Electro-triggering and electrochemical monitoring of dopamine exocytosis from a single cell by using ultrathin electrodes based on Au nanowires†
Mijeong
Kang‡
a,
Seung Min
Yoo‡
b,
Raekeun
Gwak
a,
Gayoung
Eom
a,
Jihwan
Kim
a,
Sang Yup
Lee
*b and
Bongsoo
Kim
*a
aDepartment of Chemistry, KAIST, 291 Daehak-ro, Yuseong-gu, Daejeon, 34141, Republic of Korea. E-mail: bongsoo@kaist.ac.kr
bDepartment of Chemical and Biomolecular Engineering (BK21 plus Program), KAIST, 291 Daehak-ro, Yuseong-gu, Daejeon, 34141, Republic of Korea. E-mail: leesy@kaist.ac.kr
First published on 20th November 2015
Abstract
A sophisticated set of an Au nanowire (NW) stimulator–Au NW detector system is developed for electrical cell stimulation and electrochemical analysis of subsequent exocytosis with very high spatial resolution. Dopamine release from a rat pheochromocytoma cell is more stimulated by a more negative voltage pulse. This system could help to improve the therapeutic efficacy of electrotherapies by providing valuable information on their healing mechanism.
Cell communication by delivering signaling molecules is essential for biological viability.1,2 Malfunction in such communication may cause serious diseases including Parkinson's disease, Alzheimer's disease, cancer, and schizophrenia. While electrotherapies such as deep brain stimulation have been effectively employed to cure such diseases,3,4 their healing mechanisms have not been fully elucidated yet.5–7 Detailed single-cell level studies of the biological responses to electrical stimulation could help us to understand such mechanisms and improve the therapeutic efficacy.8–12
The spatial precision of electrical cell stimulation has been improved by the technical advancement in miniaturizing the electrodes. At an early stage, an electric field was applied between two macroscopic electrodes, stimulating a population of cells lying between them. Reducing the size of electrodes down to a nm-size can enhance the precision of the stimulation point and thus could optimize the efficiency of the stimulating electrodes.13 Here, we employed ultrathin electrodes based on an Au nanowire (NW) having a diameter of 100–200 nm to stimulate a rat pheochromocytoma (PC12) cell, generally used as a model in neuroscience,14,15 with voltage pulses, and observed the subsequent release of signaling molecules (dopamine) in real time by a detector composed of another Au NW electrode (Scheme 1). Precise control of the position of Au NW electrodes in the nm scale helped us to find that the active release zones are sparsely distributed over the cell membrane. The ultrathin electrode based on Au NW having such high spatial precision could allow us to figure out in detail how each synapse of a single neuron that possesses many heterogeneous synapses responds differently to electrical stimulation by detecting the dopamine exocytosis from each synapse.16–19
With this well-controlled experiment, we observed that the PC12 cell released dopamine more frequently when the applied voltage was set more negatively. Furthermore, we observed that such release was inhibited by Cd2+, a Ca2+ channel blocker. This indicates that the opening of Ca2+ channels by electrical stimulation triggers the dopamine exocytosis. The sophisticated set of an Au NW stimulator–Au NW detector system for electrical cell stimulation and the detection of subsequent cellular responses would help to reveal the working mechanism of electrotherapies, widely employed to treat diverse diseases in cardiac, muscular, urinary as well as neural systems, leading to improved therapeutic efficacy and reduced side effects.
Ultrathin nanoscale stimulator and detector made of Au NWs were synthesized by using a vapor transport method as we previously reported (see details in the ESI†).20 Although Au NWs are extremely thin with a diameter of 100–200 nm, their strong light scattering allows for optical observation. An Au NW electrode was fabricated by picking up one of the Au NWs vertically grown on a sapphire substrate with a macroscopic tungsten tip and completely insulating the tungsten part of the combined nanowire–tip with a UV-curable polymer and nail varnish (see details in the ESI†).21,22 The scanning electron microscopy (SEM) image of the Au NW attached onto a tungsten tip is shown in Fig. 1a, which was taken before insulating the tungsten tip to avoid surface charging.
In this Au NW electrode-based platform, each electrical stimulation or the electrochemical recording part was connected to its own independently operating electrochemical workstation. The stimulating Au NW electrode (the right one in Fig. 1b–d) approaches a PC12 cell within a few μm by using a micromanipulator under optical monitoring. A saturated calomel electrode (SCE) was put in the same extracellular solution as a reference electrode to constitute a 2-electrode system. We observed that the gap between the cell and the stimulating Au NW electrode did not significantly affect the electrically triggered exocytosis in the range from ∼20 μm to ∼5 μm (see the ESI†). Generally, a 3-electrode system is preferred since it controls the potential application more accurately; therefore, the recording part was made of a 3-electrode system. When a 3-electrode configuration was employed for the stimulation, however, the amperometric trace measured by a recording Au NW electrode was severely interfered. This interference was not observed when a 2-electrode configuration was employed for stimulation. We also confirmed that the 2-electrode configuration did not significantly affect the electrochemical behavior of an Au NW electrode (see the ESI, Fig. S1†). Electrical stimulation was performed by applying voltage pulses of −0.3, −0.1, 0.1, and 0.3 V with a 1 s pulse duration to the Au NW electrode, respectively. The range of pulse voltages was set not to exceed the onset of preoxidation of Au (ca. 0.35 V) and the onset of an oxygen reduction reaction (ca. −0.3 V, see Fig. S2†). Another Au NW electrode (the left one in Fig. 1b–d), working as an electrochemical recorder, approaches the same cell more closely within 1 μm (Fig. 1b–c): as closely as possible to minimize the quantity of the released dopamine that significantly diffuses out and, consequently, is not detected by the recording Au NW electrode.23 This recording Au NW electrode was used as a working electrode. The Ag/AgCl electrode and the Pt wire were placed in the same solution as a reference and a counter electrode, respectively, to constitute a 3-electrode system (see the ESI, Fig. S1†). The monitoring of dopamine exocytosis was achieved by amperometry, measuring the current as a function of time. Since most part of the PC12 cell surface is inactive for dopamine exocytosis and only several localized zones, active release zones, are in charge of dopamine exocytosis,23 we sought out such zones by finely maneuvering the recording Au NW electrode (Fig. 1c and d).
Prior to triggering and monitoring dopamine exocytosis, we examined the electrochemical characteristics and the dopamine detection ability of Au NW electrodes. A cyclic voltammogram was obtained from an Au NW electrode in a phosphate buffer (pH 7.4) solution under a 3-electrode configuration, in which single peaks for Au oxidation and reduction were observed at ∼0.9 and ∼0.4 V, respectively (Fig. 2a). This indicates that the Au NW is enclosed by a well-defined single-crystalline surface and in good contact with the tungsten tip. Tungsten oxidation, which has strong oxidation peaks at 0.2 and 0.6 V during the anodic scan and 0.4 and 0.2 V during the cathodic scan (see Fig. S3†), was not observed, guaranteeing the complete insulation of the tungsten tip. Therefore, an undesirable reaction or noise from the tungsten surface was avoided.
Next, we examined whether Au NW electrodes were able to quantitatively detect dopamine. Dopamine oxidation was measured with increasing the dopamine concentration from 0 to 250 μM (Fig. 2b). The concentration range was set around 190 μM from the following approximation; the dopamine concentration that an Au NW electrode would detect right after exocytosis was roughly calculated to be ∼190 μM by simply considering that the electrode was positioned within the cubical space (volume: 1 μm3) right in front of the release zone and all the released dopamine (average quantity at a single exocytotic event: ∼190 zeptomole24) was in that space before significantly diffusing away from the release zone. The potential was scanned up to 0.7 V to avoid the Au oxidation reaction that occurs at over 0.75 V. Dopamine started to be oxidized from 0.1 V and the oxidation current gradually increased up to 0.7 V (Fig. 2b), at which the current intensity linearly increased with increasing the dopamine concentration (Fig. 2c). These results indicate that quantitative measurement of dopamine is possible with these Au NW electrodes (see the ESI†). For all of the following amperometric monitoring of dopamine exocytosis, the recording Au NW electrodes were polarized at 0.7 V so that the dopamine arriving at the Au NW electrode could be immediately oxidized resulting in current spikes.
For the study of cellular exocytosis, it is practically important to accurately find the active release zones.25 We applied voltage pulses to a PC12 cell using a stimulating Au NW electrode and observed dopamine release at several different sites of the cell with a recording Au NW electrode. We observed that while most of the monitored sites were inactive to dopamine exocytosis, the activity was found at some zones,23 where we analyzed the effects of electrical stimulation.
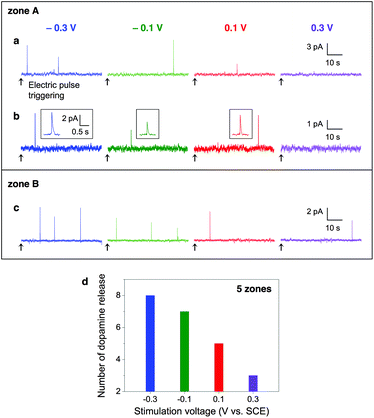
To investigate how the voltage of electric pulses affects dopamine exocytosis, we stimulated cells with different voltage pulses at an interval of 60 s. When we applied −0.3, −0.1, 0.1 and 0.3 V pulses in this order to a cell, an active release zone (zone A, Fig. 3a) had 2, 1, 1, and 0 current spikes, respectively. This means that the PC12 cell tended to release dopamine more frequently as the pulse voltage was set more negative. It might be, however, simply due to the accumulation of electrical stimulations or the depletion of intracellular dopamine as the time passed. To clarify the effect of the pulse voltage, pulses at four different voltages were applied to the same cell oppositely to the above (i.e., 0.3, 0.1, −0.1 and −0.3 V) and to another cell having an active release zone B in the same order as above (i.e., −0.3, −0.1, 0.1 and 0.3 V). In both cases, more negative pulses induced more dopamine signals (Fig. 3b and c). After we applied voltage pulses having the height from −0.3 to 0.3 V or from 0.3 to −0.3 V to five cells, we analyzed the total number of current peaks appeared. The most peaks appeared at −0.3 V (n = 8) and the least peaks appeared at 0.3 V (n = 3) (Fig. 3d). Taken together, these results indicate that the cells are likely to release dopamine more frequently by the application of more negative pulses regardless of the sequence of applied pulses, the accumulation of stimulation, the time-dependent depletion of intracellular dopamine, or cell-to-cell variation.
For the detailed information of the amperometric spikes measured by Au NW electrodes, we calculated their average height, area (charge) and half-width from the spikes shown in Fig. 3, which are 4.46 × 10−12 A, 2.71 × 10−13 C, and 46 ms, respectively. These statistical information showed no significant tendency as a function of the stimulating voltage (see ESI†). The spikes have similar shape as shown in the insets of Fig. 3b. From the charge value (Q), the number of released dopamine molecules (N) per exocytosis event was calculated to be 842![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 using Faraday's law (Q = nNF, where n is the number of electrons involved in the electrochemical reaction (2 for dopamine oxidation) and F is the Faraday's constant (96
800 using Faraday's law (Q = nNF, where n is the number of electrons involved in the electrochemical reaction (2 for dopamine oxidation) and F is the Faraday's constant (96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485 C mol−1)). This is in the same order of the reported values such as 114
485 C mol−1)). This is in the same order of the reported values such as 114![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300,24 but higher than them to some extent. Compared to the reported amperometric spikes measured by carbon electrodes, the spikes we recorded have a similar peak height and larger half-width, resulting in the higher charge value. This might be due to the different material characteristics of the electrode as well as the different instrumental setup.
300,24 but higher than them to some extent. Compared to the reported amperometric spikes measured by carbon electrodes, the spikes we recorded have a similar peak height and larger half-width, resulting in the higher charge value. This might be due to the different material characteristics of the electrode as well as the different instrumental setup.
The electrical stimulation via the Au NW electrode seems to trigger dopamine exocytosis by opening the Ca2+ channels. For exocytosis to occur, the concentration of intracellular Ca2+ should be elevated, which can be caused by the release of Ca2+ from intracellular Ca2+ stores or the influx of extracellular Ca2+. Extracellular Ca2+ enters the cell when the Ca2+ channels located at the cell membrane open. We examined if the electrical stimulation via Au NW electrodes led to opening of the Ca2+ channels of the PC12 cells. First, we found an active release zone (zone C) where dopamine was released by each of the three −0.3 V pulses (Fig. 4a). Thereafter, Cd2+, a Ca2+ channel blocker, was added to the extracellular solution26 and the same cell was stimulated by a −0.3 V pulse. The dopamine signal was no more observed at zone C (Fig. 4b). The same result was obtained from three independent experiments, suggesting that dopamine exocytosis by electrical stimulation through Au NW electrodes is related to the opening of Ca2+ channels (Scheme 1). Considering that (1) the Ca2+ channels are opened by depolarization of the cell membrane, (2) when an external electrical field is applied to a cell, the side facing the negative electrode is transiently depolarized while the side facing the positive electrode is hyperpolarized,27 (3) such effects of an external electrical field on the membrane potential are reinforced as the distance between the electrode and the cell is reduced,28 and (4) the stimulating Au NW electrode is positioned more closely to the cell than the counter electrode, it is conceived that the portion of depolarization over hyperpolarization is increased when the pulse voltage applied to the stimulating Au NW electrode becomes more negative, which would result in more dopamine exocytosis.
Conclusions
In conclusion, we stimulated a single PC12 cell with voltage pulses and electrochemically monitored the subsequent dopamine exocytosis using a set of Au NW electrode–Au NW electrode. The ultrathin electrode based on Au NW with diameters of 100–200 nm can electrically stimulate a single cell and at the same time can monitor dopamine exocytosis at the localized regions of the cell surface where dopamine is actively released, enabling precise studies on cell communication. We observed that dopamine release was promoted as the pulse voltage was negatively tuned from 0.3 V to −0.3 V and such exocytosis is attributed to the opening of the Ca2+ channel by electrical stimulation.Au NW electrodes would establish new strategies for investigating the effects of electrical stimulation on diverse cellular phenomena as well as exocytosis. We expect that Au NW electrodes could contribute to improving the therapeutic efficacy of electrotherapies used for treating paralyzed muscles, cardiac arrhythmia or overactive bladder by providing more detailed and invaluable information on their working mechanism.
Acknowledgements
The work of B. K. was supported by the NRF of Korea funded by MSIP (NRF-2012M3A2A1051686, NRF-2013R1A2A2A01069073). The work of S. Y. L. was supported by the Intelligent Synthetic Biology Center through the Global Frontier Project (2011-0031963) of the Ministry of Science, ICT & Future Planning through the NRF of Korea.Notes and references
- S. Nahavandi, S.-Y. Tang, S. Baratchi, R. Soffe, S. Nahavandi, K. Kalantar-zadeh, A. Mitchell and K. Khoshmanesh, Small, 2014, 10, 4810 CrossRef CAS PubMed.
- C. Amatore, S. Arbault, M. Guille and F. Lemaitre, Chem. Rev., 2008, 108, 2585 CrossRef CAS PubMed.
- G. D. O'Clock, Electrotherapeutic Devices: Principles, Design, and Applications, Artech House, USA, 2007 Search PubMed.
- G. G. Wallace, M. J. Higgins, S. E. Moulton and C. Wang, Nanoscale, 2012, 4, 4327 RSC.
- M. L. Kringelbach, N. Jenkinson, S. L. F. Owen and T. Z. Aziz, Nat. Rev. Neurosci., 2007, 8, 623 CrossRef CAS PubMed.
- J. S. Perlmutter and J. W. Mink, Annu. Rev. Neurosci., 2006, 29, 229 CrossRef CAS PubMed.
- A. Huttenlocher and A. R. Horwitz, N. Engl. J. Med., 2007, 356, 303 CrossRef CAS PubMed.
- D. Tsai, S. Chen, D. A. Protti, J. W. Morley, G. J. Suaning and N. H. Lovell, PLoS One, 2012, 7, e53357 CAS.
- C. Schubert, Nature, 2011, 480, 133 CrossRef CAS PubMed.
- D. Wang and S. Bodovitz, Trends Biotechnol., 2010, 28, 281 CrossRef CAS PubMed.
- R. A. Seger, P. Actis, C. Penfold, M. Maalouf, B. Vilozny and N. Pourmand, Nanoscale, 2012, 4, 5843 RSC.
- T. Huang, L. M. Browning and X. H. N. Xu, Nanoscale, 2012, 4, 2797 RSC.
- G. M. Dittami and R. D. Rabbitt, Lab Chip, 2010, 10, 30 RSC.
- R. H. S. Westerink and A. G. Ewing, Acta Physiol., 2008, 192, 273 CrossRef CAS PubMed.
- T. D. B. Nguyen-Vu, H. Chen, A. M. Cassell, R. Andrews, M. Meyyappan and J. Li, Small, 2006, 2, 89 CrossRef CAS PubMed.
- W. Wang, S.-H. Zhang, L.-M. Li, Z.-L. Wang, J.-K. Cheng and W.-H. Huang, Anal. Bioanal. Chem., 2009, 394, 17 CrossRef CAS PubMed.
- Y.-T. Li, S.-H. Zhang, L. Wang, R.-R. Xiao, W. Liu, X.-W. Zhang, Z. Zhou, C. Amatore and W.-H. Huang, Angew. Chem., Int. Ed., 2014, 53, 12456 CAS.
- A. M. Craig and H. Boudin, Nat. Neurosci., 2001, 4, 569 CrossRef CAS PubMed.
- T. Li and W. Hu, Nanoscale, 2011, 3, 166 RSC.
- Y. Yoo, K. Seo, S. Han, K. S. K. Varadwaj, H. Y. Kim, J. H. Ryu, H. M. Lee, J. P. Ahn, H. Ihee and B. Kim, Nano Lett., 2010, 10, 432 CrossRef CAS PubMed.
- S. M. Yoo, M. Kang, T. Kang, D. Kim, S. Y. Lee and B. Kim, Nano Lett., 2013, 13, 2431 CrossRef CAS PubMed.
- M. Kang, S. Jung, H. Zhang, T. Kang, H. Kang, Y. Yoo, J.-P. Hong, J.-P. Ahn, J. Kwak, D. Jeon, N. A. Kotov and B. Kim, ACS Nano, 2014, 8, 8182 CrossRef CAS PubMed.
- W.-Z. Wu, W.-H. Huang, W. Wang, Z.-L. Wang, J.-K. Cheng, T. Xu, R.-Y. Zhang, Y. Chen and J. Liu, J. Am. Chem. Soc., 2005, 127, 8914 CrossRef CAS PubMed.
- T. K. Chen, G. Luo and A. G. Ewing, Anal. Chem., 1994, 66, 3031 CrossRef CAS PubMed.
- E. D. Gundelfinger, M. M. Kessels and B. Qualmann, Nat. Rev. Mol. Cell Biol., 2003, 4, 127 CrossRef CAS PubMed.
- A. P. Neal, Y. Yuan and W. D. Atchison, Toxicol. Sci., 2010, 116, 604 CrossRef CAS PubMed.
- K. W. Horch and G. S. Dhillon, Neuroprosthetics: Theory and Practice, World Scientific, Singapore, 2004 Search PubMed.
- T. Tomita, J. Physiol., 1966, 183, 450 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5nr06021d |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2016 |