A novel 2D infinite M3L2 cage-based Cd(II) microporous coordination polymer with a tripodal carboxylic acid ligand and solvent-dependent luminescence properties†
Jian
Su‡
ab,
Liudi
Yao‡
ac,
Jun
Zhang
*ab,
Shizhen
Yuan
a,
Fazhi
Xie
a,
Yi
Ding
a,
Meng
Zhao
b,
Shichao
Wang
b,
Hao
Li
b,
Shengyi
Zhang
b,
Jieying
Wu
b and
Yupeng
Tian
*b
aKey Laboratory of Functional Molecule Design and Interface Process, Anhui Jianzhu University, Hefei 230601, P. R. China. E-mail: zhangjun@ahjzu.edu.cn
bDepartment of Chemistry, Key Laboratory of Functional Inorganic Materials Chemistry of Anhui Province, Anhui University, Hefei 230039, P. R. China. E-mail: yptian@ahu.edu.cn
cImperial College London, Department of Medicine, St Mary's Campus, London W21PG, UK
First published on 17th November 2015
Abstract
A novel M3L2 cage-based microporous coordination polymer assembled from the six-coordinated Cd(II) ion and a flexible tricarboxylic ligand has been designed and synthesized. The solvent-dependent luminescence properties revealed that the coordination polymer has an obvious, enhanced luminescence in the solvents CH2Cl2 and CHCl3.
Considering that cage-based compounds, especially metal organic complexes, can serve as very effective encapsulation devices for functional guest molecules, their self-assembly and applications have been well studied and summarized.1 M3L2-type cage-like complexes, as the smallest metal organic cages, are commonly synthesized by using a tripodal pyridine-containing or imidazole-containing ligand with low-coordinated metal ions such as Ag+, Pt2+ and Pd2+.2 Interestingly, Sun's group has firstly reported the single-crystal structure of a discrete M3L2 cage-like complex assembled with a tetrahedral zinc(II) center.3 Later, another discrete M3L2 cage was obtained by the assembly of Cu(II) salt and a flexible tri(oxamate)-base ligand.4 On the other hand, a M3L2 cage containing a silver(I) coordination polymer chain linked by SO42− through a non-coordination bond has nearly been assembled.5 However, there are no related reports on the use of a carboxyl-containing ligand even with upper 4-coordinated metal ions, such as Zn2+ and Cd2+, to assemble the 1D, 2D or 3D structures by the stronger coordination bond. This may be due to the various coordinated modes of the carboxyl and the steric hindrance of the highly coordinated metal ions. It is well known that the widely used polycarboxylic ligands play an important role in the construction of various metal organic complexes. So, making full use of polycarboxylic ligands to construct special structural and functional metal organic complexes is very urgent.
The self-assembly of microporous coordination polymers (MCPs) is a highly topical area of current research that has yielded many fascinating architectures and revealed enormous potential applications in catalysis,6 the capture and degradation of toxic gases,7 NLO optics,8 biomedical imaging9 and molecular sensors.10 Among the fruitful products of functional MCPs reported to date, luminescent MCPs can be viewed as the most promising class of materials due to their potential applications. In fact, recently, some luminescent MCPs have been successfully used in the sensing of solvents such as DMF, acetone and water.11 Considering the wide use of MCPs, the study of solvent-dependent luminescence properties in common solvents is necessary.
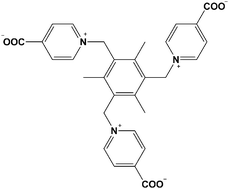
Herein, we used a bowl-shaped tripodal carboxyl acid ligand, 1,1′,1′′-((2,4,6-trimethylbenzene-1,3,5-triyl)tris(methylene))tris (pyridine-1-ium-4-carboxylate) (named L, as shown in Scheme 1),12 and cadmium ion, which has a distorted octahedral configuration, to construct a M3L2 cage expanded by 1,3,5-benzenetricarboxylic acid (H3BTC) to form a 2D infinite M3L2 cage-based framework [(BTC)2/3(L)2/3Cd]·(H2O)1.5·(DMF)0.5 (compound 1).§ The 3D structure of compound 1 is formed by the hydrogen bonds and π–π stacking between the 2D planer structures. To the best of our knowledge, this is the first MCP based on a M3L2 cage constructed by Cd(II) ions and with flexible tripodal carboxyl-containing ligands. Furthermore, analysis of the solvent-dependent luminescence properties revealed that compound 1 has an obvious, enhanced luminescence in the solvents CH2Cl2 and CHCl3, respectively.
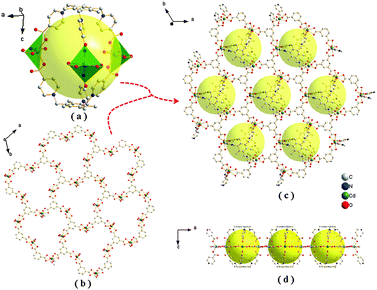
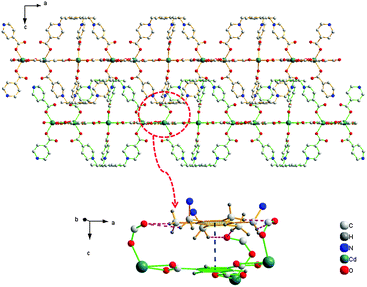
Single-crystal X-ray diffraction reveals that compound 1 crystallizes in the centrosymmetric trigonal space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c, which is associated with the point group D3d(-3m). The asymmetric unit is comprised by half Cd(II) atom, one-third L ligand, and one-third BTC3− (Fig. S8, ESI†). As shown in Fig. 1a, the Cd atom occupies the centre of a distorted octahedron defined by two carboxylates in bidentate chelating mode from two different BTC3− ligands and two carboxylates in monodentate coordination mode from two independent L ligands. The Cd–O bond lengths range from 2.27(2) to 2.49(7) Å, which are comparable with those reported in other Cd(II)–carboxylic complexes.13 The O–Cd–O angles range from 51.5(5) to 139.6(2)°, and the tetrahedron length ranges from 4.03(9) to 5.77(1) Å. In the viewpoint of the L ligand, three methylene-4-carboxylic acid groups of L are oriented towards the same side of the basic phenyl group, which results in a bowl-shaped configuration of L. Two L ligands, just facing opposite, are linked by three Cd(II) ions to form the interesting M3L2 cage (Fig. 1a). The diameter of the cage is about 12.6 Å. Then each M3L2 cage is linked by six BTC3− to form a 2D coplanar structure (Fig. 1c). In the side of BTC3−, each BTC3− is linked by three BTC3−, which are coordinated by Cd(II) ions to form a 2D plane (Fig. 1b). So, the resulting M3L2-based MCP is obtained due to the contribution of L ligand and BTC3−. Because of C–H⋯O hydrogen bonds and π–π stacking (Fig. 2), the 2D coplanar structures link with each other to form the 3D architecture.
c, which is associated with the point group D3d(-3m). The asymmetric unit is comprised by half Cd(II) atom, one-third L ligand, and one-third BTC3− (Fig. S8, ESI†). As shown in Fig. 1a, the Cd atom occupies the centre of a distorted octahedron defined by two carboxylates in bidentate chelating mode from two different BTC3− ligands and two carboxylates in monodentate coordination mode from two independent L ligands. The Cd–O bond lengths range from 2.27(2) to 2.49(7) Å, which are comparable with those reported in other Cd(II)–carboxylic complexes.13 The O–Cd–O angles range from 51.5(5) to 139.6(2)°, and the tetrahedron length ranges from 4.03(9) to 5.77(1) Å. In the viewpoint of the L ligand, three methylene-4-carboxylic acid groups of L are oriented towards the same side of the basic phenyl group, which results in a bowl-shaped configuration of L. Two L ligands, just facing opposite, are linked by three Cd(II) ions to form the interesting M3L2 cage (Fig. 1a). The diameter of the cage is about 12.6 Å. Then each M3L2 cage is linked by six BTC3− to form a 2D coplanar structure (Fig. 1c). In the side of BTC3−, each BTC3− is linked by three BTC3−, which are coordinated by Cd(II) ions to form a 2D plane (Fig. 1b). So, the resulting M3L2-based MCP is obtained due to the contribution of L ligand and BTC3−. Because of C–H⋯O hydrogen bonds and π–π stacking (Fig. 2), the 2D coplanar structures link with each other to form the 3D architecture.
The solid diffuse reflectance spectrum revealed that compound 1 was a wide-gap semiconductor with band gap of 3.45 eV (Fig. S5, ESI†). The photoluminescent properties of L ligand, H3BTC and compound 1 in solid state were investigated at ambient temperature (Fig. S6, ESI†). Upon excitation at 300 nm, peaks at 372 and 397 nm for H3BTC, 402 and 460 nm for L ligand, and 399 and 462 nm for compound 1 were found in the photoluminescence spectrum. Compared with the free L ligand, the emission band locations of compound 1 were comparable but with an increasing band height. This may be attributed to the coordination effect of the ligands to Cd ions, which increased the structural rigidity and reduced the nonradiative decay of the intraligand.12
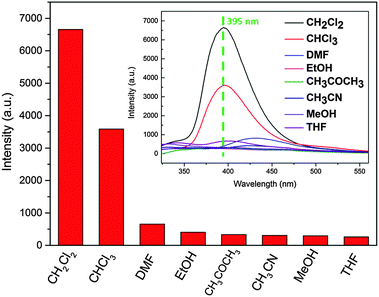
The most interesting feature of compound 1 is that its PL spectrum is largely dependent on the solvent molecules, particularly in the case of CH2Cl2 and CHCl3. As shown in Fig. 3, compared with DMF, ethanol, acetone, acetonitrile, methanol and THF, the intensities of compound 1 suspended in CH2Cl2 and CHCl3 were greatly enhanced. The other solvents did not exhibit changes at 395 nm. In order to give a possible fluorescence enhancement mechanism, 1H-NMR and TGA experiments were conducted on the solvent-exchanged samples. The results of 1H-NMR revealed that both CH2Cl2 and CHCl3 cannot exchange with the guest molecules in the framework of compound 1 (Fig. S7, ESI†). The result of TGA experiments further proved no exchange between the CH2Cl2, CHCl3 and guest molecules (Fig. S3, ESI†). So, the probable reason may be the solvent-dependent surface-enhanced fluorescence, which has been studied in capped Au or Ag metallic nanostructures, mono-layer organic structures, and quantum dots.14 Although some fluorescent MOFs relying on guest molecules have been observed previously,11 most examples are in the solvents DMF, acetone and water through solvent exchange. Here, we have presented a rare example of obvious surface-enhanced luminescence in the CH2Cl2 and CHCl3 solvents. To the best of our knowledge, such halomethane solvents for fluorescent MOFs have not previously been reported.
In summary, we have designed and synthesized a 2D infinite M3L2 cage-based MCP comprised by six-coordinated Cd(II) ion and a flexible tricarboxylic ligand. The solvent-dependent luminescence properties revealed that compound 1 has an obvious, enhanced luminescence in the CH2Cl2 and CHCl3 solvent. The results could immensely expand the design and synthesis of special cage-based MCPs assembled with well-designed ligand and medium coordinated metal ions, and hence push the application of cage-based MCPs in the functional materials field by encapsulation with functional guest molecules. The guest-functionalized MCPs will have wide applications in nonlinear optics, biological imaging and drug delivery that depend on the function of the guest molecules. Furthermore, some potential halomethane sensors may be found in the field of MCPs. Further work for preparing new, stable cage-based MCPs functionalized with nonlinear optical molecules is in progress.
This work was supported by the National Natural Science Foundation of China (grant no. 21201005, 201201004, 21271004, 21275006, 21273008 and 51372003), the Natural Science Foundation of Anhui Province (grant no. 1308085QB33 and 1208085MB22), and the China Postdoctoral Science Foundation Funded Project (grant no. 2013M541810).
Notes and references
- (a) N. Hafezi, J. M. Holcroft, K. J. Hartlieb, E. J. Dale, N. A. Vermeulen, C. L. Stern, A. A. Sarjeant and J. F. Stoddart, Angew. Chem., Int. Ed., 2015, 54, 456 CAS; (b) G. Zhang and M. Mastalerz, Chem. Soc. Rev., 2014, 43, 1934 RSC; (c) P. J. Stang and B. Olenyuk, Acc. Chem. Res., 1997, 30, 502 CrossRef CAS; (d) S. R. Seidel and P. J. Stang, Acc. Chem. Res., 2002, 35, 972 CrossRef CAS PubMed; (e) R. Custelcean, Chem. Soc. Rev., 2014, 43, 1813 RSC; (f) L. Chen, Q. Chen, M. Wu, F. Jiang and M. Hong, Acc. Chem. Res., 2015, 48, 201 CrossRef CAS PubMed; (g) T. N. Parac, D. L. Caulder and K. N. Raymond, J. Am. Chem. Soc., 1998, 120, 8003 CrossRef CAS; (h) J. Roukala, J. Zhu, C. Giri, K. Rissanen, P. Lantto and V. V. Telkki, J. Am. Chem. Soc., 2015, 137, 2464 CrossRef CAS PubMed.
- (a) A. Ikeda, M. Yoshimura, H. Udzu, C. Fukuhara and S. Shinkai, J. Am. Chem. Soc., 1999, 121, 4296 CrossRef CAS; (b) M. Hong, Y. Zhao, W. Su, R. Cao, M. Fujita, Z. Zhou and A. S. C. Chan, Angew. Chem., Int. Ed., 2000, 39, 2468 CrossRef CAS; (c) X. Sun, D. W. Johnson, K. N. Raymond and E. H. Wong, Inorg. Chem., 2001, 40, 4504 CrossRef CAS PubMed; (d) U. Radhakrishnan, M. Schweiger and P. J. Stang, Org. Lett., 2001, 3, 3141 CrossRef CAS PubMed; (e) C. G. Claessens and T. Torres, J. Am. Chem. Soc., 2002, 124, 14522 CrossRef CAS PubMed; (f) S. Hiraoka, T. Yi, M. Shiro and M. Shionoya, J. Am. Chem. Soc., 2002, 124, 14510 CrossRef CAS PubMed; (g) C. G. Claessens, M. J. Vicente-Arana and T. Torres, Chem. Commun., 2008, 6378 RSC; (h) I. Sanchez-Molina, B. Grimm, R. M. Krick Calderon, C. G. Claessens, D. M. Guldi and T. Torres, J. Am. Chem. Soc., 2013, 135, 10503 CrossRef CAS PubMed; (i) T. K. Ronson and M. J. Hardie, CrystEngComm, 2008, 10, 1731 RSC; (j) F. L. Thorp-Greenwood, T. K. Ronson and M. J. Hardie, Chem. Sci., 2015, 6, 5779 RSC; (k) J. J. Henkelis, C. J. Carruthers, S. E. Chambers, R. Clowes, A. I. Cooper, J. Fisher and M. J. Hardie, J. Am. Chem. Soc., 2014, 136, 14393 CrossRef CAS PubMed; (l) M. Wang, V. Vajpayee, S. Shanmugaraju, Y. R. Zheng, Z. Zhao, H. Kim, P. S. Mukherjee, K. W. Chi and P. J. Stang, Inorg. Chem., 2011, 50, 1506 CrossRef CAS PubMed; (m) S. Tartaggia, O. De Lucchi, A. Gambaro, R. Zangrando, F. Fabris and A. Scarso, Chem. – Eur. J., 2013, 19, 5701 CrossRef CAS PubMed; (n) J. J. Henkelis, T. K. Ronson, L. P. Harding and M. J. Hardie, Chem. Commun., 2011, 47, 6560 RSC.
- H.-K. Liu, W.-Y. Sun, D.-J. Ma, W.-X. Tang and K.-B. Yu, Chem. Commun., 2000, 591 RSC.
- M. C. Dul, X. Ottenwaelder, E. Pardo, R. Lescouezec, Y. Journaux, L. M. Chamoreau, R. Ruiz-Garcia, J. Cano, M. Julve and F. Lloret, Inorg. Chem., 2009, 48, 5244 CrossRef CAS PubMed.
- H. K. Liu, X. Huang, T. Lu, X. Wang, W. Y. Sun and B. S. Kang, Dalton Trans., 2008, 3178 RSC.
- (a) J. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450 RSC; (b) L. Ma, C. Abney and W. Lin, Chem. Soc. Rev., 2009, 38, 1248 RSC; (c) T. Zhang and W. Lin, Chem. Soc. Rev., 2014, 43, 59823 Search PubMed.
- E. Barea, C. Montoro and J. A. Navarro, Chem. Soc. Rev., 2014, 43, 5419 RSC.
- (a) O. R. Evans and W. Lin, Acc. Chem. Res., 2002, 35, 511 CrossRef CAS PubMed; (b) C. Wang, T. Zhang and W. Lin, Chem. Rev., 2012, 112, 1084 CrossRef CAS PubMed.
- J. Della Rocca, D. Liu and W. Lin, Acc. Chem. Res., 2011, 44, 957 CrossRef CAS PubMed.
- (a) L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne and J. T. Hupp, Chem. Rev., 2012, 112, 1105 CrossRef CAS PubMed; (b) Z. Hu, B. J. Deibert and J. Li, Chem. Soc. Rev., 2014, 43, 5815 RSC.
- (a) B. Chen, Y. Yang, F. Zapata, G. Lin, G. Qian and E. B. Lobkovsky, Adv. Mater., 2007, 19, 1693 CrossRef CAS; (b) Z. Guo, H. Xu, S. Su, J. Cai, S. Dang, S. Xiang, G. Qian, H. Zhang, M. O'Keeffe and B. Chen, Chem. Commun., 2011, 47, 5551 RSC; (c) S. Liu, Z. Xiang, Z. Hu, X. Zheng and D. Cao, J. Mater. Chem., 2011, 21, 6649 RSC; (d) Z. Z. Lu, R. Zhang, Y. Z. Li, Z. J. Guo and H. G. Zheng, J. Am. Chem. Soc., 2011, 133, 4172 CrossRef CAS PubMed; (e) J. Cui, Z. Lu, Y. Li, Z. Guo and H. Zheng, Chem. Commun., 2012, 48, 7967 RSC; (f) Y. Li, S. Zhang and D. Song, Angew. Chem., Int. Ed., 2013, 52, 710 CrossRef CAS PubMed; (g) C. Hou, Y. L. Bai, X. Bao, L. Xu, R. G. Lin, S. Zhu, J. Fang and J. Xu, Dalton Trans., 2015, 44, 7770 RSC; (h) L. Qin, M. X. Zheng, Z. J. Guo, H. G. Zheng and Y. Xu, Chem. Commun., 2015, 51, 2447 RSC.
- G.-Q. Kong and C.-D. Wu, Cryst. Growth Des., 2010, 10, 4590 CAS.
- (a) Y.-P. Xia, Y.-W. Li, D.-C. Li, Q.-X. Yao, Y.-C. Du and J.-M. Dou, CrystEngComm, 2015, 17, 2459 RSC; (b) B. Bhattacharya, A. Layek, M. Mehboob Alam, D. K. Maity, S. Chakrabarti, P. P. Ray and D. Ghoshal, Chem. Commun., 2014, 50, 7858 RSC; (c) R. Haldar, S. Bonakala, P. Kanoo, S. Balasubramanian and T. K. Maji, CrystEngComm, 2014, 16, 4877 RSC.
- (a) J. Zhang, J. Malicka, I. Gryczynski and J. R. Lakowicz, J. Phys. Chem. B, 2005, 109, 7643 CrossRef CAS PubMed; (b) P. A. Antunes, C. J. L. Constantino, R. F. Aroca and J. Duff, Langmuir, 2001, 17, 2958 CrossRef CAS; (c) P. A. Antunes, C. J. L. Constantino, R. F. Aroca and J. Duff, Langmuir, 2001, 17, 2958 CrossRef CAS; (d) K. T. Shimizu, W. K. Woo, B. R. Fisher, H. J. Eisler and M. G. Bawendi, Phys. Rev. Lett., 2002, 89, 117401 CrossRef CAS PubMed; (e) E. Fort and S. Grésillon, J. Phys. D: Appl. Phys., 2008, 41, 013001 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic conditions, PXRD setup and patterns, CIF file for compound 1, TGA data, solid-state diffuse reflectance spectra, solid-state PL spectra, additional picture of compound 1. CCDC 1402379. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5nj02144h |
| ‡ These authors contributed equally. |
| § To the solution of L ligand (0.0340 g, 0.065 mmol) and H3BTC (0.0210 g, 0.100 mmol) in 4 mL DMF and 1 mL H2O was added the solution of Cd(NO3)2·4H2O (0.0462 g, 0.150 mmol). Then 1 drop of 6 mol L−1 HCl was added. The mixture was heated to 90 °C in a sealed vial for 72 h, then allowed to cool down to room temperature. The colorless block crystals (0.0174 g) of 1 were obtained by filtration and washed with DMF and CH2Cl2 three times, respectively. Yield was 60.42% (based on L ligand). Calcd for C27.5H26.5O10CdN2.5 (Mr = 664.4): C, 49.71; H, 4.02; N, 5.27%. Found: C, 49.90; H, 3.85; N, 5.41%. FT-IR (KBr pellet, cm−1): 3416 s, b, 3114 w, 3053 w, 1619 vs, 1576 vs, 1438 m, 1370 vs, 1250 w, 1206 w, 1161 w, 1129 w, 1104 w, 1043 w, 935 w, 876 w, 799 w, 770 m, 732 m, 696 w. |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2016 |