Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Reduced graphene oxide nanosheets decorated with Au–Pd bimetallic alloy nanoparticles towards efficient photocatalytic degradation of phenolic compounds in water
Gitashree
Darabdhara
ab,
Purna K.
Boruah
ab,
Priyakshree
Borthakur
ab,
Najrul
Hussain
ab,
Manash R.
Das
*ab,
Tansir
Ahamad
c,
Saad M.
Alshehri
*c,
Victor
Malgras
d,
Kevin C.-W.
Wu
*e and
Yusuke
Yamauchi
d
aMaterials Science Division, CSIR-North East Institute of Science and Technology, Jorhat 785006, Assam, India. E-mail: mnshrdas@yahoo.com
bAcademy of Scientific and Innovative Research, India
cDepartment of Chemistry, College of Science, King Saud University, Riyadh 11451, Saudi Arabia. E-mail: alshehri@ksu.edu.sa
dNational Institute for Materials Science (NIMS), 1-1 Namiki, Tsukuba, Ibaraki 305-0044, Japan
eDepartment of Chemical Engineering, National Taiwan University, No. 1, Sec. 4, Roosevelt Road, Taipei 10617, Taiwan. E-mail: kevinwu@ntu.edu.tw
First published on 7th November 2016
Abstract
Correction for ‘Reduced graphene oxide nanosheets decorated with Au–Pd bimetallic alloy nanoparticles towards efficient photocatalytic degradation of phenolic compounds in water’ by Gitashree Darabdhara, et al., Nanoscale, 2016, 8, 8276–8287.
The authors would like to draw the attention of the readers to some corrected aspects of the published article:
On page 8281, section 3.2: the pH value in the sentence beginning “About 94.4% phenol degradation…” should be corrected to 7.
On page 8282, section 3.2.1: the five different catalyst loadings should be corrected to 0.1, 0.3, 0.5, 0.8 and 1 g L−1.
On page 8282, section 3.2.2: the four different concentrations of the phenolic components should be corrected to 0.2, 0.3, 0.5 and 0.8 mM.
On page 8282, section 3.2.2: the degradation efficiencies of the phenolic compounds at various initial concentrations for a fixed amount of catalyst and pH are actually displayed in Fig. S4, not Fig. S3.
On page 8283, section 3.2.4: the two sentences “The electron donating –NO2 group….reduces it” should be corrected to “The electron donating group on the aromatic ring possessing an (+I) inductive effect (i.e., an electron donating effect) increases the negative charge on the aromatic ring of phenol. On the other hand, the presence of a –Cl and –NO2 group having an (−I) inductive effect (i.e., electron accepting effect) reduces it”.
On page 8284, section 3.2.6: all instances of H2O˙ should be corrected to 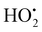 in the discussion of the mechanism.
in the discussion of the mechanism.
On page 8284, section 3.2.6: eqn (2) and (3) should be corrected to:
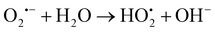 | (2) |
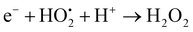 | (3) |
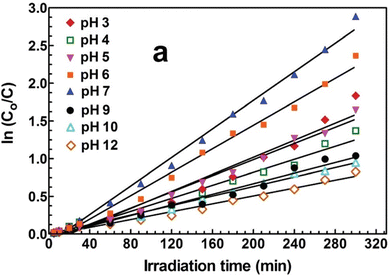
On page 8284: Fig. 12(a), with corrected legend, should be:
15. A. Fujishima and K. Honda, Nature, 1972, 238, 37.
37. P. Zhu, A. S. Nair, P. Shengjie, Y. Shengyuan and S. Ramakrishna, ACS Appl. Mater. Interfaces, 2012, 4, 581.
63. O. Akhavan, E. Ghaderi and A. Esfandiar, J. Phys. Chem. B, 2011, 115, 6279.
67. M. Aslam, I. M. I. Ismail, S. Chandrasekaran and A. Hameed, J. Hazard. Mater., 2014, 276, 120.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2016 |