Dual-responsive vesicles formed by an amphiphile containing two tetrathiafulvalene units in aqueous solution†
Xiao-Jun
Wang
ab,
Ling-Bao
Xing
ac,
Bin
Chen
a,
Ying
Quan
b,
Chen-Ho
Tung
a and
Li-Zhu
Wu
*a
aKey Laboratory of Photochemical Conversion and Optoelectronic Materials, Technical Institute of Physics and Chemistry, The Chinese Academy of Sciences, Beijing 100190, P. R. China. E-mail: lzwu@mail.ipc.ac.cn; Fax: +86 108254 3580; Tel: +86 108254 3580
bJiangsu Key Laboratory of Green Synthetic Chemistry for Functional Materials, School of Chemistry and Chemical Engineering, Jiangsu Normal University, Xuzhou 221116, P. R. China
cSchool of Chemical Engineering, Shandong University of Technology, Zibo 255049, P. R. China
First published on 19th November 2015
Abstract
The first example of tetrathiafulvalene (TTF)-based vesicle fabricated in water solution with 1 vol.% tetrahydrofuran that could be prevented by chemical oxidant Fe(ClO4)3 or electron-deficient cyclobis(paraquat-p-phenylene) tetracation cyclophane (CBPQT4+) is described.
Vesicles as enclosed compartments constructed by self-assembly membranes of amphiphiles, have attracted extensive attention in recent years because they are crucial building blocks for all living systems and have various potential applications in drug and gene delivery, nanomaterials, and artificial cell membranes.1–8 Encouraged by Mother Nature, chemists have employed various amphiphilic polymers and small surfactants to construct artificial vesicles with defined sizes and functions. For example, calixarenes,9 cucurbiturils,10 cyclodextrin,11,12 cyclophane,13,14 foldamers,15 as well as block copolymers16,17 have been reported. On the other hand, several strategies have been developed to endow the artificial vesicles “stimuli-responsive” characteristics by a range of photo-,18,19 electro-,20–22 pH-,23–26 thermo-,27,28 or enzyme-stimuli.29,30 However, most of them could only respond to one single external stimulus. There are only a few examples regarding the multi-stimuli responsive supramolecular vesicles.31–35
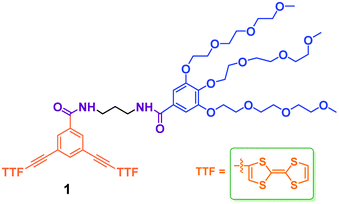
Tetrathiafulvalene and its derivatives (TTFs) have attracted extensive research interest as strong electron donors for the development of organic electrical conductors and superconductors over the past decades.35–37 In addition, they are also used as building blocks in macromolecular and supramolecular systems for various applications.38,39 Zhao and Li reported the first self-assembling vesicles from TTF derivatives in organic solvents.40 However, their self-assembling behaviors and applications in aqueous environments have been far less explored41 owing to the pronounced hydrophobicity of TTFs. Recently, we reported that a water-soluble small molecular weight amphiphile with one TTF unit can self-assemble into micelles in water.42 The encouraging result prompted us to design a new different amphiphile 1 containing two TTF units and to explore further its assembly behaviors in aqueous environment (Scheme 1). In comparison to the reported amphiphilic molecule,42 the introduction of one more TTF units in 1 results in the whole molecular structural shape changing from wedge and dumbbell (Fig. S1†).43,44 More importantly, the self-assembly of dumbbell-shaped 1 in aqueous solution results in TTF-based vesicles and shows different self-assembly aggregates as a result of the new balance and orientation of hydrophobic moiety and hydrophilic chain.
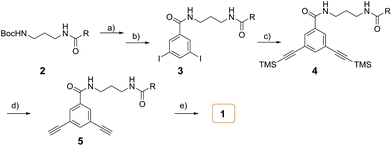
The synthesis of amphiphile 1 was carried out as described in Scheme 2. The starting N-Boc protected compound 2 with hydrophilic group was deprotected under excess trifluoroacetic acid (TFA). Subsequently, an excess of triethylamine (TEA) was slowly added to neutralize TFA. The generated amine was directly coupled with 3,5-diiodobenzoic acid with the aid of PyBOP to give compound 3. The Sonogashira reaction of 3 with an excess (trimethylsilyl)acetylene (TMSA) yielded 4, which was then deprotected by excess K2CO3 to afford the terminal alkyne compound 5. Then, 5 was reacted with 2-iodotetrahiafulvalene to produce target amphiphilic compound 1. The target compound was identified by 1H NMR and 13C NMR spectroscopy, matrix-assisted laser desorption/ionization mass spectrometry (MALDI-TOF MS).
Due to the very limited solubility of 1 in water, it cannot directly dissolve into water for self-assembly. Therefore, a little amount of organic solvent tetrahydrofuran (THF) that may have some solvation or even templation effect was used to aid the solvation.6 Specifically, to initiate the self-assembly of 1 in aqueous solution, a schematic representation of the preparation procedure is shown in Fig. S2.† In a typical experiment, a concentrated THF solution of 1 (2 × 10−3 M, 20 μL) was injected into a rigoursly stirred water (2 mL) to induce aggregate formation.
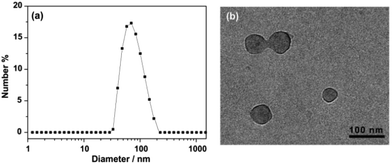
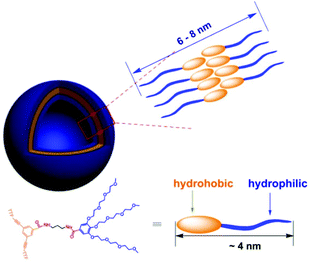
The aggregated species of 1 in aqueous solution was corroborated by dynamic light scattering (DLS) experiments. CONTIN analysis of the DLS autocorrelation function of 1 shows a broad distribution of hydrodynamic diameter (Dh) of the aggregates centered at ∼70 nm in aqueous solution (Fig. 1a). The observation suggested the presence of large aggregates. Cryogenic transmission electron microscopy (cryo-TEM) was performed to gain a direct visualization of the size and morphology (Fig. 1b and S3†). As shown in Fig. 1b, these aggregate species have a typical vesicular structure by the distinct contrast between the dark periphery and the light central part with a diameter of 50–90 nm, which are consistent with the results of DLS measurement. The wall thickness (dark part) is estimated to be about 4–7 nm, which indicated the hydrophobic segments consisting of bilayer of amphiphilic molecules (Fig. 2). The driving force for the formation of vesicles of 1 lies with the balance of π–π and S–S interactions45–51 and solvophobic interactions between TTF cores and interior hydrophilic interaction. Moreover, the absorption spectra of 1 in water exhibited a weaker absorbance with a red shift than that in CH3CN (Fig. S4†), confirming the π–π interactions between TTF cores. We postulated, based on the evidence to be shown, that 1 self-assembled in a bilayer fashion with the hydrophilic ethoxy chains exposed both internally and externally to the polar solvent with the hydrophobic TTF groups interacting in the middle of the bilayer.
Then, we explore the possibility of prevention of such vesicular aggregates by oxidation or complexation with TTF unit. Firstly, we found it was difficult to fully oxidize the TTF units of 1 in aqueous solution to its cation TTF2+, as shown in Fig. S5.† Whereas only 4 equiv. of Fe(ClO4)3 was needed to produce dication 12+ (TTF2+) in CH3CN. Such differences could be interpreted by the fact that the TTF moiety is buried in the hydrophobic core of vesicular aggregate that is not easily accessible for Fe(ClO4)3. Due to the low solubility of 1 in water, we first oxidized the TTF unit to its dication TTF2+ in CH3CN, and then added it to deionized water for cryo-TEM measurement (Fig. 3, left). As shown in Fig. 3, no vesicular structures could be observed, indicating the prevention of such vesicular aggregates by the oxidation of TTF units to more hydrophilic TTF2+, together with the electrostatic repulsion between the oxidized species. However, the trial for regeneration of vesicles by addition of reductant, such as Vitamin C and NaBH4, was unsuccessful mainly due to the limited solubility of 1 in water.
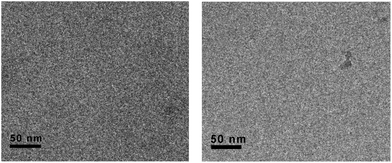 | ||
| Fig. 3 The representative cryo-TEM images of 1 with TTF units being fully oxidized by Fe3+ (left) or complexation with CBPQT4+ (right). | ||
Due to the high π-electron donating ability, TTFs can form stable charge-transfer complexes with electron-deficient species, tetracationic cyclobis(paraquat-p-phenylene) (CBPQT4+). Therefore, we took advantage of the complexation of 1 and CBPQT4+ in solution to disassemble the vesicular architectures. When amphiphile 1 and CBPQT4+ were mixed in water and CH3CN, respectively (Fig S6†), a green solution appeared immediately accompanied with a very broad charge-transfer band around 800 nm in the absorption spectra. Additional insight into the complexation of 1 and CBPQT4+ was obtained by means of 1H NMR spectroscopy studies in CD3CN (Fig. S7†). A mixture of 1 and CBPQT4+ (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) exhibited a quite different spectrum from that of amphiphile 1 itself in CD3CN. Upon addition of CBPQT4+ to solution, the proton H (●, ■) resonance of TTF unit shifted to upfield position, whereas the aromatic protons H (◆, ◊) neighboring the TTF unit shifted to downfield. These results suggested the formation of charge-transfer complex between 1 and CBPQT4+, and thereby leading to the strong shielding and deshielding effect of the aromatic rings in CBPQT4+. The disassembling vesicles of 1 upon complexation with CBPQT4+ were further studied using cryo-TEM techniques (Fig. 3, right). Fig. 3 (right) showed the typical cryo-TEM images of 1 after complexation with CBPQT4+. Also, no vesicular structures were observed, indicating the damage of vesicles. This prevention may be explained by considering the two factors: (1) the original hydrophobic part of 1 became more hydrophilic upon complexation with CBPQT4+; and (2) the strong electrostatic repulsion interactions between these terminus tetracationic species caused the separations of charge-transfer complexes.
2) exhibited a quite different spectrum from that of amphiphile 1 itself in CD3CN. Upon addition of CBPQT4+ to solution, the proton H (●, ■) resonance of TTF unit shifted to upfield position, whereas the aromatic protons H (◆, ◊) neighboring the TTF unit shifted to downfield. These results suggested the formation of charge-transfer complex between 1 and CBPQT4+, and thereby leading to the strong shielding and deshielding effect of the aromatic rings in CBPQT4+. The disassembling vesicles of 1 upon complexation with CBPQT4+ were further studied using cryo-TEM techniques (Fig. 3, right). Fig. 3 (right) showed the typical cryo-TEM images of 1 after complexation with CBPQT4+. Also, no vesicular structures were observed, indicating the damage of vesicles. This prevention may be explained by considering the two factors: (1) the original hydrophobic part of 1 became more hydrophilic upon complexation with CBPQT4+; and (2) the strong electrostatic repulsion interactions between these terminus tetracationic species caused the separations of charge-transfer complexes.
Conclusions
In summary, we have designed and synthesized a dumb-bell shaped amphiphile having two redox-active TTF units by Sonogashira reaction in a good chemical yield. It can self-assemble in aqueous solution to form vesicular aggregates. More interestingly, the vesicular architectures could be prevented either by the addition of chemical oxidant Fe(ClO4)3 or by the complexation with electron-deficient ring CBPQT4+. This is, to the best of our knowledge, the first example of vesicles in aqueous media based on a small organic molecule featuring electro-active TTF unit. This work also has demonstrated that the aggregates’ morphologies and functions of amphiphilic TTFs are dependent on their molecular shapes and the number of TTF units. Furthermore, it is anticipated that this research line can lead to the rational design of new smarter and more complicated aggregates in aqueous environments.Acknowledgements
We are grateful for financial support from the Ministry of Science and Technology of China (2013CB834505 and 2014CB239402), the National Science Foundation of China (21302072 and 91427303), Natural Science Foundation of Jiangsu Province (BK20130226), Jiangsu Normal University (12XLR015 and 12XLR018), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and the Chinese Academy of Sciences.Notes and references
- Y. Wang, H. Xu and X. Zhang, Adv. Mater., 2009, 21, 2849–2864 CrossRef CAS.
- H.-J. Kim, T. Kim and M. Lee, Acc. Chem. Res., 2010, 44, 72–82 CrossRef PubMed.
- Y. Hizume, K. Tashiro, R. Charvet, Y. Yamamoto, A. Saeki, S. Seki and T. Aida, J. Am. Chem. Soc., 2010, 132, 6628–6629 CrossRef CAS PubMed.
- X. Zhang, S. Rehm, M. M. Safont-Sempere and F. Würthner, Nat. Chem., 2009, 1, 623–629 CrossRef CAS PubMed.
- J. Voskuhl and B. Ravoo, Chem. Soc. Rev., 2009, 38, 495–505 RSC.
- D. Jiao, J. Geng, X. J. Loh, D. Das, T.-C. Lee and O. A. Scherman, Angew. Chem., Int. Ed., 2012, 51, 9633–9637 CrossRef CAS PubMed.
- X.-D. Xu, L. Zhao, Q. Qu, J.-G. Wang, H. Shi and Y. Zhao, ACS Appl. Mater. Interfaces, 2015, 7, 17371–17380 CAS.
- L.-B. Meng, W. Zhang, D. Li, Y. Li, X.-Y. Hu, L. Wang and G. Licd, Chem. Commun., 2015, 51, 14381–14384 RSC.
- Y. Tanaka, M. Miyachi and Y. Kobuke, Angew. Chem., Int. Ed., 1999, 38, 504–506 CrossRef CAS.
- Y. J. Jeon, P. K. Bharadwaj, S. W. Choi, J. W. Lee and K. Kim, Angew. Chem., Int. Ed., 2002, 41, 4474–4476 CrossRef CAS.
- B. J. Ravoo and R. Darcy, Angew. Chem., Int. Ed., 2000, 39, 4324–4326 CrossRef CAS.
- C. W. Lim, O. Crespo-Biel, M. C. A. Stuart, D. N. Reinhoudt, J. Huskens and B. J. Ravoo, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 6986–6991 CrossRef CAS PubMed.
- S. H. Seo, J. Y. Chang and G. N. Tew, Angew. Chem., Int. Ed., 2006, 45, 7526–7530 CrossRef CAS PubMed.
- Y. Murakami, J. Kikuchi, T. Ohno, O. Hayashida and M. Kojima, J. Am. Chem. Soc., 1990, 112, 7672–7681 CrossRef CAS.
- W. Cai, G.-T. Wang, Y.-X. Xu, X.-K. Jiang and Z.-T. Li, J. Am. Chem. Soc., 2008, 130, 6936–6937 CrossRef CAS PubMed.
- Y. Zhou and D. Yan, Angew. Chem., Int. Ed., 2004, 43, 4896–4899 CrossRef CAS PubMed.
- Q. Yan, J. Yuan, Z. Cai, Y. Xin, Y. Kang and Y. Yin, J. Am. Chem. Soc., 2010, 132, 9268–9270 CrossRef CAS PubMed.
- S. K. M. Nalluri, J. Voskuhl, J. B. Bultema, E. J. Boekema and B. J. Ravoo, Angew. Chem., Int. Ed., 2011, 50, 9747–9751 CrossRef CAS PubMed.
- G. Yu, C. Han, Z. Zhang, J. Chen, X. Yan, B. Zheng, S. Liu and F. Huang, J. Am. Chem. Soc., 2012, 134, 8711–8717 CrossRef CAS PubMed.
- C. Wang, Y. Guo, Y. Wang, H. Xu and X. Zhang, Chem. Commun., 2009, 5380–5382 RSC.
- Q. Yan, J. Yuan, Z. Cai, Y. Xin, Y. Kang and Y. Yin, J. Am. Chem. Soc., 2010, 132, 9268–9270 CrossRef CAS PubMed.
- H. Kim, S.-M. Jeong and J.-W. Park, J. Am. Chem. Soc., 2011, 133, 5206–5209 CrossRef CAS PubMed.
- Q. Duan, Y. Cao, Y. Li, X. Hu, T. Xiao, C. Lin, Y. Pan and L. Wang, J. Am. Chem. Soc., 2013, 135, 10542–10549 CrossRef CAS PubMed.
- G. Yu, X. Zhou, Z. Zhang, C. Han, Z. Mao, C. Gao and F. Huang, J. Am. Chem. Soc., 2012, 134, 19489–19497 CrossRef CAS PubMed.
- M. Lee, S. J. Lee and L. H. Jiang, J. Am. Chem. Soc., 2004, 126, 12724–12725 CrossRef CAS PubMed.
- Y. Yao, M. Xue, J. Chen, M. Zhang and F. Huang, J. Am. Chem. Soc., 2012, 134, 15712–15715 CrossRef CAS PubMed.
- T. Ta, A. J. Convertine, C. R. Reyes, P. S. Stayton and T. M. Porter, Biomacromolecules, 2010, 11, 1915–1920 CrossRef CAS PubMed.
- W. M. Park and J. A. Champion, J. Am. Chem. Soc., 2014, 136, 17906–17909 CrossRef CAS PubMed.
- D.-S. Guo, K. Wang, Y.-X. Wang and Y. Liu, J. Am. Chem. Soc., 2012, 134, 10244–10250 CrossRef CAS PubMed.
- C. Wang, Q. Chen, Z. Wang and X. Zhang, Angew. Chem., Int. Ed., 2010, 49, 8612–8615 CrossRef CAS PubMed.
- Y. Cao, X.-Y. Hu, Y. Li, X. Zou, S. Xiong, C. Lin, Y.-Z. Shen and L. Wang, J. Am. Chem. Soc., 2014, 136, 10762–10769 CrossRef CAS PubMed.
- K. Wang, D.-S. Guo, X. Wang and Y. Liu, ACS Nano, 2011, 5, 2880–2894 CrossRef CAS PubMed.
- L.-B. Xing, S. Yu, X.-J. Wang, G.-X. Wang, B. Chen, L.-P. Zhang, C.-H. Tung and L.-Z. Wu, Chem. Commun., 2012, 48, 10886–10888 RSC.
- L. Gao, B. Zheng, Y. Yao and F. Huang, Soft Matter, 2013, 9, 7314–7319 RSC.
- F. Wudl, D. Wobschall and E. J. Hufnagel, J. Am. Chem. Soc., 1972, 94, 670–672 CrossRef CAS.
- J. Puigmartí-Luis, D. Schaffhauser, B. R. Burg and P. S. Dittrich, Adv. Mater., 2010, 22, 2255–2259 CrossRef PubMed.
- Y. Geng, X.-J. Wang, B. Chen, H. Xue, Y.-P. Zhao, S. Lee, C.-H. Tung and L.-Z. Wu, Chem. – Eur. J., 2009, 15, 5124–5129 CrossRef CAS PubMed.
- D. Canevet, M. Salle, G. Zhang, D. Zhang and D. Zhu, Chem. Commun., 2009, 2245–2269 RSC.
- J. L. Segura and N. Martín, Angew. Chem., Int. Ed., 2001, 40, 1372–1409 CrossRef CAS.
- K.-D. Zhang, G.-T. Wang, X. Zhao, X.-K. Jiang and Z.-T. Li, Langmuir, 2010, 26, 6878–6882 CrossRef CAS PubMed.
- J. Bigot, B. Charleux, G. Cooke, F. O. Delattre, D. Fournier, J. L. Lyskawa, L. N. Sambe, F. O. Stoffelbach and P. Woisel, J. Am. Chem. Soc., 2010, 132, 10796–10801 CrossRef CAS PubMed.
- X.-J. Wang, L.-B. Xing, F. Wang, G.-X. Wang, B. Chen, C.-H. Tung and L.-Z. Wu, Langmuir, 2011, 27, 8665–8671 CrossRef CAS PubMed.
- T. Shimizu, M. Masuda and H. Minamikawa, Chem. Rev., 2005, 105, 1401–1443 CrossRef CAS PubMed.
- X. Zhang, Z. Chen and F. Wuerthner, J. Am. Chem. Soc., 2007, 129, 4886–4887 CrossRef CAS PubMed.
- C. Wang, D. Q. Zhang and D. B. Zhu, J. Am. Chem. Soc., 2005, 127, 16372–16373 CrossRef CAS PubMed.
- T. Kitahara, M. Shirakawa, S. Kawano, U. Beginn, N. Fujita and S. Shinkai, J. Am. Chem. Soc., 2005, 127, 14980–14981 CrossRef CAS PubMed.
- H. Enozawa, M. Hasegawa, D. Takamatsu, K. Fukui and M. Iyoda, Org. Lett., 2006, 8, 1917–1920 CrossRef CAS PubMed.
- M. Hasegawa, H. Enozawa, Y. Kawabata and M. Iyoda, J. Am. Chem. Soc., 2007, 129, 3072–3073 CrossRef CAS PubMed.
- J. Luis Lopez, E. M. Perez, P. M. Viruela, R. Viruela, E. Orti and N. Martin, Org. Lett., 2009, 11, 4524–4527 CrossRef PubMed.
- X.-J. Wang, L.-B. Xing, W.-N. Cao, X.-B. Li, B. Chen, C.-H. Tung and L.-Z. Wu, Langmuir, 2011, 27, 774–781 CrossRef PubMed.
- U. Kauscher, K. Bartels, I. Schrader, V. A. Azov and B. J. Ravoo, J. Mater. Chem. B, 2015, 3, 475–480 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, and other supporting data. See DOI: 10.1039/c5ob02214b |
| This journal is © The Royal Society of Chemistry 2016 |