef CAS PubMed; (c) R. Sordella, D. W. Bell, D. A. Haber and J. Settleman, Science, 2004, 305, 1163 CrossRef CAS PubMed; (d) M. W. N. Deininger and B. J. Druker, Pharmacol. Rev., 2003, 55, 401 CrossRef CAS PubMed.
- For Pd-catalyzed nucleophilic amination for diarylamine synthesis, see: (a) F. Paul, J. Patt and J. F. Hartwig, Organometallics, 1995, 14, 3030 CrossRef CAS; (b) A. S. Guram, R. A. Rennels and S. L. Buchwald, Angew. Chem., 1995, 107, 1456 ( Angew. Chem. Int. Ed. Engl. , 1995 , 34 , 1348 ) CrossRef; (c) J. Louie and J. F. Hartwig, Tetrahedron Lett., 1995, 36, 3609 CrossRef CAS; (d) F. Paul, J. Patt and J. F. Hartwig, J. Am. Chem. Soc., 1994, 116, 5969 CrossRef CAS. For Cu-catalyzed nucleophilic amination for diarylamine synthesis, see: (e) D. M. T. Chan, K. L. Monaco, R.-P. Wang and M. P. Winters, Tetrahedron Lett., 1998, 39, 2933 CrossRef CAS; (f) D. A. Evans, J. L. Katz and T. R. West, Tetrahedron Lett., 1998, 39, 2937 CrossRef CAS; (g) P. Y. S. Lam, C. G. Clark, S. Saubern, J. Adams, M. P. Winters, D. M. T. Chan and A. Combs, Tetrahedron Lett., 1998, 39, 2941 CrossRef CAS.
- For transition metal-catalyzed electrophilic amination for diarylamine synthesis: for stoichiometric addition of organoaluminum reagents reacting with O-protected hydroxamic acid, see: (a) S. Zhou, Z. Yang, X. Chen, Y. Li, L. Zhang, H. Fang, W. Wang, X. Zhu and S. Wang, J. Org. Chem., 2015, 80, 6323 CrossRef CAS PubMed; (b) H. Yoon and Y. Lee, J. Org. Chem., 2015, 80, 10244 CrossRef CAS PubMed. For Cu-catalyzed electrophilic amination for diarylamine synthesis, see: (c) R. Sakae, K. Hirano and M. Miura, J. Am. Chem. Soc., 2015, 137, 6460 CrossRef CAS PubMed; (d) T. Kawano, K. Hirano, T. Satoh and M. Miura, J. Am. Chem. Soc., 2010, 132, 6900 CrossRef CAS PubMed; (e) C. He, C. Chen, J. Cheng, C. Liu, W. Liu, Q. Li and A. Lei, Angew. Chem., Int. Ed., 2008, 47, 6414 CrossRef CAS PubMed. For Rh-catalyzed electrophilic amination for diarylamine synthesis, see: (f) S. H. Park, J. Kwak, K. Shin, J. Ryu, Y. Park and S. Chang, J. Am. Chem. Soc., 2014, 136, 2492 CrossRef CAS PubMed; (g) K. Shin, Y. Baek and S. Chang, Angew. Chem., Int. Ed., 2013, 52, 1 CrossRef PubMed; (h) J. Ryu, K. Shin, S. H. Park, J. Y. Kim and S. Chang, Angew. Chem., Int. Ed., 2012, 51, 9904 CrossRef CAS PubMed.
- For arylrhodium(III)-mediated catalytic C–N bond coupling reactions, see: (a) G. Song, F. Wang and X. Li, Chem. Soc. Rev., 2012, 41, 3651 RSC; (b) K. Shin, Y. Baek and S. Chang, Angew. Chem., Int. Ed., 2013, 52, 1 CrossRef PubMed; (c) C. Grohmann, H. Wang and F. Glorius, Org. Lett., 2012, 14, 656 CrossRef CAS PubMed; (d) J. Y. Kim, S. H. Park, J. Ryu, S. H. Cho, S. H. Kim and S. Chang, J. Am. Chem. Soc., 2012, 134, 9110 CrossRef CAS PubMed; (e) C. Grohmann, H. Wang and F. Glorius, Org. Lett., 2013, 15, 3014 CrossRef CAS PubMed. For arylrhodium(III)-mediated catalytic carbenoid C–C bond coupling reactions, see: (f) Y.-S. Lu and W.-Y. Yu, Org. Lett., 2016, 18, 1350 CrossRef CAS PubMed; (g) F.-N. Ng, Y.-F. Lau, Z. Zhou and W.-Y. Yu, Org. Lett., 2015, 17, 1676 CrossRef CAS PubMed.
- J. W. Kang, K. Moseley and P. M. Maitlis, J. Am. Chem. Soc., 1969, 91, 5970 CrossRef CAS.
- E. J. Farrington, C. F. J. Barnard, E. Rowsell and J. M. Brown, Adv. Synth. Catal., 2005, 347, 185 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1455768–1455771. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ob00719h |
| This journal is © The Royal Society of Chemistry 2016 |