Molecular modeling and synthesis of some new 2-imino-4-thiazolidinone derivatives with promising TNF-α inhibitory activity†
Yakub
Ali
a,
Mohammad Sarwar
Alam
*a,
Hinna
Hamid
a,
Asif
Husain
b,
Abhijeet
Dhulap
c,
Firasat
Hussain
d,
Sameena
Bano
a and
Chetna
Kharbanda
a
aDepartment of Chemistry, Faculty of Science, JamiaHamdard (Hamdard University), New Delhi-110 062, India. E-mail: msalam@jamiahamdard.ac.in; msalam5555@gmail.com; Fax: +91 11 26059663; Tel: +91 11 26059688 (5555)
bDepartment of Pharmaceutical Chemistry, Faculty of Pharmacy, JamiaHamdard (Hamdard University), New Delhi-110 062, India
cCSIR Unit for Research and Development of Information Products, Pune-411038, India
dDepartment of Chemistry, Faculty of Science, Delhi University, New Delhi, India
First published on 26th November 2015
Abstract
A new series of thirty two 2-imino-4-thiazolidinone derivatives were synthesized, and the synthesized compounds were docked for in silico studies against the TNF-α target. The predicted results were confirmed through an in vitro TNF-α study which revealed that compounds 3f and 3g showed better TNF-α inhibition as compared to the standard drug indomethacin without causing any cytotoxicity. Fourteen compounds exhibiting significant in vitro TNF-α activity were further tested for in vivo anti-inflammatory activity by a carrageenan induced method. Compounds 3f and 3g showed better inhibition of inflammation in vivo as compared to the standard drug without causing any damage to the stomach. Furthermore, an immunohistochemical study showed that the compounds 3f and 3g exhibited better reduction in protein expression of TNF-α as compared to indomethacin. The in silico, in vitro and in vivo studies suggested that the compounds 3f and 3g might be considered as potent anti-inflammatory agents.
Introduction
Inflammation is a dynamic process with the tumor necrosis factor TNF-α playing a central role in pathogenesis. It is associated with many chronic diseases, including atherosclerosis, allergy, arthritis and auto-immune diseases. A large percentage of our population depends on NSAIDs for the treatment of inflammatory diseases. But the long term use of these NSAIDs leads to adverse side effects such as gastrointestinal ulceration, kidney damage and bleeding.1–3 Thiazolidinones belong to an important group of heterocyclic compounds containing sulfur and nitrogen in a five-membered ring. Thiazolidinones possess a wide range of biological activities such as antimicrobial,4,5 anti-HIV,6 anti-cancer,7 antitubercular,8,9 anti-histaminic,10 amoebicidal11 and anaesthetic12 activities. Recently, 4-thiazolidinone derivatives have been found to show promising anti-inflammatory and TNF-α antagonistic activities.13–16 For example, Geronikaki et al.17 identified thiazolidinones such as I exhibiting potent anti-inflammatory activity via dual COX/LOX inhibition. Similarly, 5-benzylidene-2-phenylthiazolinones (II) were found to possess promising anti-inflammatory potential through 5-LO inhibition.18 More recently, thiazolidinone derivatives such as III and IV were synthesised which exhibited significant anti-inflammatory activities via the inhibition of pro-inflammatory cytokines such as TNF-α and IL6.16 It was proposed that modulating the functions of pro-inflammatory cytokines (such as TNF-α) involved in systemic inflammation can provide a target for controlling inflammatory diseases.16 On the other hand, structurally related furan-containing thiazolidinediones19 (V) and rhodanines20 (VI) have also been found to possess significant activities against inflammation targets. Hence, considering the biological importance of 4-thiazolidinones, specifically towards the design of anti-inflammatory agents, and the importance of the furan scaffold, we decided to combine these two structural features and herein report the synthesis of novel 5-furanyl-2-imino-4-thiazolidinone derivatives as an anti-inflammatory TNF-α antagonistic agent with better gastric tolerance.Results and discussion
Chemistry
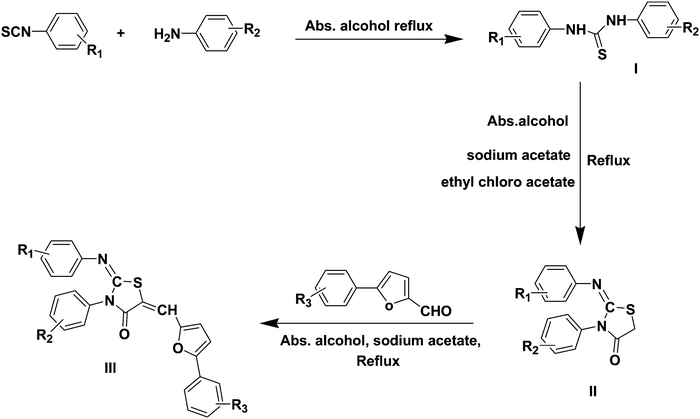
The synthetic route used to synthesize substituted-2-imino-4-thiazolidinone derivatives is outlined in Scheme 1. The key starting materials i.e. different aromatic thiosemicarbazides (I) were prepared in good yield by condensation of different aromatic amines with appropriate aromatic isothiocynates in the presence of absolute alcohol. Substituted-2-imino-4-thiazolidinones (II) were synthesized by the reaction of I in ethyl chloroacetate and sodium acetate in the presence of absolute alcohol. The final compounds were prepared by the reaction of (II) with different 5-(substituted-phenyl)-furan-2-carbaldehydes in the presence of absolute alcohol and sodium acetate. The structural confirmation of final compounds was done by 1H NMR, 13C NMR, IR and mass spectroscopy. The formation of final compounds was confirmed by the appearance of singlets for the olefinic hydrogen at δ 7.43 to δ 7.63 and by the disappearance of CH2 proton signals of intermediate II of the thiazolidinone ring at δ 4.2 to δ 4.6 in the 1H NMR spectra of the final compounds. The remaining protons appeared in the aromatic region as expected. Mass spectra of all the compounds showed [M]+, [M + 1]+ and [M + 2]+ peaks with reasonable intensity confirming the structures. Crystallographic data for the compound (3e) confirmed the Z-stereochemistry around both the exocyclic olefinic and imino linkages (Fig. 2). The list of substituents is shown in Table 1.| Compounds | R1 | R2 | R3 |
|---|---|---|---|
| 1a | p-OC2H5 | H | p-Cl |
| 1b | p-OC2H5 | p-F | p-Cl |
| 1c | p-OC2H5 | p-Cl | p-Cl |
| 1d | p-OC2H5 | p-Br | p-Cl |
| 1e | p-OC2H5 | p-OCH3 | p-Cl |
| 1f | p-OC2H5 | p-OC2H5 | p-Cl |
| 1g | p-OC2H5 | o-OCH3 | p-Cl |
| 1h | p-OC2H5 | o-CH3 | p-Cl |
| 2a | p-OC2H5 | H | o-Cl |
| 2b | p-OC2H5 | p-F | o-Cl |
| 2c | p-OC2H5 | p-Cl | o-Cl |
| 2d | p-OC2H5 | p-Br | o-Cl |
| 2e | p-OC2H5 | p-OCH3 | o-Cl |
| 2f | p-OC2H5 | p-OC2H5 | o-Cl |
| 2g | p-OC2H5 | o-OCH3 | o-Cl |
| 2h | p-OC2H5 | o-CH3 | o-Cl |
| 3a | p-OCH3 | H | o-Cl |
| 3b | p-OCH3 | p-F | o-Cl |
| 3c | p-OCH3 | p-Cl | o-Cl |
| 3d | p-OCH3 | p-Br | o-Cl |
| 3e | o-OCH3 | p-OCH3 | o-Cl |
| 3f | p-OCH3 | p-OC2H5 | o-Cl |
| 3g | p-OCH3 | o-OCH3 | o-Cl |
| 3h | p-OCH3 | o-CH3 | o-Cl |
| 4a | p-OCH3 | H | p-Cl |
| 4b | p-OCH3 | p-F | p-Cl |
| 4c | p-OCH3 | p-Cl | p-Cl |
| 4d | p-OCH3 | p-Br | p-Cl |
| 4e | p-OCH3 | p-OCH3 | p-Cl |
| 4f | p-OCH3 | p-OC2H5 | p-Cl |
| 4g | p-OCH3 | o-OCH3 | p-Cl |
| 4h | p-OCH3 | o-CH3 | p-Cl |
In silico molecular docking
The molecular docking study was performed by placing all the synthesized compounds inside the binding site of TNF-α (2AZ5 protein). All docking runs were carried out using Maestro (Schrödinger). Molecular docking studies provided insights of molecular binding modes of molecules inside the pocket of the TNF-α receptor. The crystallized structure of the 2AZ5 protein was chosen from protein data bank and used as the target for molecular docking studies with the reference ligand indomethacin.21–23 The synthesized compounds docked against the optimized grid showed good binding energies ranging from −30.17 to −49.26 kcal mol−1. Among all the synthesized molecules, some molecules showed better glide scores as compared to the reference ligand (indomethacin) and the other reference compound-V. The most promising molecules were 3f, 3g, 2f and 2h with the glide scores −6.27, −6.07, −6.06 and −6.05, respectively. The binding modes of compounds 3f, 3g, 2f, 2h and indomethacin with TNF-α are shown in Fig. S1 (ESI†). Compounds 3g, 2f and 2h were found to be aligned perfectly with the hydrophobic pocket and had π–π stacking interactions with TYR-A59, TYR-B59 and TYR-B119 of the target protein, respectively. However, the compound 3f was found to form a hydrogen bond with GLY-B121. On the other hand, indomethacin was found to be aligned perfectly with the hydrophobic pocket of the TNF-α protein showing a glide score of −5.02 and no other interactions were found. Compound-V which is structurally related to the synthesized derivatives was also used as a reference compound and docked in the protein binding site showing a π–π stacking interaction with TYR-A59 and H-bonding interactions with GLN-A161 and GLY-A121. The glide score and binding energies of all the synthesized compounds are shown in Table 2.| Ligands | Glide score | Glide energy | QPlog Po/w | QPlogS | PSA |
|---|---|---|---|---|---|
| 1a | −4.01 | −42.31 | 6.944 | −7.526 | 50.138 |
| 1b | −5.73 | −37.75 | 7.157 | −7.792 | 50.146 |
| 1c | −5.65 | −42.44 | 7.332 | −7.857 | 50.175 |
| 1d | −5.47 | −41.91 | 7.371 | −7.902 | 50.388 |
| 1e | −4.72 | −50.08 | 6.754 | −6.754 | 58.566 |
| 1f | −5.08 | −41.66 | 6.832 | −7.048 | 55.173 |
| 1g | −5.22 | −40.68 | 7.148 | −7.214 | 58.18 |
| 1h | −3.86 | −38.55 | 7.118 | −7.662 | 47.441 |
| 2a | −5.77 | −47.85 | 6.812 | −7.211 | 50.603 |
| 2b | −5.68 | −36.37 | 7.031 | −7.486 | 50.609 |
| 2c | −5.21 | −41.44 | 7.378 | −8.388 | 50.639 |
| 2d | −5.18 | −36.37 | 7.296 | −7.54 | 50.651 |
| 2e | −5.24 | −42.21 | 6.633 | −6.454 | 59.034 |
| 2f | −6.06 | −42.73 | 6.843 | −7.776 | 58.425 |
| 2g | −5.78 | −44.29 | 7.093 | −7.119 | 58.443 |
| 2h | −6.05 | −48.94 | 7.06 | −7.613 | 47.591 |
| 3a | −5.73 | −46.67 | 6.343 | −6.509 | 51.142 |
| 3b | −5.67 | −41.36 | 6.662 | −7.053 | 50.956 |
| 3c | −5.34 | −44.24 | 6.765 | −6.985 | 51.172 |
| 3d | −5.50 | −37.90 | 6.831 | −7.051 | 51.179 |
| 3e | −4.55 | −41.80 | 6.276 | −6.171 | 59.518 |
| 3f | −6.27 | −44.05 | 6.244 | −6.089 | 56.117 |
| 3g | −6.07 | −41.22 | 6.948 | −7.044 | 58.969 |
| 3h | −4.32 | −30.17 | 6.573 | −6.793 | 49.645 |
| 4a | −4.95 | −41.84 | 6.471 | −6.816 | 50.677 |
| 4b | −5.30 | −43.17 | 6.776 | −7.408 | 50.697 |
| 4c | −5.13 | −41.84 | 6.934 | −7.371 | 50.69 |
| 4d | −5.05 | −42.56 | 6.96 | −7.357 | 50.716 |
| 4e | −5.05 | −45.89 | 6.404 | −6.493 | 59.056 |
| 4f | −4.97 | −49.26 | 6.331 | −6.279 | 56.294 |
| 4g | −5.76 | −47.55 | 6.894 | −7.205 | 58.536 |
| 4h | −3.47 | −40.75 | 6.722 | −7.136 | 49.189 |
| Indomethacin | −5.02 | −33.09 | 4.28 | −5.31 | 84.32 |
| Reference-compound-V | −5.2 | −33.6 | 2.031 | −3.556 | 99.29 |
In vitro TNF-α assay
Twenty four compounds showing better glide scores than indomethacin in molecular docking studies were further subjected to in vitro TNF-α level studies in order to confirm their mode of action. The results of an in vitro TNF-α study are shown in Table 3. From the data, it is clear that compounds 3f (75.85%), 3g (73.89%), 2f (72.68%), 2h (71.56%) and 2b (71.01%) showed significant inhibition of the TNF-α level as compared to indomethacin (69.89%). Compounds 1b, 2g, 3b, 4b and 4g exhibited TNF-α inhibition comparable to the standard drug.| Compounds | % Inhibition |
|---|---|
| Standard (indomethacin) | 69.89 |
| 1b | 63.58 |
| 1c | 58.10 |
| 1d | 56.15 |
| 1f | 52.74 |
| 1g | 57.98 |
| 2a | 42.67 |
| 2b | 71.01 |
| 2c | 64.10 |
| 2d | 60.35 |
| 2e | 64.51 |
| 2f | 72.68 |
| 2g | 66.18 |
| 2h | 71.56 |
| 3a | 46.98 |
| 3b | 67.51 |
| 3c | 62.50 |
| 3d | 59.65 |
| 3f | 75.85 |
| 3g | 73.89 |
| 4b | 64.53 |
| 4c | 57.95 |
| 4d | 54.46 |
| 4e | 55.45 |
| 4g | 60.97 |
Compounds 3f, 3g, 2f, 2h and 2b showed better in vitro TNF-α activity as compared to the standard drug indomethacin. This is well supported by docking studies which show 3f molecules forming a hydrogen bond with the GLY-121 residue, which is deeply buried into the hydrophobic binding pocket of TNF-α wherein the reference molecule indomethacin shows only hydrophobic and shape driven interactions. Similarly 3g, 2f and 2h were found to show additional hydrophobic interactions with TYR-A59, TYR-B59 and TYR-B119 residues in the binding pocket of TNF-α. The molecules 1b, 2c, 2d, 2e, 2g, 3b, 3c, 3d, 4b, and 4g showed comparable glide scores with respect to indomethacin but were found to show slightly less in vitro percent inhibition with respect to indomethacin (59.65–67.51%). The molecules 1c, 2d, 2f, 1g, 2a, 4e, 4d, 4c and 3a showed better glide scores but were unable to show a comparable percent inhibition in vitro (46.98–58.51%). These effects could be attributed to the docking methodology. All docking programs currently in use exploit empirically based algorithms, avoiding a systematic search for the conformational space, and scoring is done using simple equations, to speed up the process, thereby making it necessary to verify the results by subsequent in vitro studies. Our docking as well as in vitro studies show correlation although not exact. There are deviations in the observed values of TNF-α inhibition and docking results because the hydrophobic pocket of the TNF-α molecule is buried by about 330 angstroms into the protein surface. This gives rise to a Y-shaped binding pocket and makes it difficult for the ligands to bind to the pocket if they are not flexible.
Cytotoxicity assay
In order to evaluate the cytotoxicity effect of the most active compounds 3f, 3g, 2f, 2h and 2b, we carried out MTT proliferation assay. The results of cytotoxicity assay are shown in Table 4. The results clearly indicate that the cell viability of compounds 3f, 3g, 2f, 2h and 2b was more than 85% i.e. these compounds did not cause any abnormal cell death as compared to the standard drug indomethacin which has a cell viability of 60%.| Compounds | % Cytotoxicity |
|---|---|
| Standard drug (indomethacin) | 42.41 |
| 2b | 12.18 |
| 2f | 5.65 |
| 2h | 9.73 |
| 3f | 2.98 |
| 3g | 4.18 |
In vivo anti-inflammatory activity
Fourteen compounds showing significant in vitro TNF-α suppression were further evaluated for their in vivo anti-inflammatory activity (Table 5). It was clear that compounds 3f (76.32% at 3 h, 80.92% at 5 h) and 3g (74.51% at 3 h, 78.42% at 5 h) showed better anti-inflammatory activity as compared to the standard drug indomethacin which showed an inhibition of 72.14% at 3 h and 78.11% at 5 h. Other compounds 2h (73.02%), 2b (72.07%), 2f (70.27%) and 3b (68.52%) showed anti-inflammatory activity comparable to the standard drug at 5 h.24–27 Compounds 3f and 3g showed better docking results as well as better in vitro and in vivo anti-inflammatory activities. Compounds 2h and 2f showed better docking results and in vitro TNF-α activity but these compounds showed less anti-inflammatory activity as compared to the standard drug indomethacin.| Compounds | Change in paw volume (ml) mean ± (SEM) | % Inhibition | ||
|---|---|---|---|---|
| 3 h | 5 h | 3 h | 5 h | |
| Data are analyzed by one-way ANOVA followed by Dunnett's ‘t’ test and expressed as mean ± SEM from five observations where *p < 0.05, **p < 0.01. | ||||
| Control | 1.67 ± 0.021 | 1.70 ± 0.019 | — | — |
| Indomethacin | 0.60 ± 0.023** | 0.51 ± 0.023** | 72.14 | 78.11 |
| 1b | 1.03 ± 0.020* | 1.01 ± 0.021* | 42.06 | 44.55 |
| 2b | 0.65 ± 0.013* | 0.66 ± 0.014** | 67.96 | 72.07 |
| 2c | 0.91 ± 0.022* | 0.87 ± 0.016* | 49.58 | 53.47 |
| 2d | 0.98 ± 0.018* | 1.04 ± 0.024* | 47.03 | 43.59 |
| 2e | 0.92 ± 0.017* | 0.95 ± 0.024* | 49.16 | 48.09 |
| 2f | 0.68 ± 0.021** | 0.64 ± 0.014** | 66.57 | 70.27 |
| 2g | 0.96 ± 0.017* | 1.03 ± 0.018* | 46.51 | 42.77 |
| 2h | 0.66 ± 0.018** | 0.61 ± 0.020** | 69.08 | 73.02 |
| 3b | 0.70 ± 0.019* | 0.65 ± 0.011* | 64.48 | 68.52 |
| 3c | 0.70 ± 0.014* | 0.76 ± 0.016* | 64.00 | 61.58 |
| 3f | 0.54 ± 0.020** | 0.48 ± 0.013** | 76.32 | 80.92 |
| 3g | 0.55 ± 0.015** | 0.50 ± 0.016** | 74.51 | 78.42 |
| 4b | 0.80 ± 0.022** | 0.74 ± 0.017** | 58.99 | 63.62 |
| 4g | 0.81 ± 0.022* | 0.79 ± 0.016* | 56.82 | 58.99 |
Immunohistochemistry
The protein expression of the pro-inflammatory mediator TNF-α in the presence of active compounds 3f and 3g was checked using immunohistochemistry. The modulation of the cellular signaling network involving the induction and activation of pro-inflammatory cytokines like TNF-α has been considered a paradigm for preventing inflammation.28 Therefore in the present study the potential of the active compounds in suppressing the TNF-α cytokine was studied. The results of the protein expression study of active compounds 3f and 3g in the paw tissue of animals are shown in Fig. 1. For immunohistochemical analyses, brown colour indicates specific immunostaining of TNF-α expression. The intensity of brown colour in the animals treated with carrageenan only (group II) clearly indicates more number of cells having TNF-α expression as compared to that of control group (I). Administration of active compounds 3f and 3g and the standard drug indomethacin reduced the expression of TNF-α significantly as compared to the carrageenan induced group. However the reduction of TNF-α expression was more in the presence of 3f and 3g in comparison to the standard drug.Analgesic activity
The compounds 2b, 2f, 2h, 3f and 3g showing most potent anti-inflammatory activity (in vivo) were further tested for their analgesic activity. From Table 6, it is clear that compound 3f showed significant analgesic activity (54.58%) as compared to indomethacin which showed 57.91% activity. Compound 3g also showed activity comparable to the standard drug.| Group | Number of writhes in 10 min | % Protection |
|---|---|---|
| Data are analyzed by one way ANOVA followed by Dunnett's ‘t’ test and expressed as mean ± SEM from five observations where *p < 0.05, **p < 0.01. | ||
| Control | 96.0 ± 2.20 | — |
| Standard | 40.4 ± 1.63** | 57.91 |
| 2b | 49.2 ± 1.24** | 48.95 |
| 2f | 57.0 ± 1.37* | 40.62 |
| 2h | 53.0 ± 2.12* | 44.79 |
| 3f | 43.6 ± 1.60** | 54.58 |
| 3g | 45.8 ± 1.98** | 52.29 |
Ulcerogenic activity
Compounds 2b, 2f, 2h, 3f and 3g showing potent anti-inflammatory activity were further checked for their ulcerogenic risk (Fig. S2, ESI†). Compounds 3f, 3g and 2f did not cause any damage to the stomach as compared to the standard drug indomethacin. Whereas, the compounds 2b and 2h caused a slight damage to the epithelium tissue which was lesser as compared to the standard drug causing significant epithelial mucosal damage to the stomach of tested animals.Structure–activity relationship
On the basis of docking score and biological activity, the following SAR could be determined:• The presence of hydrogen at the R2 position in the synthesized compounds resulted in poor in vitro and in vivo activities regardless of the glide scores.
• Halogen substitution at the R2 position showed better glide scores as well as in vitro and in vivo activities in the order of F > Cl > Br regardless of any substitution at R1 and R3.
• With R2 as o-OCH3 and R1 = p-OC2H5 the in vitro and in vivo activities decreased with para substitution of the chloro group in comparison to ortho substitution at the R3 position. Whereas the pattern reversed when R1 was substituted with p-OCH3.
• If R2 = p-OC2H5 and R3 = o-Cl, the glide scores for in vitro and iv vivo activities are high regardless of substitution at R1 as p-OC2H5 or p-OCH3.
Crystallographic study
Intensity data were collected at 183(2) K using an Oxford Xcalibur Sapphire 3 diffractometer (a single wavelength Enhance X-ray source with MoKα radiation, λ = 0.71073 Å).29 The selected suitable single crystals were mounted using paratone oil on the top of a glass fiber fixed on a goniometer head and immediately transferred to the diffractometer. Pre-experiment, data collection, data reduction and analytical absorption corrections30 were performed using the Oxford program suite CrysAlisPro.31 The crystal structures were solved using SHELXS-9732 using direct methods. The structure refinements were performed by full-matrix least-squares on F2 using SHELXL-97.32 All programs used for the crystal structure determination process are included in WINGX software.33The chemical formula and the ring labeling system are shown in Fig. 2. Crystal data for compound 3e: C28H21ClN2O4S, Mr, 516.98; system, monoclinic; space group, P21/c; unit cell dimensions, a = 17.4889(14) Å; b = 17.3814(11) Å; c = 8.2889(6) Å; β = 99.779(8)°; V = 2483.1(3) Å3; Z = 4; T = 298 K; Rint, 0.0948; R(all), 0.2044; Gof = 0.988; Δρmax = 0.23 e Å3; Δρmin = −0.23 e Å3. The resolution obtained for the structure of the compounds was limited by the poor quality of the available crystals.
All hydrogen atoms were calculated after each cycle of refinement using a riding model, with C–H = 0.93 Å + Uiso(H) = 1.2Ueq(C) for aromatic H atoms and C–H = 0.97 Å + Uiso(H) = 1.2Ueq(C) for methylene H atoms.
Crystallographic data for structure 3e have been deposited with the Cambridge Crystallographic Data Center (CCDC) under the number CCDC-1058214.
Conclusion
Thirty two 2-imino 4-thiazolidinone derivatives were subjected to in silico molecular docking studies and evaluated for the effect on the in vitro TNF-α target. The preliminary in vitro TNF-α activity on these synthesized derivatives suggested that the compounds 3f and 3g showed significant inhibition of TNF-α levels. These compounds (3f and 3g) also showed a significant in vivo anti-inflammatory activity as compared to indomethacin without causing cytotoxicity and damage to the stomach. Moreover, compounds 3f and 3g also significantly suppressed the protein expression of TNF-α in the carrageenan induced paw tissue of animals. Therefore, these compounds may be considered as potential candidates for the development of new anti-inflammatory agents.Experimental
Materials and methods
All the reagents and starting materials were purchased from Sigma Aldrich and were used as such. The progress of the reaction was monitored by TLC (E. Merck Kieselgel 60 F254) and visualization was accomplished using UV light and iodine. The structural confirmation of the synthesized compounds was done by 1H NMR (Bruker Avance II 300 NMR spectrometer), 13C NMR (Bruker Avance II 400 NMR spectrometer), IR (Bio-Rad FTS-135) and mass spectroscopy (ES-MS, LCQ Fleet,) as well as by elemental analysis (Elementar GmBH). Melting points were recorded using Veego melting point apparatus (model VHP-DS), and are uncorrected.5-[5-(4-Chloro-phenyl)-furan-2-ylmethylene]-2-(4-ethoxy-phenylimino)-3-phenyl-thiazolidin-4-one (1a). Light yellow solid; yield: 71%; M.p.: 161–162 °C; M.W.: 501.00; Rf: 0.56; FT-IR (νmax; cm−1 KBr): 1578 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1695 (C
N), 1695 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (400 MHz, CDCl3-d6, TMS): δ: 1.42 (t, 3H, OCH2
O); 1H-NMR (400 MHz, CDCl3-d6, TMS): δ: 1.42 (t, 3H, OCH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3J = 7.1 Hz), 4.05 (q, 2H,
3J = 7.1 Hz), 4.05 (q, 2H, ![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH3J = 7.2 Hz), 6.73–7.03 (m, 7H, Ar-H), 7.19–7.54 (m, 8H, Ar-H), 7.55 (s, 1H, olefinic proton); 13C-NMR (120 MHz, CDCl3): δ: 14.96, 63.52, 114.29, 114.07, 114.67, 114.90, 115.13, 116.16, 118.12, 120.12, 122.52, 127.80,127.89, 128.12, 129.10, 129.15, 130.16, 130.70, 141.96, 149.48, 153.19, 156.37, 156.99, 159.10, 166.66. Mass: m/z: 503.6 [M + 2]+. Elemental analysis for C28H21ClN2O3S: calculated: C, 67.13; H, 4.22; N, 5.59; S, 6.40. Found: C, 67.89; H, 4.31; N, 5.62; S, 6.47.
2CH3J = 7.2 Hz), 6.73–7.03 (m, 7H, Ar-H), 7.19–7.54 (m, 8H, Ar-H), 7.55 (s, 1H, olefinic proton); 13C-NMR (120 MHz, CDCl3): δ: 14.96, 63.52, 114.29, 114.07, 114.67, 114.90, 115.13, 116.16, 118.12, 120.12, 122.52, 127.80,127.89, 128.12, 129.10, 129.15, 130.16, 130.70, 141.96, 149.48, 153.19, 156.37, 156.99, 159.10, 166.66. Mass: m/z: 503.6 [M + 2]+. Elemental analysis for C28H21ClN2O3S: calculated: C, 67.13; H, 4.22; N, 5.59; S, 6.40. Found: C, 67.89; H, 4.31; N, 5.62; S, 6.47.
5-[5-(4-Chloro-phenyl)-furan-2-ylmethylene]-2-(4-ethoxy-phenylimino)-3-(4-fluoro-phenyl)-thiazolidin-4-one (1b). Yellow solid; yield: 69%; M.p.: 134–135 °C; M.W.: 518.99; Rf: 0.55; FT-IR (νmax; cm−1 KBr): 1582 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1688 (C
N), 1688 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (300 MHz, CDCl3-d6, TMS): δ: 1.52 (t, 3H, OCH2
O); 1H-NMR (300 MHz, CDCl3-d6, TMS): δ: 1.52 (t, 3H, OCH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3J = 7.2 Hz), 4.09 (q, 2H,
3J = 7.2 Hz), 4.09 (q, 2H, ![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH3J = 5.1 Hz) 6.80–7.13 (m, 7H, Ar-H), 7.34–7.39 (m, 4H, Ar-H), 7.46–7.62 (m, 4H, Ar-H); 13C-NMR (120 MHz, CDCl3): δ: 14.52, 63.72, 114.29, 114.35, 114.67, 114.90, 115.13, 116.61, 117.12, 120.10, 122.15, 127.60, 127.96, 128.70, 129.99, 130.29, 141.09, 149.12, 153.19, 156.37, 156.66, 159.44, 159.99, 166.10. Mass: m/z: 520.8 [M + 1]+. Elemental analysis for C28H20ClFN2O3S: calculated: C, 64.80; H, 3.88; N, 5.40; S, 6.18. Found: C, 64.94; H, 4.12; N, 5.52; S, 6.29.
2CH3J = 5.1 Hz) 6.80–7.13 (m, 7H, Ar-H), 7.34–7.39 (m, 4H, Ar-H), 7.46–7.62 (m, 4H, Ar-H); 13C-NMR (120 MHz, CDCl3): δ: 14.52, 63.72, 114.29, 114.35, 114.67, 114.90, 115.13, 116.61, 117.12, 120.10, 122.15, 127.60, 127.96, 128.70, 129.99, 130.29, 141.09, 149.12, 153.19, 156.37, 156.66, 159.44, 159.99, 166.10. Mass: m/z: 520.8 [M + 1]+. Elemental analysis for C28H20ClFN2O3S: calculated: C, 64.80; H, 3.88; N, 5.40; S, 6.18. Found: C, 64.94; H, 4.12; N, 5.52; S, 6.29.
3-(4-Chloro-phenyl)-5-[5-(4-chloro-phenyl)-furan-2-ylmethylene]-2-(4-ethoxy-phenylimino)-thiazolidin-4-one (1c). Yellow solid; yield: 78%; M.p.: 156–157 °C; M.W.: 535.44; Rf: 0.55; FT-IR (νmax; cm−1 KBr); 1567 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1681 (C
N), 1681 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (400 MHz, CDCl3-d6, TMS,): δ: 1.44 (t, 3H, OCH2
O); 1H-NMR (400 MHz, CDCl3-d6, TMS,): δ: 1.44 (t, 3H, OCH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3J = 6.0 Hz), 4.09 (q, 2H,
3J = 6.0 Hz), 4.09 (q, 2H, ![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH3J = 6.9 Hz), 6.80–6.99 (m, 6H, Ar-H), 7.02–7.08 (m, 2H, Ar-H), 7.28–7.56 (m, 6H, Ar-H), 7.59 (s, 1H, olefinic proton); 13C-NMR (120 MHz, CDCl3): δ: 14.18, 63.57, 114.02, 114.03, 114.23, 115.74, 116.74, 118.65, 120.15, 122.15, 127.25, 127.35, 129.51, 129.51, 130.22, 130.52, 141.22, 149.22, 153.36, 156.31, 156.41, 159.41, 159.41, 159.51, 166.57. Mass: m/z: 536.20 [M + 1]+. Elemental analysis for C28H20Cl2N2O3S: calculated: 62.81; H, 3.76; N, 5.23; S, 5.99. Found: C, 62.89; H, 3.86; N, 5.34; S, 6.12.
2CH3J = 6.9 Hz), 6.80–6.99 (m, 6H, Ar-H), 7.02–7.08 (m, 2H, Ar-H), 7.28–7.56 (m, 6H, Ar-H), 7.59 (s, 1H, olefinic proton); 13C-NMR (120 MHz, CDCl3): δ: 14.18, 63.57, 114.02, 114.03, 114.23, 115.74, 116.74, 118.65, 120.15, 122.15, 127.25, 127.35, 129.51, 129.51, 130.22, 130.52, 141.22, 149.22, 153.36, 156.31, 156.41, 159.41, 159.41, 159.51, 166.57. Mass: m/z: 536.20 [M + 1]+. Elemental analysis for C28H20Cl2N2O3S: calculated: 62.81; H, 3.76; N, 5.23; S, 5.99. Found: C, 62.89; H, 3.86; N, 5.34; S, 6.12.
3-(4-Bromo-phenyl)-5-[5-(4-chloro-phenyl)-furan-2-ylmethylene]-2-(4-ethoxy-phenylimino)-thiazolidin-4-one (1d). Yellow solid; yield: 78%; M.p.: 190–191 °C; M.W.: 579.89; Rf: 0.53; FT-IR (νmax; cm−1 KBr): 1589 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1691 (C
N), 1691 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (300 MHz, CDCl3-d6, TMS,): δ: 1.50 (t, 3H, OCH2
O); 1H-NMR (300 MHz, CDCl3-d6, TMS,): δ: 1.50 (t, 3H, OCH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3J = 6.6 Hz), 4.09(q, 2H,
3J = 6.6 Hz), 4.09(q, 2H, ![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH3J = 5.4 Hz), 6.32–6.63 (m, 5H, Ar-H), 6.87–7.04 (m, 2H, Ar-H), 7.28–7.81 (m, 8H, Ar-H); 13C-NMR (120 MHz, CDCl3): δ: 14.22, 63.72, 114.22, 114.22, 114.32, 114.33, 115.43, 116.44, 118.54, 120.55, 127.55, 127.55, 128.60, 129.16, 129.18, 130.20, 130.22, 130.22, 141.41, 149.27, 153.66, 156.14, 156.14, 159.14, 159.14, 166.15. Mass: m/z: 580.9 [M + 1]+. Elemental analysis for C28H20BrClN2O3S: calculated: C, 57.99; H, 3.48; N, 4.83; S, 5.53. Found: C, 58.13; H, 3.88; N, 4.89; S, 5.61.
2CH3J = 5.4 Hz), 6.32–6.63 (m, 5H, Ar-H), 6.87–7.04 (m, 2H, Ar-H), 7.28–7.81 (m, 8H, Ar-H); 13C-NMR (120 MHz, CDCl3): δ: 14.22, 63.72, 114.22, 114.22, 114.32, 114.33, 115.43, 116.44, 118.54, 120.55, 127.55, 127.55, 128.60, 129.16, 129.18, 130.20, 130.22, 130.22, 141.41, 149.27, 153.66, 156.14, 156.14, 159.14, 159.14, 166.15. Mass: m/z: 580.9 [M + 1]+. Elemental analysis for C28H20BrClN2O3S: calculated: C, 57.99; H, 3.48; N, 4.83; S, 5.53. Found: C, 58.13; H, 3.88; N, 4.89; S, 5.61.
5-[5-(4-Chloro-phenyl)-furan-2-ylmethylene]-2-(4-ethoxy-phenylimino)-3-(4-methoxy-phenyl)-thiazolidin-4-one (1e). Light yellow solid; yield: 81%; M.p.: 146–147 °C; M.W.: 531.03; Rf: 0.55; FT-IR (νmax; cm−1 KBr): 1549 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1696 (C
N), 1696 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (300 MHz, CDCl3-d6, TMS): δ: 1.47 (t, 3H, OCH2
O); 1H-NMR (300 MHz, CDCl3-d6, TMS): δ: 1.47 (t, 3H, OCH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3J = 6.9 Hz), 3.83 (s, 3H, OCH3), 4.09 (q, 2H,
3J = 6.9 Hz), 3.83 (s, 3H, OCH3), 4.09 (q, 2H, ![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH3J = 6.9 Hz), 6.78(s, 2H, Ar-H), 6.85–7.34 (m, 8H, Ar-H), 7.36–7.55 (m, 4H, Ar-H), 7.58 (s, 1H, olefinic proton); 13C-NMR (120 MHz, CDCl3): δ: 14.18, 55.22, 63.15, 114.26, 114.28, 114.30, 114.32, 11s5.47, 116.47, 118.56, 120.54, 122.54, 127.54, 128.62, 129.15, 130.20, 130.22, 141.22, 149.22, 153.13, 156.14, 156.63, 159.14, 159.15, 166.75. Mass: m/z: 532.3 [M + 1]+. Elemental analysis for C29H23ClN2O4S: calculated: C, 65.59; H, 4.37; N, 5.28; S, 6.04. Found: C, 65.83; H, 4.57; N, 5.45; S, 6.24.
2CH3J = 6.9 Hz), 6.78(s, 2H, Ar-H), 6.85–7.34 (m, 8H, Ar-H), 7.36–7.55 (m, 4H, Ar-H), 7.58 (s, 1H, olefinic proton); 13C-NMR (120 MHz, CDCl3): δ: 14.18, 55.22, 63.15, 114.26, 114.28, 114.30, 114.32, 11s5.47, 116.47, 118.56, 120.54, 122.54, 127.54, 128.62, 129.15, 130.20, 130.22, 141.22, 149.22, 153.13, 156.14, 156.63, 159.14, 159.15, 166.75. Mass: m/z: 532.3 [M + 1]+. Elemental analysis for C29H23ClN2O4S: calculated: C, 65.59; H, 4.37; N, 5.28; S, 6.04. Found: C, 65.83; H, 4.57; N, 5.45; S, 6.24.
5-[5-(4-Chloro-phenyl)-furan-2-ylmethylene]-3-(4-ethoxy-phenyl)-2-(4-ethoxy-phenylimino)-thiazolidin-4-one (1f). Light yellow solid; yield: 79%; M.p.: 201–202 °C; M.W.: 545.05; Rf: 0.56; FT-IR (νmax; cm−1 KBr): 1540 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1624 (C
N), 1624 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (400 MHz, CDCl3-d6, TMS): δ: 1.42 (t, 6H, 2-OCH2
O); 1H-NMR (400 MHz, CDCl3-d6, TMS): δ: 1.42 (t, 6H, 2-OCH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3J = 6.88 Hz), 4.05 (q, 4H, 2-
3J = 6.88 Hz), 4.05 (q, 4H, 2-![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH3J = 6.88 Hz), 6.81 (d, 1H Ar-H, J = 3.72 Hz), 6.88–7.04 (m, 6H, Ar-H), 7.21–7.44 (m, 6H Ar-H), 7.57(s, 1H, olefinic proton), 7.76–7.79 (m, 1H, Ar-H); 13C-NMR (120 MHz, CDCl3): δ: 14.85, 14.97, 63.70, 114.07, 114.90, 115.12, 116.56, 118.09, 120.07, 122.51, 127.05, 127.16, 128.11, 129.02, 129.11, 130.58, 130.96, 141.60, 149.49, 152.23, 153.15, 156.36, 159.08, 166.42. Mass: m/z: 545.10 [M+]+. Elemental analysis for C30H25ClN2O4S: calculated: C, 66.11; H, 4.62; N, 5.14; S, 5.88. Found: C, 66.21; H, 4.69; N, 5.34; S, 5.91.
2CH3J = 6.88 Hz), 6.81 (d, 1H Ar-H, J = 3.72 Hz), 6.88–7.04 (m, 6H, Ar-H), 7.21–7.44 (m, 6H Ar-H), 7.57(s, 1H, olefinic proton), 7.76–7.79 (m, 1H, Ar-H); 13C-NMR (120 MHz, CDCl3): δ: 14.85, 14.97, 63.70, 114.07, 114.90, 115.12, 116.56, 118.09, 120.07, 122.51, 127.05, 127.16, 128.11, 129.02, 129.11, 130.58, 130.96, 141.60, 149.49, 152.23, 153.15, 156.36, 159.08, 166.42. Mass: m/z: 545.10 [M+]+. Elemental analysis for C30H25ClN2O4S: calculated: C, 66.11; H, 4.62; N, 5.14; S, 5.88. Found: C, 66.21; H, 4.69; N, 5.34; S, 5.91.
5-[5-(4-Chloro-phenyl)-furan-2-ylmethylene]-2-(4-ethoxy-phenylimino)-3-(2-methoxy-phenyl)-thiazolidin-4-one (1g). Light brownish solid; yield: 75%; M.p.: 181–182 °C; M.W.: 531.02; Rf: 0.55; FT-IR (νmax; cm−1 KBr): 1549 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1656 (C
N), 1656 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (400 MHz, CDCl3-d6, TMS): δ: 1.43 (t, 3H, OCH2
O); 1H-NMR (400 MHz, CDCl3-d6, TMS): δ: 1.43 (t, 3H, OCH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3J = 6.96 Hz), 3.86 (s, 3H, OCH3), 4.04 (q, 2H,
3J = 6.96 Hz), 3.86 (s, 3H, OCH3), 4.04 (q, 2H, ![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH3, J = 6.96 Hz), 6.74 (d 2H, Ar-H, J = 3.08 Hz) 6.89–7.10 (m, 6H, Ar-H), 7.17–7.36 (m, 3H, Ar-H), 7.41–7.54 (m, 3H, Ar-H), 7.55 (s, 1H, olefinic proton); 13C-NMR (120 MHz, CDCl3): δ: 14.11, 55.18, 63.22, 114.62, 114.82, 114.03, 114.23, 115.74, 116.74, 118.65, 120.15, 122.15, 127.15, 127.15, 128.56, 129.51, 129.51, 130.52, 141.22, 149.22, 153.36, 156.31, 156.41, 159.71, 166. 57. Mass: m/z: 533.2 [M + 2]+. Elemental analysis for C29H23ClN2O4S: calculated: C, 65.59; H, 4.37; N, 5.28; S, 6.04. Found: C, 65.82; H, 4.41; N, 5.56; S, 6.14.
2CH3, J = 6.96 Hz), 6.74 (d 2H, Ar-H, J = 3.08 Hz) 6.89–7.10 (m, 6H, Ar-H), 7.17–7.36 (m, 3H, Ar-H), 7.41–7.54 (m, 3H, Ar-H), 7.55 (s, 1H, olefinic proton); 13C-NMR (120 MHz, CDCl3): δ: 14.11, 55.18, 63.22, 114.62, 114.82, 114.03, 114.23, 115.74, 116.74, 118.65, 120.15, 122.15, 127.15, 127.15, 128.56, 129.51, 129.51, 130.52, 141.22, 149.22, 153.36, 156.31, 156.41, 159.71, 166. 57. Mass: m/z: 533.2 [M + 2]+. Elemental analysis for C29H23ClN2O4S: calculated: C, 65.59; H, 4.37; N, 5.28; S, 6.04. Found: C, 65.82; H, 4.41; N, 5.56; S, 6.14.
5-[5-(4-Chloro-phenyl)-furan-2-ylmethylene]-2-(4-ethoxy-phenylimino)-3-o-tolyl-thiazolidin-4-one (1h). Light brownish solid; yield: 71%; M.p.: 151–152 °C; M.W.: 515.04; Rf: 0.55; FT-IR (νmax; cm−1 KBr): 1556 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1674 (C
N), 1674 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (400 MHz, CDCl3-d6, TMS): δ: 1.41 (t, 3H, OCH2
O); 1H-NMR (400 MHz, CDCl3-d6, TMS): δ: 1.41 (t, 3H, OCH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3J = 6.9 Hz), 2.41 (s, 3H, CH3), 4.06 (q, 2H,
3J = 6.9 Hz), 2.41 (s, 3H, CH3), 4.06 (q, 2H, ![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH3J = 6.90 Hz), 6.77–6.79 (s, 2H, Ar-H), 6.91–7.15 (m, 5H, Ar-H), 7.15–7.31 (m, 3H, Ar-H), 7.41–7.53 (m, 4H, Ar-H), 7.57 (s, 1H, olefinic proton); 13C-NMR (120 MHz, CDCl3): δ: 14.22, 63.11, 114.12, 114.12, 114.15, 114.23, 115.14, 116.14, 118.15, 120.12, 122.12, 127.13, 128.24, 129.55, 130.52, 130.76, 141.22, 149.22, 153.36, 156.33, 156.44, 159.44, 159.55. 166.52. Mass: m/z: 517.10 [M + 2]+. Elemental analysis for C29H23ClN2O3S: calculated: C, 67.63; H, 4.50; N, 5.44; S, 6.23. Found: C, 67.83; H, 4.59; N, 5.49; S, 6.56.
2CH3J = 6.90 Hz), 6.77–6.79 (s, 2H, Ar-H), 6.91–7.15 (m, 5H, Ar-H), 7.15–7.31 (m, 3H, Ar-H), 7.41–7.53 (m, 4H, Ar-H), 7.57 (s, 1H, olefinic proton); 13C-NMR (120 MHz, CDCl3): δ: 14.22, 63.11, 114.12, 114.12, 114.15, 114.23, 115.14, 116.14, 118.15, 120.12, 122.12, 127.13, 128.24, 129.55, 130.52, 130.76, 141.22, 149.22, 153.36, 156.33, 156.44, 159.44, 159.55. 166.52. Mass: m/z: 517.10 [M + 2]+. Elemental analysis for C29H23ClN2O3S: calculated: C, 67.63; H, 4.50; N, 5.44; S, 6.23. Found: C, 67.83; H, 4.59; N, 5.49; S, 6.56.
5-[5-(2-Chloro-phenyl)-furan-2-ylmethylene]-2-(4-ethoxy-phenylimino)-3-phenyl-thiazolidin-4-one (2a). Light yellow solid; yield: 78%; M.p.: 115–116 °C; M.W.: 501.00; Rf: 0.57; FT-IR (νmax; cm−1 KBr): 1559 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1678 (C
N), 1678 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (300 MHz, CDCl3-d6, TMS): δ: 1.50 (t, 3H, OCH2
O); 1H-NMR (300 MHz, CDCl3-d6, TMS): δ: 1.50 (t, 3H, OCH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3J = 6.0 Hz), 4.10 (q, 2H,
3J = 6.0 Hz), 4.10 (q, 2H, ![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH3J = 6.9 Hz), 6.80 (s, 2H, Ar-H), 6.92–7.08 (m, 4H, Ar-H), 7.22–7.59 (m, 9H, Ar-H), 7.60 (s, 1H, olefinic proton); 13C-NMR (120 MHz, CDCl3): δ: 14.85, 63.72, 108.67, 114.90, 115.14, 116.57, 116.79, 118.21, 118.39, 118.50, 119.40, 119.57, 119.66, 121.39, 122.51, 124.88, 125.51, 125.55, 127.04, 127.14, 127.81, 127.85, 128.09, 128.87, 128.93, 129.12, 129.14, 129.31, 134.31, 144.72, 148.57, 149.85, 149.89, 149.93, 152.39, 152.50, 155.66, 155.76, 156.42, 159.08, 159.12, 166.23, 166.39, 166.44. Mass: m/z: 502.8 [M + 1]+. Elemental analysis for C28H21ClN2O3S: calculated: C, 67.13; H, 4.22; N, 5.59; S, 6.40. Found: 67.34; H, 4.31; N, 5.65; S, 6.48.
2CH3J = 6.9 Hz), 6.80 (s, 2H, Ar-H), 6.92–7.08 (m, 4H, Ar-H), 7.22–7.59 (m, 9H, Ar-H), 7.60 (s, 1H, olefinic proton); 13C-NMR (120 MHz, CDCl3): δ: 14.85, 63.72, 108.67, 114.90, 115.14, 116.57, 116.79, 118.21, 118.39, 118.50, 119.40, 119.57, 119.66, 121.39, 122.51, 124.88, 125.51, 125.55, 127.04, 127.14, 127.81, 127.85, 128.09, 128.87, 128.93, 129.12, 129.14, 129.31, 134.31, 144.72, 148.57, 149.85, 149.89, 149.93, 152.39, 152.50, 155.66, 155.76, 156.42, 159.08, 159.12, 166.23, 166.39, 166.44. Mass: m/z: 502.8 [M + 1]+. Elemental analysis for C28H21ClN2O3S: calculated: C, 67.13; H, 4.22; N, 5.59; S, 6.40. Found: 67.34; H, 4.31; N, 5.65; S, 6.48.
5-[5-(2-Chloro-phenyl)-furan-2-ylmethylene]-2-(4-ethoxy-phenylimino)-3-(4-fluoro-phenyl)-thiazolidin-4-one (2b). Light yellow solid; yield: 71%; M.p.: 126–127 °C; M.W.: 518.99; Rf: 0.56; FT-IR (νmax; cm−1 KBr): 1579 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1671 (C
N), 1671 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (300 MHz, CDCl3-d6, TMS): δ: 1.47 (t, 3H, OCH2
O); 1H-NMR (300 MHz, CDCl3-d6, TMS): δ: 1.47 (t, 3H, OCH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3J = 4.8 Hz), 4.08 (q, 2H,
3J = 4.8 Hz), 4.08 (q, 2H, ![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH3J = 6.9 Hz), 6.84–7.12 (m, 5H, Ar-H), 7.25–7.49 (m, 8H, Ar-H), 7.63 (s, 1H, olefinic proton), 7.75–7.83 (m, 1H, Ar-H); 13C-NMR (120 MHz, CDCl3): δ: 14.72, 63.96, 114.12, 114.14, 114.87, 114.93, 115.31, 116.31, 116.57, 116.72, 117.85, 117.89, 117.93, 122.66, 123.76, 124.08, 124.42, 127.12, 127.23, 127.39, 127.44, 128.67, 128.90, 128.93, 129.14, 129.57, 129.69, 134.79, 141.21, 148.39, 149.50, 149.57, 152.39, 152.66, 155.51, 155.88, 156.51, 159.04, 159.55, 166.41, 166.81, 166.85. Mass: m/z: 519.8 [M+]+. Elemental analysis for C28H20ClFN2O3S: calculated: C, 64.80; H, 3.88; N, 5.40; S, 6.18. Found: C, 64.97; H, 3.91; N, 5.49; S, 6.56.
2CH3J = 6.9 Hz), 6.84–7.12 (m, 5H, Ar-H), 7.25–7.49 (m, 8H, Ar-H), 7.63 (s, 1H, olefinic proton), 7.75–7.83 (m, 1H, Ar-H); 13C-NMR (120 MHz, CDCl3): δ: 14.72, 63.96, 114.12, 114.14, 114.87, 114.93, 115.31, 116.31, 116.57, 116.72, 117.85, 117.89, 117.93, 122.66, 123.76, 124.08, 124.42, 127.12, 127.23, 127.39, 127.44, 128.67, 128.90, 128.93, 129.14, 129.57, 129.69, 134.79, 141.21, 148.39, 149.50, 149.57, 152.39, 152.66, 155.51, 155.88, 156.51, 159.04, 159.55, 166.41, 166.81, 166.85. Mass: m/z: 519.8 [M+]+. Elemental analysis for C28H20ClFN2O3S: calculated: C, 64.80; H, 3.88; N, 5.40; S, 6.18. Found: C, 64.97; H, 3.91; N, 5.49; S, 6.56.
3-(4-Chloro-phenyl)-5-[5-(2-chloro-phenyl)-furan-2-ylmethylene]-2-(4-ethoxy-phenylimino)-thiazolidin-4-one (2c). Light yellow solid; yield: 68%; M.p.: 158–159 °C; M.W.: 535.44; Rf: 0.55; FT-IR (νmax; cm−1 KBr): 1589 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1678 (C
N), 1678 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (300 MHz, CDCl3-d6, TMS): δ: 1.50 (t, 3H, OCH2
O); 1H-NMR (300 MHz, CDCl3-d6, TMS): δ: 1.50 (t, 3H, OCH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3J = 5.7 Hz), 4.09 (q, 2H,
3J = 5.7 Hz), 4.09 (q, 2H, ![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH3J = 6.9 Hz), 6.80–7.07 (m, 7H, Ar-H), 7.28–7.47 (m, 5H, Ar-H), 7.51–7.56 (m, 2H, Ar-H), 7.58 (s, 1H, olefinic proton); 13C-NMR (120 MHz, CDCl3): δ: 15.51, 63.96, 114.12, 114.14, 114.72, 114.87, 114.93, 115.31, 115.39, 115.66, 115.72, 116.51, 116.88, 121.51, 124.04, 124.14, 127.85, 127.89, 127.93, 128.39, 128.50, 128.66, 129.08, 129.76, 129.42, 134.12, 141.23, 148.39, 149.44, 149.67, 149.90, 152.14, 152.93, 155.57, 155.69, 156.79, 159.21, 159.39, 166.40, 166.50, 166.57. Mass: m/z: 536.11 [M + 1]+. Elemental analysis for C28H20Cl2N2O3S: calculated: C, 62.81; H, 3.76; N, 5.23; S, 5.99. Found: C, 62.89; H, 3.86; N, 5.45; S, 6.10.
2CH3J = 6.9 Hz), 6.80–7.07 (m, 7H, Ar-H), 7.28–7.47 (m, 5H, Ar-H), 7.51–7.56 (m, 2H, Ar-H), 7.58 (s, 1H, olefinic proton); 13C-NMR (120 MHz, CDCl3): δ: 15.51, 63.96, 114.12, 114.14, 114.72, 114.87, 114.93, 115.31, 115.39, 115.66, 115.72, 116.51, 116.88, 121.51, 124.04, 124.14, 127.85, 127.89, 127.93, 128.39, 128.50, 128.66, 129.08, 129.76, 129.42, 134.12, 141.23, 148.39, 149.44, 149.67, 149.90, 152.14, 152.93, 155.57, 155.69, 156.79, 159.21, 159.39, 166.40, 166.50, 166.57. Mass: m/z: 536.11 [M + 1]+. Elemental analysis for C28H20Cl2N2O3S: calculated: C, 62.81; H, 3.76; N, 5.23; S, 5.99. Found: C, 62.89; H, 3.86; N, 5.45; S, 6.10.
5-[5-(2-Chloro-phenyl)-furan-2-ylmethylene]-2-(4-ethoxy-phenylimino)-3-(4-bromo-phenyl)-thiazolidin-4-one (2d). Light brown solid; yield: 63%; M.p.: 166–167 °C; M.W.: 579.89; Rf: 0.54; FT-IR (νmax; cm−1 KBr): 1579 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1694 (C
N), 1694 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (300 MHz, CDCl3-d6, TMS): δ: 1.45 (t, 3H, OCH2
O); 1H-NMR (300 MHz, CDCl3-d6, TMS): δ: 1.45 (t, 3H, OCH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3J = 6.9 Hz), 4.09 (q, 2H,
3J = 6.9 Hz), 4.09 (q, 2H, ![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH3J = 6.9 Hz) 6.80–7.07 (m, 7H, Ar-H), 7.35–7.57 (m, 6H, Ar-H), 7.59 (s, 1H, olefinic proton), 7.60–7.71 (m, 1H, Ar-H); 13C-NMR (120 MHz): δ: 15.39, 114.81, 114.85, 114.89, 114.93, 115.31, 115.66, 115.72, 116.51, 116.88, 121.51, 124.08, 124.14, 124.55, 124.81, 127.85, 127.89, 127.93, 128.39, 128.50, 128.66, 129.08, 129.76, 134.14, 141.23, 148.39, 149.44, 149.67, 149.90, 152.14, 152.93, 155.57, 155.69, 156.79, 159.12, 159.21, 166.14, 166.72, 166.87. Mass: m/z: 580.2 [M + 1]+. Elemental analysis for C28H20BrClN2O3S: calculated: C, 57.99; H, 3.48; N, 4.83; S, 5.53. Found: C, 58.13; H, 3.54; N, 4.89; S, 5.58.
2CH3J = 6.9 Hz) 6.80–7.07 (m, 7H, Ar-H), 7.35–7.57 (m, 6H, Ar-H), 7.59 (s, 1H, olefinic proton), 7.60–7.71 (m, 1H, Ar-H); 13C-NMR (120 MHz): δ: 15.39, 114.81, 114.85, 114.89, 114.93, 115.31, 115.66, 115.72, 116.51, 116.88, 121.51, 124.08, 124.14, 124.55, 124.81, 127.85, 127.89, 127.93, 128.39, 128.50, 128.66, 129.08, 129.76, 134.14, 141.23, 148.39, 149.44, 149.67, 149.90, 152.14, 152.93, 155.57, 155.69, 156.79, 159.12, 159.21, 166.14, 166.72, 166.87. Mass: m/z: 580.2 [M + 1]+. Elemental analysis for C28H20BrClN2O3S: calculated: C, 57.99; H, 3.48; N, 4.83; S, 5.53. Found: C, 58.13; H, 3.54; N, 4.89; S, 5.58.
5-[5-(2-Chloro-phenyl)-furan-2-ylmethylene]-2-(4-ethoxy-phenylimino)-3-(4-methoxy-phenyl)-thiazolidin-4-one (2e). Light yellow solid; yield: 69%; M.p.: 171–172 °C; M.W.: 531.04; Rf: 0.56; FT-IR (νmax; cm−1 KBr): 1592 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1698 (C
N), 1698 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (400 MHz, CDCl3-d6, TMS): δ: 1.45 (t, 3H, OCH2
O); 1H-NMR (400 MHz, CDCl3-d6, TMS): δ: 1.45 (t, 3H, OCH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3J = 4.5 Hz), 3.87 (s, 3H, OCH3), 4.07 (q, 2H,
3J = 4.5 Hz), 3.87 (s, 3H, OCH3), 4.07 (q, 2H, ![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH3J = 6.9 Hz), 6.85–7.05 (m, 6H, Ar-H), 7.25–7.48 (m, 8H, Ar-H), 7.61 (s, 1H, olefinic proton); 13C-NMR (120 MHz, CDCl3): δ: 14.64, 46.28, 66.75, 114.81, 119.65, 121.17, 124.87, 127.43, 127.88, 128.51, 128.99, 129.37, 130.34, 131.85, 133.32, 136.06, 138.37, 148.14, 150.04, 151.64, 158.74, 166.79. Mass: m/z: 532.4 [M + 1]+. Elemental analysis for C29H23ClN2O4S: Calculated: C, 65.59; H, 4.37; N, 5.28; S, 6.04. Found: C, 65.78; H, 4.43; N, 5.34; S, 6.17.
2CH3J = 6.9 Hz), 6.85–7.05 (m, 6H, Ar-H), 7.25–7.48 (m, 8H, Ar-H), 7.61 (s, 1H, olefinic proton); 13C-NMR (120 MHz, CDCl3): δ: 14.64, 46.28, 66.75, 114.81, 119.65, 121.17, 124.87, 127.43, 127.88, 128.51, 128.99, 129.37, 130.34, 131.85, 133.32, 136.06, 138.37, 148.14, 150.04, 151.64, 158.74, 166.79. Mass: m/z: 532.4 [M + 1]+. Elemental analysis for C29H23ClN2O4S: Calculated: C, 65.59; H, 4.37; N, 5.28; S, 6.04. Found: C, 65.78; H, 4.43; N, 5.34; S, 6.17.
5-[5-(2-Chloro-phenyl)-furan-2-ylmethylene]-3-(4-ethoxy-phenyl)-2-(4-ethoxy-phenylimino)-thiazolidin-4-one (2f). Light yellow solid; yield: 67%; M.p.: 175–176 °C; M.W.: 545.05; Rf: 0.54; FT-IR (νmax; cm−1 KBr): 1598 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1656 (C
N), 1656 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (300 MHz, CDCl3-d6, TMS): δ: 1.47 (t, 6H, 2-OCH2
O); 1H-NMR (300 MHz, CDCl3-d6, TMS): δ: 1.47 (t, 6H, 2-OCH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3J = 4.8 Hz), 4.10 (q, 4H, 2-
3J = 4.8 Hz), 4.10 (q, 4H, 2-![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH3J = 6.9 Hz), 6.84 (d, 1H Ar-H, J = 3.7 Hz), 6.84–7.06 (m, 6H, Ar-H), 7.24–7.48 (m, 7H Ar-H), 7.61 (s, 1H, olefinic proton), 7.80–7.83 (m, 1H, Ar-H); 13C-NMR (120 MHz, CDCl3): δ: 14.97, 63.97, 114.06, 114.74, 115.38, 116.58, 118.96, 120.64, 122.49, 127.23, 127.15, 127.36, 128.08, 129.07, 130.12, 130.90, 141.56, 149.09, 152.07, 153.51, 156.05, 159.15, 166.88. Mass: m/z: 547.06 [M + 2]+. Elemental analysis for C30H25ClN2O4S: calculated: C, 66.11; H, 4.62; N, 5.14; S, 5.88. Found: C, 66.41; H, 4.72; N, 5.34; S, 5.94.
2CH3J = 6.9 Hz), 6.84 (d, 1H Ar-H, J = 3.7 Hz), 6.84–7.06 (m, 6H, Ar-H), 7.24–7.48 (m, 7H Ar-H), 7.61 (s, 1H, olefinic proton), 7.80–7.83 (m, 1H, Ar-H); 13C-NMR (120 MHz, CDCl3): δ: 14.97, 63.97, 114.06, 114.74, 115.38, 116.58, 118.96, 120.64, 122.49, 127.23, 127.15, 127.36, 128.08, 129.07, 130.12, 130.90, 141.56, 149.09, 152.07, 153.51, 156.05, 159.15, 166.88. Mass: m/z: 547.06 [M + 2]+. Elemental analysis for C30H25ClN2O4S: calculated: C, 66.11; H, 4.62; N, 5.14; S, 5.88. Found: C, 66.41; H, 4.72; N, 5.34; S, 5.94.
5-[5-(2-Chloro-phenyl)-furan-2-ylmethylene]-2-(4-ethoxy-phenylimino)-3-(2-methoxy-phenyl)-thiazolidin-4-one (2g). Light yellow solid; yield: 69%; M.p.: 120–121 °C; M.W.: 531.02; Rf: 0.56; FT-IR (νmax; cm−1 KBr); 1567 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1696 (C
N), 1696 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (400 MHz, CDCl3-d6, TMS): δ: 1.44 (t, 3H, OCH2
O); 1H-NMR (400 MHz, CDCl3-d6, TMS): δ: 1.44 (t, 3H, OCH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3J = 6.9 Hz), 3.96 (s, 3H, OCH3), 4.06 (q, 2H,
3J = 6.9 Hz), 3.96 (s, 3H, OCH3), 4.06 (q, 2H, ![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH3J = 6.9 Hz), 6.74 (d, 2H, Ar-H, J = 3.08 Hz) 6.80–7.17 (m, 5H, Ar-H), 7.12–7.37 (m, 4H, Ar-H), 7.43–7.52 (m, 3H, Ar-H), 7.56 (s, 1H, olefinic proton); 13C-NMR (120 MHz, CDCl3): δ: 14.95, 55.88, 63.74, 108.64, 112.04, 112.48, 112.86, 115.13, 115.21, 116.43, 118.02, 118.18, 119.86, 120.09, 121.08, 122.01, 122.54, 123.62, 125.50, 125.53, 125.81, 127.07, 127.89, 128.97, 129.04, 129.09, 129.12, 130.02, 130.86, 134.23, 137.77, 141.91, 149.95, 150.01, 151.59, 153.26, 155.54, 155.63, 156.34, 159.03, 166.06, 166.48. Mass: m/z: 533.4 [M + 2]+. Elemental analysis for C29H23ClN2O4S: calculated: C, 65.59; H, 4.37; N, 5.28; S, 6.04. Found: C, 65.72; H, 4.47; N, 5.45; S, 6.20.
2CH3J = 6.9 Hz), 6.74 (d, 2H, Ar-H, J = 3.08 Hz) 6.80–7.17 (m, 5H, Ar-H), 7.12–7.37 (m, 4H, Ar-H), 7.43–7.52 (m, 3H, Ar-H), 7.56 (s, 1H, olefinic proton); 13C-NMR (120 MHz, CDCl3): δ: 14.95, 55.88, 63.74, 108.64, 112.04, 112.48, 112.86, 115.13, 115.21, 116.43, 118.02, 118.18, 119.86, 120.09, 121.08, 122.01, 122.54, 123.62, 125.50, 125.53, 125.81, 127.07, 127.89, 128.97, 129.04, 129.09, 129.12, 130.02, 130.86, 134.23, 137.77, 141.91, 149.95, 150.01, 151.59, 153.26, 155.54, 155.63, 156.34, 159.03, 166.06, 166.48. Mass: m/z: 533.4 [M + 2]+. Elemental analysis for C29H23ClN2O4S: calculated: C, 65.59; H, 4.37; N, 5.28; S, 6.04. Found: C, 65.72; H, 4.47; N, 5.45; S, 6.20.
5-[5-(2-Chloro-phenyl)-furan-2-ylmethylene]-2-(4-ethoxy-phenylimino)-3-o-tolyl-thiazolidin-4-one (2h). Light yellow solid; yield: 86%; M.p.: 129–130 °C; Rf: 0.57; M.W.: 515.02; FT-IR (νmax; cm−1 KBr): 1587 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1686 (C
N), 1686 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (300 MHz, CDCl3-d6, TMS): δ: 1.48 (t, 3H, OCH2
O); 1H-NMR (300 MHz, CDCl3-d6, TMS): δ: 1.48 (t, 3H, OCH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3J = 4.5 Hz), 2.32 (s, 3H, CH3), 4.08 (q, 2H,
3J = 4.5 Hz), 2.32 (s, 3H, CH3), 4.08 (q, 2H, ![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH3J = 6.9 Hz), 6.84–7.03 (m, 5H Ar-H), 7.06–7.37 (m, 5H, Ar-H), 7.40–7.76 (m, 5H, Ar-H); 13C-NMR (120 MHz, CDCl3): δ: 14.97, 23.28, 63.14, 114.28, 114.29, 115.29, 116.30, 118.30, 120.41, 122.49, 127.52, 127.53, 128.59, 129.11, 129.66, 130.58, 141.64, 149.49, 152.23, 153.15, 156.36, 159.08, 166.42. Mass: m/z: 517.10 [M + 2]+. Elemental analysis for C29H23ClN2O3S: calculated: C, 67.63; H, 4.50; N, 5.44; S, 6.23. Found: C, 67.78; H, 4.56; N, 5.49; S, 6.45.
2CH3J = 6.9 Hz), 6.84–7.03 (m, 5H Ar-H), 7.06–7.37 (m, 5H, Ar-H), 7.40–7.76 (m, 5H, Ar-H); 13C-NMR (120 MHz, CDCl3): δ: 14.97, 23.28, 63.14, 114.28, 114.29, 115.29, 116.30, 118.30, 120.41, 122.49, 127.52, 127.53, 128.59, 129.11, 129.66, 130.58, 141.64, 149.49, 152.23, 153.15, 156.36, 159.08, 166.42. Mass: m/z: 517.10 [M + 2]+. Elemental analysis for C29H23ClN2O3S: calculated: C, 67.63; H, 4.50; N, 5.44; S, 6.23. Found: C, 67.78; H, 4.56; N, 5.49; S, 6.45.
5-[5-(2-Chloro-phenyl)-furan-2-ylmethylene]-2-(4-methoxy-phenylimino)-3-phenyl-thiazolidin-4-one (3a). Light yellow solid; yield: 67%; M.p.: 124–125 °C; Rf: 0.58; M.W.: 486.97; FT-IR (νmax; cm−1 KBr): 1543 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1646 (C
N), 1646 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (300 MHz, CDCl3-d6, TMS): δ: 3.88 (s, 3H, OCH3), 6.84–7.13 (m, 6H, Ar-H), 7.15–7.62 (m, 8H, Ar-H), 7.73 (s, 1H, olefinic proton), 7.80–7.83 (m, 1H, Ar-H), 13C-NMR (120 MHz, CDCl3):δ: 55.64, 114.34, 119.85, 121.32, 124.05, 127.14, 127.37, 128.05, 128.64, 129.74, 130.79, 131.81, 133.65, 136.11, 138.81, 148.42, 150.82, 151.52, 158.92, 166.32. Mass: m/z: 487.1 [M + 1]+. Elemental analysis for C27H19ClN2O3S: calculated: C, 66.59; H, 3.93; N, 5.75; S, 6.58. Found: C, 66.81; H, 3.99; N, 5.81; S, 6.68.
O); 1H-NMR (300 MHz, CDCl3-d6, TMS): δ: 3.88 (s, 3H, OCH3), 6.84–7.13 (m, 6H, Ar-H), 7.15–7.62 (m, 8H, Ar-H), 7.73 (s, 1H, olefinic proton), 7.80–7.83 (m, 1H, Ar-H), 13C-NMR (120 MHz, CDCl3):δ: 55.64, 114.34, 119.85, 121.32, 124.05, 127.14, 127.37, 128.05, 128.64, 129.74, 130.79, 131.81, 133.65, 136.11, 138.81, 148.42, 150.82, 151.52, 158.92, 166.32. Mass: m/z: 487.1 [M + 1]+. Elemental analysis for C27H19ClN2O3S: calculated: C, 66.59; H, 3.93; N, 5.75; S, 6.58. Found: C, 66.81; H, 3.99; N, 5.81; S, 6.68.
5-[5-(2-Chloro-phenyl)-furan-2-ylmethylene]-3-(4-fluoro-phenyl)-2-(4-methoxy-phenylimino)-thiazolidin-4-one (3b). Yellow solid; yield: 73%; M.p.: 147–149 °C; Rf: 0.54; M.W.: 504.96; FT-IR (νmax; cm−1 KBr): 1567 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1649 (C
N), 1649 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (300 MHz, CDCl3-d6, TMS): δ: 3.89 (s, 3H, OCH3), 6.80–7.13 (m, 6H, Ar-H), 7.21–7.42 (m, 5H, Ar-H), 7.46 (s, 1H, olefinic proton), 7.48–761 (m, 3H, Ar-H); 13C-NMR (120 MHz, CDCl3): δ: 55.73, 108.53, 114.05, 114.73, 115.25, 116.24, 116.47, 116.98, 117.12, 118.58, 118.66, 118.90, 119.99, 122.38, 122.43, 122.50, 125.57, 125.60, 127.76, 127.78, 127.82, 129.07, 129.17, 129.76, 129.90, 129.96, 134.16, 134.33, 141.92, 144.11, 144.45, 149.76, 149.84, 151.51, 153.58, 155.15, 155.76, 157.81, 159.13, 166.17, 166.19. Mass: m/z: 504.9 [M+]+. Elemental analysis for C27H18ClFN2O3S: calculated: C, 64.22; H, 3.59; N, 5.55; S, 6.35. Found: C, 64.32; H, 3.71; N, 5.65; S, 6.56.
O); 1H-NMR (300 MHz, CDCl3-d6, TMS): δ: 3.89 (s, 3H, OCH3), 6.80–7.13 (m, 6H, Ar-H), 7.21–7.42 (m, 5H, Ar-H), 7.46 (s, 1H, olefinic proton), 7.48–761 (m, 3H, Ar-H); 13C-NMR (120 MHz, CDCl3): δ: 55.73, 108.53, 114.05, 114.73, 115.25, 116.24, 116.47, 116.98, 117.12, 118.58, 118.66, 118.90, 119.99, 122.38, 122.43, 122.50, 125.57, 125.60, 127.76, 127.78, 127.82, 129.07, 129.17, 129.76, 129.90, 129.96, 134.16, 134.33, 141.92, 144.11, 144.45, 149.76, 149.84, 151.51, 153.58, 155.15, 155.76, 157.81, 159.13, 166.17, 166.19. Mass: m/z: 504.9 [M+]+. Elemental analysis for C27H18ClFN2O3S: calculated: C, 64.22; H, 3.59; N, 5.55; S, 6.35. Found: C, 64.32; H, 3.71; N, 5.65; S, 6.56.
3-(4-Chloro-phenyl)-5-[5-(2-chloro-phenyl)-furan-2-ylmethylene]-2-(4-methoxy-phenylimino)-thiazolidin-4-one (3c). Light yellow solid; yield: 62%; M.p.: 154–156 °C; Rf: 0.53; M.W.: 521.41 FT-IR (νmax; cm−1 KBr): 1567 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1649 (C
N), 1649 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (300 MHz, CDCl3-d6, TMS): δ: 3.86 (s, 3H, OCH3), 6.84–7.08 (m, 6H, Ar-H), 7.28–7.48 (m, 7H, Ar-H), 7.61 (s, 1H, olefinic proton), 7.80–7.83 (m, 1H, Ar-H); 13C-NMR (120 MHz, CDCl3): δ: 55.95, 114.34, 114.74, 116.26, 116.46, 118.55, 118.55, 120.17, 120.77, 122.03, 124.19, 126.12, 127.21, 127.23, 127.27, 127.29, 127.33, 127.75, 128.95, 128.98, 129.19, 129.42, 129.72, 130.00, 130.14, 130.12, 130.25, 130.26, 141.21, 147.28, 149.25, 149.26, 151.15, 152.17, 153.14, 153.17, 157.18, 159.12, 159.18, 166.16. Mass: m/z: 521.9 [M+]+. Elemental analysis for C27H18Cl2N2O3S: calculated: C, 62.19; H, 3.48; N, 5.37; S, 6.15. Found: C, 62.34; H, 3.58; N, 5.89; S, 6.25.
O); 1H-NMR (300 MHz, CDCl3-d6, TMS): δ: 3.86 (s, 3H, OCH3), 6.84–7.08 (m, 6H, Ar-H), 7.28–7.48 (m, 7H, Ar-H), 7.61 (s, 1H, olefinic proton), 7.80–7.83 (m, 1H, Ar-H); 13C-NMR (120 MHz, CDCl3): δ: 55.95, 114.34, 114.74, 116.26, 116.46, 118.55, 118.55, 120.17, 120.77, 122.03, 124.19, 126.12, 127.21, 127.23, 127.27, 127.29, 127.33, 127.75, 128.95, 128.98, 129.19, 129.42, 129.72, 130.00, 130.14, 130.12, 130.25, 130.26, 141.21, 147.28, 149.25, 149.26, 151.15, 152.17, 153.14, 153.17, 157.18, 159.12, 159.18, 166.16. Mass: m/z: 521.9 [M+]+. Elemental analysis for C27H18Cl2N2O3S: calculated: C, 62.19; H, 3.48; N, 5.37; S, 6.15. Found: C, 62.34; H, 3.58; N, 5.89; S, 6.25.
3-(4-Bromo-phenyl)-5-[5-(2-chloro-phenyl)-furan-2-ylmethylene]-2-(4-methoxy-phenylimino)-thiazolidin-4-one (3d). Light yellow solid; yield: 64%; M.p.: 185–186 °C; Rf: 0.51; M.W.: 565.87; FT-IR (νmax; cm−1 KBr): 1581 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1688 (C
N), 1688 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (400 MHz, CDCl3-d6, TMS): δ: 3.89 (s, 3H, OCH3), 6.81–7.01 (m, 6H, Ar-H), 7.39–7.60 (m, 7H, Ar-H), 7.61 (s, 1H, olefinic proton), 7.71 (s, 1H, Ar-H); 13C-NMR (120 MHz, CDCl3): δ: 55.08, 108.73, 114.76, 115.08, 115.39, 116.08, 118.18, 119.18, 119.19, 120.29, 122.20, 124.22, 125.24, 125.25, 126.26, 127.21, 127.22, 127.25, 127.29, 129.29, 129.41, 130.29, 130.30, 134.30, 141.34, 147.41, 149.47, 149.49, 151.49, 152.51, 155.52, 155.55, 156.59, 159.56, 159.59, 166.66. Mass: m/z: 567.5 [M + 1]+. Elemental analysis for C27H18BrClN2O3S: calculated: C, 57.31; H, 3.21; N, 4.95; S, 5.67. Found: C, 57.39; H, 3.41; N, 5.10; S, 5.87.
O); 1H-NMR (400 MHz, CDCl3-d6, TMS): δ: 3.89 (s, 3H, OCH3), 6.81–7.01 (m, 6H, Ar-H), 7.39–7.60 (m, 7H, Ar-H), 7.61 (s, 1H, olefinic proton), 7.71 (s, 1H, Ar-H); 13C-NMR (120 MHz, CDCl3): δ: 55.08, 108.73, 114.76, 115.08, 115.39, 116.08, 118.18, 119.18, 119.19, 120.29, 122.20, 124.22, 125.24, 125.25, 126.26, 127.21, 127.22, 127.25, 127.29, 129.29, 129.41, 130.29, 130.30, 134.30, 141.34, 147.41, 149.47, 149.49, 151.49, 152.51, 155.52, 155.55, 156.59, 159.56, 159.59, 166.66. Mass: m/z: 567.5 [M + 1]+. Elemental analysis for C27H18BrClN2O3S: calculated: C, 57.31; H, 3.21; N, 4.95; S, 5.67. Found: C, 57.39; H, 3.41; N, 5.10; S, 5.87.
5-[5-(2-Chloro-phenyl)-furan-2-ylmethylene]-3-(4-methoxy-phenyl)-2-(2-methoxy-phenylimino)-thiazolidin-4-one (3e). Light yellow solid; yield: 69%; M.p.: 191–192 °C; Rf: 0.56; M.W.: 517.00; FT-IR (νmax; cm−1 KBr): 1588 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1698 (C
N), 1698 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (300 MHz, CDCl3-d6, TMS):δ: 3.89 (s, 3H, OCH3), 3.97 (s, 3H, OCH3), 6.82–7.09 (m 6H, Ar-H), 7.17–7.49 (m, 7H, Ar-H), 7.61 (s, IH, olefinic proton), 7.73–7.82 (m, 1H, Ar-H); 13C-NMR (120 MHz, CDCl3): δ: 55.82, 55.94, 108.65, 112.02, 114.26, 114.67, 116.58, 118.22, 119.81, 120.83, 121.08, 122.00, 122.54, 125.51, 125.83, 127.27, 127.85, 129.09, 134.24, 137.72, 149.93, 150.88, 153.27, 155.66, 159.61, 166.49. Mass: m/z: 518.4 [M + 1]+. Elemental analysis for C28H21ClN2O4S: calculated: C, 65.05; H, 4.09; N, 5.42. S, 6.20. found: C, 65.88; H, 4.16; N, 5.49; S, 6.30.
O); 1H-NMR (300 MHz, CDCl3-d6, TMS):δ: 3.89 (s, 3H, OCH3), 3.97 (s, 3H, OCH3), 6.82–7.09 (m 6H, Ar-H), 7.17–7.49 (m, 7H, Ar-H), 7.61 (s, IH, olefinic proton), 7.73–7.82 (m, 1H, Ar-H); 13C-NMR (120 MHz, CDCl3): δ: 55.82, 55.94, 108.65, 112.02, 114.26, 114.67, 116.58, 118.22, 119.81, 120.83, 121.08, 122.00, 122.54, 125.51, 125.83, 127.27, 127.85, 129.09, 134.24, 137.72, 149.93, 150.88, 153.27, 155.66, 159.61, 166.49. Mass: m/z: 518.4 [M + 1]+. Elemental analysis for C28H21ClN2O4S: calculated: C, 65.05; H, 4.09; N, 5.42. S, 6.20. found: C, 65.88; H, 4.16; N, 5.49; S, 6.30.
5-[5-(2-Chloro-phenyl)-furan-2-ylmethylene]-3-(4-ethoxy-phenyl)-2-(4-methoxy-phenylimino)-thiazolidin-4-one (3f). Light yellow solid; yield: 69%; M.p.: 176–177 °C; Rf: 0.56; M.W.: 531.02; FT-IR (νmax; cm−1 KBr): 1589 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1681(C
N), 1681(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (400 MHz, CDCl3-d6, TMS): δ: 1.43 (t, 3H, OCH2
O); 1H-NMR (400 MHz, CDCl3-d6, TMS): δ: 1.43 (t, 3H, OCH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3, J = 6.6 Hz), 3.84 (s, 3H, OCH3), 4.05 (q, 2H,
3, J = 6.6 Hz), 3.84 (s, 3H, OCH3), 4.05 (q, 2H, ![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH3, J = 7.1 Hz), 6.82–7.05 (m, 7H, Ar-H), 7.22–7.45 (m, 6H, Ar-H), 7.59 (s, 1H, olefinic proton), 7.78 (s, 1H, Ar-H); 13C-NMR (120 MHz, CDCl3): δ: 14.96, 55.52, 63.71, 114.07, 114.29, 114.67, 114.90, 115.13, 116.61, 118.12, 120.02, 122.52, 127.89, 128.12, 129.10, 129.15, 130.60, 141.70, 149.48, 153.19, 156.37, 156.99, 159.10, 159.66, 166.44. Mass: m/z: 531.12 [M+]+. Elemental analysis for C29H23ClN2O4S: calculated: C, 65.59; H, 4.37; N, 5.28; S, 6.04. Found: C, 65.91; H, 4.43; N, 5.58; S, 6.49.
2CH3, J = 7.1 Hz), 6.82–7.05 (m, 7H, Ar-H), 7.22–7.45 (m, 6H, Ar-H), 7.59 (s, 1H, olefinic proton), 7.78 (s, 1H, Ar-H); 13C-NMR (120 MHz, CDCl3): δ: 14.96, 55.52, 63.71, 114.07, 114.29, 114.67, 114.90, 115.13, 116.61, 118.12, 120.02, 122.52, 127.89, 128.12, 129.10, 129.15, 130.60, 141.70, 149.48, 153.19, 156.37, 156.99, 159.10, 159.66, 166.44. Mass: m/z: 531.12 [M+]+. Elemental analysis for C29H23ClN2O4S: calculated: C, 65.59; H, 4.37; N, 5.28; S, 6.04. Found: C, 65.91; H, 4.43; N, 5.58; S, 6.49.
5-[5-(2-Chloro-phenyl)-furan-2-ylmethylene]-3-(2-methoxy-phenyl)-2-(4-methoxy-phenylimino)-thiazolidin-4-one (3g). Light brown solid; yield: 61%; M.p.: 179–180 °C; Rf: 0.57; M.W.: 517; FT-IR (νmax; cm−1 KBr); 1595 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1688(C
N), 1688(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (400 MHz, CDCl3-d6, TMS): δ: 3.83 (s, 6H, 2OCH3), 6.79(d 1H, Ar-H, J = 3.72 Hz), 6.90 (d, 2H, Ar-H, J = 8.88 Hz), 6.92 (d, 2H, Ar-H, J = 6.92 Hz), 7.04 (d, 2H, Ar-H, J = 8.88), 7.19–7.24 (m, 2H, Ar-H), 7.28 (d, 1H, Ar-H, J = 3.72), 7.37–7.43 (m, 3H, Ar-H), 7.56 (s, 1H, olefinic proton), 7.74–7.76 (m, 1H, Ar-H); 13C-NMR (120 MHz, CDCl3): δ: 55.51, 55.52, 114.09, 114.31, 114.67, 116.61, 118.16, 119.99, 122.54, 127.02, 128.09, 129.07, 129.17, 130.58, 130.97, 141.77, 149.96, 152.36, 153.17, 157.02, 159.67, 166.39. Mass: m/z: 517.21 [M+]+. Elemental analysis for C28H21ClN2O4S: calculated: C, 65.05; H, 4.09; N, 5.42; S, 6.20. Found: C, 65.42; H, 4.69; N, 5.82; S, 6.85.
O); 1H-NMR (400 MHz, CDCl3-d6, TMS): δ: 3.83 (s, 6H, 2OCH3), 6.79(d 1H, Ar-H, J = 3.72 Hz), 6.90 (d, 2H, Ar-H, J = 8.88 Hz), 6.92 (d, 2H, Ar-H, J = 6.92 Hz), 7.04 (d, 2H, Ar-H, J = 8.88), 7.19–7.24 (m, 2H, Ar-H), 7.28 (d, 1H, Ar-H, J = 3.72), 7.37–7.43 (m, 3H, Ar-H), 7.56 (s, 1H, olefinic proton), 7.74–7.76 (m, 1H, Ar-H); 13C-NMR (120 MHz, CDCl3): δ: 55.51, 55.52, 114.09, 114.31, 114.67, 116.61, 118.16, 119.99, 122.54, 127.02, 128.09, 129.07, 129.17, 130.58, 130.97, 141.77, 149.96, 152.36, 153.17, 157.02, 159.67, 166.39. Mass: m/z: 517.21 [M+]+. Elemental analysis for C28H21ClN2O4S: calculated: C, 65.05; H, 4.09; N, 5.42; S, 6.20. Found: C, 65.42; H, 4.69; N, 5.82; S, 6.85.
5-[5-(2-Chloro-phenyl)-furan-2-ylmethylene]-2-(4-methoxy-phenylimino)-3-o-tolyl-thiazolidin-4-one (3h). Light yellow solid; yield: 55%; M.p.: 164–165 °C; Rf: 0.58; M.W.: 501.00; FT-IR (νmax; cm−1 KBr): 1570 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1675 (C
N), 1675 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (300 MHz, CDCl3-d6, TMS): δ: 2.19 (s, 3H, CH3), 3.87 (s, 3H, OCH3), 6.79(s 2H, Ar-H), 6.91–7.07 (m, 6H, Ar-H), 7.32–7.41 (m, 4H, Ar-H), 7.54 (s, 1H, olefinic proton), 7.57–7.58 (m, 2H, Ar-H); 13C-NMR (120 MHz, CDCl3): δ: 17.94, 55.53, 114.08, 114.10, 114.31, 114.68, 114.72, 116.60, 116.73, 118.17, 118.22, 120.00, 120.03, 120.17, 122.56, 124.90, 126.38, 127.03, 128.04, 129.07, 129.13, 129.18, 130.12, 130.52, 130.70, 130.93, 13097, 141.78, 147.28, 149.36, 149.45, 151.98, 152.37, 153.16, 153.20, 157.03, 159.67, 159.74, 166.39. Mass: m/z: 502.8 [M + 1]+. Elemental analysis for C28H21ClN2O3S: calculated: C, 67.13; H, 4.22; N, 5.59. S, 6.40; found: C, 67.59; H, 4.30; N, 5.96; S, 6.59.
O); 1H-NMR (300 MHz, CDCl3-d6, TMS): δ: 2.19 (s, 3H, CH3), 3.87 (s, 3H, OCH3), 6.79(s 2H, Ar-H), 6.91–7.07 (m, 6H, Ar-H), 7.32–7.41 (m, 4H, Ar-H), 7.54 (s, 1H, olefinic proton), 7.57–7.58 (m, 2H, Ar-H); 13C-NMR (120 MHz, CDCl3): δ: 17.94, 55.53, 114.08, 114.10, 114.31, 114.68, 114.72, 116.60, 116.73, 118.17, 118.22, 120.00, 120.03, 120.17, 122.56, 124.90, 126.38, 127.03, 128.04, 129.07, 129.13, 129.18, 130.12, 130.52, 130.70, 130.93, 13097, 141.78, 147.28, 149.36, 149.45, 151.98, 152.37, 153.16, 153.20, 157.03, 159.67, 159.74, 166.39. Mass: m/z: 502.8 [M + 1]+. Elemental analysis for C28H21ClN2O3S: calculated: C, 67.13; H, 4.22; N, 5.59. S, 6.40; found: C, 67.59; H, 4.30; N, 5.96; S, 6.59.
5-[5-(4-Chloro-phenyl)-furan-2-ylmethylene]-2-(4-methoxy-phenylimino)-3-phenyl-thiazolidin-4-one (4a). Light yellow solid; yield: 59%; M.p.: 118–119 °C; Rf: 0.59; M.W.: 486.97; FT-IR (νmax; cm−1 KBr): 1562 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1671 (C
N), 1671 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (300 MHz, CDCl3-d6, TMS): δ: 3.88 (s, 3H, OCH3), 6.80–7.08 (m, 9H, Ar-H), 7.34–7.42 (m, 4H, Ar-H), 7.56 (s, 1H, olefinic proton), 7.59–7.7.69 (m, 2H, Ar-H); 13C-NMR (120 MHz, CDCl3): δ: 55.64, 114.81, 119.65, 121.17, 124.87, 127.43, 127.88, 128.99, 129.37, 130.34, 131.85, 133.32, 136.05, 138.37, 148.14, 150.05, 151.64, 158.74, 166.79. Mass: m/z: 487.6 [M + 1]+. Elemental analysis for C27H19ClN2O3S: calculated: C, 66.59; H, 3.93; N, 5.75; S, 6.58. Found: C, 66.98; H, 3.53; N, 5.65; S, 6.91.
O); 1H-NMR (300 MHz, CDCl3-d6, TMS): δ: 3.88 (s, 3H, OCH3), 6.80–7.08 (m, 9H, Ar-H), 7.34–7.42 (m, 4H, Ar-H), 7.56 (s, 1H, olefinic proton), 7.59–7.7.69 (m, 2H, Ar-H); 13C-NMR (120 MHz, CDCl3): δ: 55.64, 114.81, 119.65, 121.17, 124.87, 127.43, 127.88, 128.99, 129.37, 130.34, 131.85, 133.32, 136.05, 138.37, 148.14, 150.05, 151.64, 158.74, 166.79. Mass: m/z: 487.6 [M + 1]+. Elemental analysis for C27H19ClN2O3S: calculated: C, 66.59; H, 3.93; N, 5.75; S, 6.58. Found: C, 66.98; H, 3.53; N, 5.65; S, 6.91.
5-[5-(4-Chloro-phenyl)-furan-2-ylmethylene]-3-(4-fluoro-phenyl)-2-(4-methoxy-phenylimino)-thiazolidin-4-one (4b). Dark yellow solid; yield: 65%; M.p.: 137–138 °C; Rf: 0.58; M.W.: 504.96; FT-IR (νmax; cm−1 KBr): 1568 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1678 (C
N), 1678 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (400 MHz, CDCl3-d6, TMS): δ: 3.86 (s, 3H, OCH3), 6.77–6.80 (m, 2H, Ar-H), 6.92–7.10 (m, 5H, Ar-H), 7.20–7.25 (m, 1H, Ar-H), 7.32–7.39 (m, 2H, Ar-H), 7.43 (s, 1H, olefinic proton), 7.44–7.59 (m, 4H, Ar-H);13C-NMR (120 MHz, CDCl3):δ: 55.51 108.39, 114.73, 114.72, 115.75, 115.97, 116.24, 116.47, 116.98, 117.12, 118.58, 118.92, 119.11, 122.45, 122.76, 122.84, 125.51, 125.58, 127.51, 127.76, 127.81, 129.13, 129.17, 129.19, 129.90, 129.99, 134.43, 141.38, 144.57, 144.60, 149.78, 149.82, 151.76, 153.07, 155.90, 155.96, 157.17, 159.76, 166.16, 166.33. Mass: m/z: 504.6 [M+]+. Elemental analysis for C27H18ClFN2O3S: calculated: C, 64.22; H, 3.59; N, 5.55; S, 6.35. Found: C, 64.78; H, 3.68; N, 5.89; S, 6.93.
O); 1H-NMR (400 MHz, CDCl3-d6, TMS): δ: 3.86 (s, 3H, OCH3), 6.77–6.80 (m, 2H, Ar-H), 6.92–7.10 (m, 5H, Ar-H), 7.20–7.25 (m, 1H, Ar-H), 7.32–7.39 (m, 2H, Ar-H), 7.43 (s, 1H, olefinic proton), 7.44–7.59 (m, 4H, Ar-H);13C-NMR (120 MHz, CDCl3):δ: 55.51 108.39, 114.73, 114.72, 115.75, 115.97, 116.24, 116.47, 116.98, 117.12, 118.58, 118.92, 119.11, 122.45, 122.76, 122.84, 125.51, 125.58, 127.51, 127.76, 127.81, 129.13, 129.17, 129.19, 129.90, 129.99, 134.43, 141.38, 144.57, 144.60, 149.78, 149.82, 151.76, 153.07, 155.90, 155.96, 157.17, 159.76, 166.16, 166.33. Mass: m/z: 504.6 [M+]+. Elemental analysis for C27H18ClFN2O3S: calculated: C, 64.22; H, 3.59; N, 5.55; S, 6.35. Found: C, 64.78; H, 3.68; N, 5.89; S, 6.93.
3-(4-Chloro-phenyl)-5-[5-(4-chloro-phenyl)-furan-2-ylmethylene]-2-(4-methoxy-phenylimino)-thiazolidin-4-one (4c). Light yellow solid; yield: 62%; M.p.: 165–166 °C; Rf: 0.57; M.W.: 521.41 FT-IR (νmax; cm−1 KBr): 1592 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1693 (C
N), 1693 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (300 MHz, CDCl3-d6, TMS): δ: 3.87 (s, 3H, OCH3), 6.80 (s, 2H, Ar-H), 6.93–7.09 (m, 5H, Ar-H), 7.31–7.44 (m, 5H, Ar-H), 7.51 (s, 1H, olefinic proton), 7.54–7.59 (m, 2H, Ar-H); 13C-NMR (120 MHz, CDCl3): δ: 55.15, 108.74, 114.34, 114.74, 115.75, 115.95, 116.76, 116.86, 116.96, 118.58, 118.68, 118.98, 119.15, 122.42, 122.77, 122.87, 125.55, 127.17, 127.77, 127.87, 129.15, 129.16, 129.19, 129.97, 134.44, 141.38, 144.54, 144.64, 147.79, 147.89, 151.17, 153.83, 155.93, 157.17, 159.79, 166.16. 166.39. Mass: m/z: 521.4 [M+]+. Elemental analysis for C27H18Cl2N2O3S: calculated: C, 62.19; H, 3.48; N, 5.37; S, 6.15. Found: C, 62.69; H, 3.89; N, 5.77; S, 6.69.
O); 1H-NMR (300 MHz, CDCl3-d6, TMS): δ: 3.87 (s, 3H, OCH3), 6.80 (s, 2H, Ar-H), 6.93–7.09 (m, 5H, Ar-H), 7.31–7.44 (m, 5H, Ar-H), 7.51 (s, 1H, olefinic proton), 7.54–7.59 (m, 2H, Ar-H); 13C-NMR (120 MHz, CDCl3): δ: 55.15, 108.74, 114.34, 114.74, 115.75, 115.95, 116.76, 116.86, 116.96, 118.58, 118.68, 118.98, 119.15, 122.42, 122.77, 122.87, 125.55, 127.17, 127.77, 127.87, 129.15, 129.16, 129.19, 129.97, 134.44, 141.38, 144.54, 144.64, 147.79, 147.89, 151.17, 153.83, 155.93, 157.17, 159.79, 166.16. 166.39. Mass: m/z: 521.4 [M+]+. Elemental analysis for C27H18Cl2N2O3S: calculated: C, 62.19; H, 3.48; N, 5.37; S, 6.15. Found: C, 62.69; H, 3.89; N, 5.77; S, 6.69.
3-(4-Bromo-phenyl)-5-[5-(4-chloro-phenyl)-furan-2-ylmethylene]-2-(4-methoxy-phenylimino)-thiazolidin-4-one (4d). Light yellow solid; yield: 69%; M.p.: 166–167 °C; Rf: 0.57; M.W.: 565.86; FT-IR (νmax; cm−1 KBr): 1599 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1673 (C
N), 1673 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (300 MHz, CDCl3-d6, TMS): δ: 3.87 (s, 3H, OCH3), 6.81–7.09 (m, 6H, Ar-H), 7.35–7.60 (m, 7H, Ar-H), 7.61 (s, 1H, olefinic proton), 7.67–7.71 (m, 1H, Ar-H); 13C-NMR (120 MHz, CDCl3): δ: 55.73, 108.86, 114.06, 115.11, 116.08, 116.70, 118.30, 118.72, 119.21, 120.94, 122.81, 124.39, 125.66, 125.75, 126.42, 127.08, 127.15, 127.42, 127.65, 129.16, 129.10, 130.57, 130.77, 134.21, 141.33, 147.57, 149. 10, 149.67,151.51, 152.93, 155.51, 155.55, 156.39, 159.12, 159.14, 166.80. Mass: m/z: 567.1 [M + 2]+. Elemental analysis for C27H18BrClN2O3S: calculated: C, 57.31; H, 3.21; N, 4.95; S, 5.67. Found: C, 57.67; H, 3.56; N, 5.08; S, 5.56.
O); 1H-NMR (300 MHz, CDCl3-d6, TMS): δ: 3.87 (s, 3H, OCH3), 6.81–7.09 (m, 6H, Ar-H), 7.35–7.60 (m, 7H, Ar-H), 7.61 (s, 1H, olefinic proton), 7.67–7.71 (m, 1H, Ar-H); 13C-NMR (120 MHz, CDCl3): δ: 55.73, 108.86, 114.06, 115.11, 116.08, 116.70, 118.30, 118.72, 119.21, 120.94, 122.81, 124.39, 125.66, 125.75, 126.42, 127.08, 127.15, 127.42, 127.65, 129.16, 129.10, 130.57, 130.77, 134.21, 141.33, 147.57, 149. 10, 149.67,151.51, 152.93, 155.51, 155.55, 156.39, 159.12, 159.14, 166.80. Mass: m/z: 567.1 [M + 2]+. Elemental analysis for C27H18BrClN2O3S: calculated: C, 57.31; H, 3.21; N, 4.95; S, 5.67. Found: C, 57.67; H, 3.56; N, 5.08; S, 5.56.
5-[5-(4-Chloro-phenyl)-furan-2-ylmethylene]-3-(4-methoxy-phenyl)-2-(4-methoxy-phenylimino)-thiazolidin-4-one (4e). Light brown solid; yield: 71%; M.p.: 150–151 °C; Rf: 0.57; M.W.: 517; FT-IR (νmax; cm−1 KBr): 1589 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1683 (C
N), 1683 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (300 MHz, CDCl3-d6, TMS): δ: 3.86 (s, 3H, OCH3) 3.87 (s, 3H, OCH3), 6.85–7.08 (m, 7H, Ar-H), 7.24–7.48 (m, 7H, Ar-H), 7.61 (s, 1H, olefinic proton); 13C-NMR (120 MHz, CDCl3): δ: 55.87, 55.94, 108.65, 112.02, 114.26, 114.67, 116.58, 118.22, 119.81, 120.83, 121.08, 122.00, 122.54, 125.51, 125.83, 127.27, 127.85, 129.09, 134.24, 137.72, 149.93, 150.88, 153.27, 155.66, 159.61, 166.49. Mas: m/z: 518.9 [M + 1]+. Elemental analysis for C28H21ClN2O4S: calculated: C, 65.05; H, 4.09; N, 5.42; S, 6.20. Found: C, 65.89; H, 4.30; N, 5.92; S, 6.45.
O); 1H-NMR (300 MHz, CDCl3-d6, TMS): δ: 3.86 (s, 3H, OCH3) 3.87 (s, 3H, OCH3), 6.85–7.08 (m, 7H, Ar-H), 7.24–7.48 (m, 7H, Ar-H), 7.61 (s, 1H, olefinic proton); 13C-NMR (120 MHz, CDCl3): δ: 55.87, 55.94, 108.65, 112.02, 114.26, 114.67, 116.58, 118.22, 119.81, 120.83, 121.08, 122.00, 122.54, 125.51, 125.83, 127.27, 127.85, 129.09, 134.24, 137.72, 149.93, 150.88, 153.27, 155.66, 159.61, 166.49. Mas: m/z: 518.9 [M + 1]+. Elemental analysis for C28H21ClN2O4S: calculated: C, 65.05; H, 4.09; N, 5.42; S, 6.20. Found: C, 65.89; H, 4.30; N, 5.92; S, 6.45.
5-[5-(4-Chloro-phenyl)-furan-2-ylmethylene]-3-(4-ethoxy-phenyl)-2-(4-methoxy-phenylimino)-thiazolidin-4-one (4f). Light yellow solid; yield: 71%; M.p.: 160–161 °C; Rf: 0.56; M.W.: 531.02; FT-IR (νmax; cm−1 KBr): 1591 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1688 (C
N), 1688 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (400 MHz, CDCl3-d6, TMS): δ: 1.43(s, 3H, OCH2
O); 1H-NMR (400 MHz, CDCl3-d6, TMS): δ: 1.43(s, 3H, OCH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3J = 6.96 Hz), 3.86 (s, 3H, OCH3), 4.06 (q 2H,
3J = 6.96 Hz), 3.86 (s, 3H, OCH3), 4.06 (q 2H, ![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2 CH3J = 7.1 Hz), 6.77(s, 2H, Ar-H), 6.90–7.05 (m, 6H, Ar-H), 7.31–7.39 (m, 4H, Ar-H), 7.52 (s,1H, olefinic proton), 7.53–7.56 (m, 2H, Ar-H); 13C-NMR (120 MHz, CDCl3): δ: 14.96, 55.12, 63.52, 114.04, 114.35, 114.72, 114.90, 115.13, 116.61, 118.12, 120.04, 122.54, 127.84, 128.15, 129.16, 129.18, 130.60, 130.96, 141.78, 149.49, 153.53, 156.54, 156.55, 159.56, 159.59, 166.66. Mass: m/z: 532.9 [M + 1]+. Elemental analysis for C29H23ClN2O4S: calculated: C, 65.59; H, 4.37; N, 5.28; S, 6.04. Found: C, 65.78; H, 4.57; N, 5.45; S, 6.56.
2 CH3J = 7.1 Hz), 6.77(s, 2H, Ar-H), 6.90–7.05 (m, 6H, Ar-H), 7.31–7.39 (m, 4H, Ar-H), 7.52 (s,1H, olefinic proton), 7.53–7.56 (m, 2H, Ar-H); 13C-NMR (120 MHz, CDCl3): δ: 14.96, 55.12, 63.52, 114.04, 114.35, 114.72, 114.90, 115.13, 116.61, 118.12, 120.04, 122.54, 127.84, 128.15, 129.16, 129.18, 130.60, 130.96, 141.78, 149.49, 153.53, 156.54, 156.55, 159.56, 159.59, 166.66. Mass: m/z: 532.9 [M + 1]+. Elemental analysis for C29H23ClN2O4S: calculated: C, 65.59; H, 4.37; N, 5.28; S, 6.04. Found: C, 65.78; H, 4.57; N, 5.45; S, 6.56.
5-[5-(4-Chloro-phenyl)-furan-2-ylmethylene]-3-(2-methoxy-phenyl)-2-(4-methoxy-phenylimino)-thiazolidin-4-one (4g). Dark yellow solid; yield: 76%; M.p.: 166–167 °C; Rf: 0.56; M.W.: 517.00; FT-IR (νmax; cm−1 KBr): 1581 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1678 (C
N), 1678 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (300 MHz, CDCl3-d6, TMS): δ: 3.83 (s, 3H, OCH3), 3.87 (s, 3H, OCH3), 6.79 (s, 2H, Ar-H), 6.95–7.08 (m, 5H, Ar-H), 7.19–7.37 (m, 3H, Ar-H), 7.46–7.55 (m, 4H, Ar-H), 7.59 (s, 1H, olefinic proton); 13C-NMR (120 MHz, CDCl3): δ: 55.51, 55.52, 114.09, 114.31, 114.67, 116.61, 118.16, 119.99, 122.54, 127.02, 128.09, 129.07, 129.17, 130.58, 130.97, 141.77, 149.96, 152.36, 153.17, 157.02, 159.67, 166.39. Mass: m/z: 518.9 [M+]+. Elemental analysis for C28H21ClN2O4S: calculated: C, 65.05; H, 4.09; N, 5.42; S, 6.20. Found: C, 65.34; H, 4.69; N, 5.92; S, 6.80.
O); 1H-NMR (300 MHz, CDCl3-d6, TMS): δ: 3.83 (s, 3H, OCH3), 3.87 (s, 3H, OCH3), 6.79 (s, 2H, Ar-H), 6.95–7.08 (m, 5H, Ar-H), 7.19–7.37 (m, 3H, Ar-H), 7.46–7.55 (m, 4H, Ar-H), 7.59 (s, 1H, olefinic proton); 13C-NMR (120 MHz, CDCl3): δ: 55.51, 55.52, 114.09, 114.31, 114.67, 116.61, 118.16, 119.99, 122.54, 127.02, 128.09, 129.07, 129.17, 130.58, 130.97, 141.77, 149.96, 152.36, 153.17, 157.02, 159.67, 166.39. Mass: m/z: 518.9 [M+]+. Elemental analysis for C28H21ClN2O4S: calculated: C, 65.05; H, 4.09; N, 5.42; S, 6.20. Found: C, 65.34; H, 4.69; N, 5.92; S, 6.80.
5-[5-(4-Chloro-phenyl)-furan-2-ylmethylene]-2-(4-methoxy-phenylimino)-3-o-tolyl-thiazolidin-4-one (4h). Light yellow solid; yield: 58%; M.p.: 185–186 °C; Rf: 0.56; M.W.: 501.00; FT-IR (νmax; cm−1 KBr): 1588 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1692 (C
N), 1692 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (300 MHz, CDCl3-d6, TMS): δ: 2.19 (s, 3H, CH3) 3.87(s, 3H, OCH3) 6.84–7.10 (m, 6H, Ar-H), 7.13–7.48 (m, 7H, Ar-H), 7.62 (s, 1H, olefinic proton), 7.73–7.82 (m, 1H, Ar-H); 13C-NMR (120 MHz, CDCl3): δ: 17.53, 55.94, 114.00, 114.03, 114.17, 114.68, 114.72, 116.60, 116.73, 118.17, 120.08, 120.10, 120.31, 122.56, 124.90, 126.38, 127.07, 127.12, 127.52, 127.57, 127.70, 127.84, 128.04, 128.08, 129.07, 129.13, 130.93, 130.97, 141.78, 147.28, 149.36, 149.45, 151.98, 152.37, 153.16, 153.20, 157.03, 159.67, 159.74, 166.39. Mass: m/z: 501.2 [M+]+. Elemental analysis for C28H21ClN2O3S: calculated: C, 67.13; H, 4.22; N, 5.59; S, 6.40. Found: C, 67.89; H, 4.67; N, 5.69; S, 6.49.
O); 1H-NMR (300 MHz, CDCl3-d6, TMS): δ: 2.19 (s, 3H, CH3) 3.87(s, 3H, OCH3) 6.84–7.10 (m, 6H, Ar-H), 7.13–7.48 (m, 7H, Ar-H), 7.62 (s, 1H, olefinic proton), 7.73–7.82 (m, 1H, Ar-H); 13C-NMR (120 MHz, CDCl3): δ: 17.53, 55.94, 114.00, 114.03, 114.17, 114.68, 114.72, 116.60, 116.73, 118.17, 120.08, 120.10, 120.31, 122.56, 124.90, 126.38, 127.07, 127.12, 127.52, 127.57, 127.70, 127.84, 128.04, 128.08, 129.07, 129.13, 130.93, 130.97, 141.78, 147.28, 149.36, 149.45, 151.98, 152.37, 153.16, 153.20, 157.03, 159.67, 159.74, 166.39. Mass: m/z: 501.2 [M+]+. Elemental analysis for C28H21ClN2O3S: calculated: C, 67.13; H, 4.22; N, 5.59; S, 6.40. Found: C, 67.89; H, 4.67; N, 5.69; S, 6.49.
In silico molecular docking
The crystallized structure of 2AZ5 was chosen from Protein Data Bank and used as a target for molecular docking studies. The 2AZ5 structure was imported into Schrodinger using the Protein Preparation Wizard. Missing hydrogen atoms were added using a prime interface. Undesired water molecules were removed. The protein was then optimized and minimized to give the low energy and structurally correct target protein. As the target protein already had the site for the reference ligand, a grid was generated by selecting the ligand as the reference ligand. Finally the grid was validated and was used for further docking with new unknown ligands to predict their docking score. Chemical structures were drawn in Maestro (Schrodinger software) and geometrically refined by the LigPrep module.35 In this module 2-D structures were converted into 3-D structures, which were further subjected to the OPLS-2005 force field to generate a single low energy 3-D structure for each input structure. During this step chiralities were maintained. Docking was carried out using Schrodinger Glide software with Extra precision and XP descriptor information. This generates favourable ligand poses which are further screened through filters to examine the spatial fit of the ligand in the active site. Ligand poses which pass through initial screening are subjected to the evaluation and minimization of grid approximation. Scoring is then done on energy minimized poses to generate glide scores.Drugs and chemicals
Indomethacin, carrageenan, and carboxymethylcellulose were purchased from Chemicals Pvt. Limited (Bangalore, India), and ELISA kits of TNF-α were purchased from eBioscience (San Diego, CA, USA).TNF-α assay
RAW 264.7 cells were cultured in RPMI-1640 medium supplemented with 10% FBS, penicillin (100 units per ml), and streptomycin sulfate (100 mg ml−1) in a humidified atmosphere of 5% CO2. The cells were harvested with trypsin–EDTA and diluted to a suspension in fresh medium. These cells were seeded in 96-well plates with 2.1 × 105 cells per well, and allowed to adhere for 1 h. Then the medium was induced with 100 μg ml−1 LPS (lipopolysaccharide), test samples (20 μM), and incubated for 24 h. The supernatant (50 μL) was transferred into a 96-well ELISA plate and the TNF-α level was quantified by ELISA kits according to the manufacturer's instructions.36Cytotoxicity
Cell culture was carried out in triplicate following the same protocol as that of TNF-α assay. RAW 264.7 cells (2 × 105) were cultured in a 96-well plate containing DMEM supplemented with 10% FBS to obtain the required confluency. These cells were stimulated with 20 μM test compounds in the presence of 100 μg ml−1 LPS for 24 h. After that, the cells were washed twice with DPBS and incubated with 100 μl of 0.5 mg ml−1 MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) for 2 h at 37 °C for testing the cell viability. The medium was then discarded and 100 μl of dimethyl sulfoxide (DMSO) was added. After 30 min of incubation, absorbance at 570 nm was recorded using a microplate reader.36Anti-inflammatory activity
The anti-inflammatory activity was carried out by the reported method.37Animals were divided into 16 groups of five animals each. One group kept as a positive control group was orally administered with standard indomethacin (20 mg kg−1) and the other group kept as a negative control group was administered with 0.5% carboxymethyl cellulose solution. The remaining groups were test groups and administered orally with synthesized compounds at a dose of 20 mg kg−1 b.w. A freshly prepared solution of carrageenan (1.0% in sterile 0.9% NaCl solution) in a volume of 0.1 ml was injected subcutaneously into the subplantar region of the right hind paw after 1 h of administration of the test sample. The right hind paw volume was measured at 3 h and 5 h after carrageenan injection with the help of a digital plethysmometer. The percent anti-inflammatory activity was calculated according to the formula given below.| % Anti-inflammatory activity = [VC − Vt/VC] × 100 |
Immunohistochemistry
The paw tissues were fixed in formalin and embedded in paraffin. Sections of 5 μm thickness were cut into poly-lysine coated glass slides. Sections were deparaffinized three times (5 min) in xylene followed by dehydration in graded ethanol and finally rehydrated in running tap water. For antigen retrieval, sections were boiled in 10 mM citrate buffer (pH 6.0) for 5–7 min. Sections were incubated with hydrogen peroxide for 15 min to minimize non-specific staining and then rinsed three times (5 min each) with 1X PBST (0.05% Tween-20). The blocking solution was applied for 10 min then sections were incubated with diluted (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) primary antibodies, purified rabbit polyclonal anti-TNF-α antibody (BioLegend), overnight at 4 °C in a humid chamber. Further processing was done according to the instructions of the Ultra Vision plus Detection System Anti-Polyvalent, HRP/DAB (Ready-To-Use) staining kit (Thermo scientific system). The peroxidase complex was visualized with 3,3’-diaminobenzidine (DAB). Lastly the slides were counterstained with haematoxylin, cleaned in xylene, dehydrated with ethanol and after DPX mounting microscopic (BX 51 Olympus) analysis was done at 40× magnification.38
100) primary antibodies, purified rabbit polyclonal anti-TNF-α antibody (BioLegend), overnight at 4 °C in a humid chamber. Further processing was done according to the instructions of the Ultra Vision plus Detection System Anti-Polyvalent, HRP/DAB (Ready-To-Use) staining kit (Thermo scientific system). The peroxidase complex was visualized with 3,3’-diaminobenzidine (DAB). Lastly the slides were counterstained with haematoxylin, cleaned in xylene, dehydrated with ethanol and after DPX mounting microscopic (BX 51 Olympus) analysis was done at 40× magnification.38
Analgesic activity
The analgesic activity was carried out by a writhing test method using the previously reported method.39 Swiss albino mice (35–40 g) of either sex were divided into seven different groups and each group contained five animals. Group I was taken as control and received CMC suspension only, group II received the standard drug indomethacin and rest of the groups were orally administered with synthesized compounds at a dose of 20 mg kg−1. After 30 min of test sample administration, 0.1% acetic acid solution was given to mice intraperitoneally. The number of muscular contractions was counted over a period of 10 min after acetic acid injection. The data represent the total number of writhes observed during 10 min and is expressed as number of writhes.Ulcerogenic activity
The ulcerogenic study was carried out by an earlier reported method.40 The compounds which showed significant anti-inflammatory activity were further evaluated for their ulcerogenic effects. Control group rats were orally administered with a suspension of 1% carboxymethylcellulose only. The standard group and test samples were orally administered at a dose of 60 mg kg−1 b.w. which is three times the dose used for anti-inflammatory activity. Animals were sacrificed after 5 h dosing of standard group and test samples.Conflict of interest
Authors declare no conflict of interest.Acknowledgements
The authors wish to express their thanks to Dr G N Qazi, Vice Chancellor, JamiaHamdard for providing necessary research facilities. The authors are thankful to Dr Javed Iqbal, former Director, Institute of Life Sciences (ILS), Hyderabad, for providing necessary in vitro facilities and Dr Parimal Misra for his help in performing in vitro studies. One of the authors, YA, is also thankful to Hamdard National Foundation for providing financial assistance.References
- A. A. Ibrahim, O. A. Mahrous, K. A. Fars, E. H. E. Kamal, M. A. Abdulmalik and H. N. Steven, Int. J. Med. Sci., 2010, 7, 232–239 Search PubMed.
- G. R. Jorge and T. L. David, Geriatr. Nephrol. Urol., 1997, 7, 51–57 CrossRef.
- H. H. Tan, W. M. C. Ong, S. H. Lai and W. C. Chow, Singapore Med. J., 2007, 48, 582–585 CAS.
- S. K. Sonwane and S. D. Srivastava, Proc. Natl. Acad. Sci., India, 2008, 78, 129–136 CAS.
- S. K. Sonwane, S. D. Srivastava and S. K. Srivastava, J. Indian Counc. Chem., 2008, 25, 15–18 CAS.
- R. K. Rawal, T. Srivastava, W. Haq and S. B. Katti, J. Chem. Res., 2004, 5, 368 CrossRef.
- M. M. Kamel, H. I. Ali, M. M. Anwar, N. A. Mohamed and A. M. Soliman, Eur. J. Med. Chem., 2010, 45, 572 CrossRef CAS PubMed.
- S. Jaju, M. Palkar, V. Maddi, P. K. Ronad, S. Mamledesai, D. Satyanarayana and M. Ghatole, Arch. Pharm. Chem. Life Sci., 2009, 342, 723 CrossRef CAS PubMed.
- H. H. Parekh, K. H. Parikh and A. R. Parikh, J. Sci., Islamic Repub. Iran, 2004, 15, 143 CAS.
- T. Shrivastava, A. K. Gaikwad, W. Haq, S. Sinha and S. Katti, ARKIVOC, 2005, 120–130 Search PubMed.
- A. Rao, A. Carbone, A. Chimirri, E. D. Clereq and A. M. Monforte, Farmaco, 2002, 57, 747–751 CrossRef CAS PubMed.
- M. L. Barreca, A. Chimirri, L. D. Luca, A. M. Monforte, P. Monforte and A. Rao, Bioorg. Med. Chem. Lett., 2011, 11, 1793–1796 CrossRef.
- A. Kumar, C. S. Rajput and S. K. Bhati, Bioorg. Med. Chem., 2007, 15, 3089 CrossRef CAS PubMed.
- A. Deep, S. Jain and P. C. Sharma, Acta Pol. Pharm., 2010, 67, 63 CAS.
- M. E. Voss, P. H. Carter, A. J. Tebben, P. A. Scherle, G. D. Brown, L. A. Thompson, M. Xu, Y. C. Lo, G. Yang and R. Liu, Bioorg. Med. Chem. Lett., 2003, 13, 533 CrossRef CAS PubMed.
- J. Hu, Y. Wang, X. Wei, X. Wu, G. Chen, G. Cao, X. Shen, X. Zhang, Q. Tang, G. Liang and X. Li, Eur. J. Chem., 2013, 64, 292–301 CrossRef CAS PubMed.
- A. G. Athina, A. L. Alexey, I. H. L. Dimitra, T. E. Phaedra, A. F. Dmitrii, V. P. Vladimir, A. Intekhab and K. S. Anil, J. Med. Chem., 2008, 51, 1601–1609 CrossRef PubMed.
- H. Bettina, B. Sebastian, B. R. Carmen, K. Andreas, B. Julia, Z. Aleksandra, S. Holger, S. Gisbert and S. Dieter, J. Med. Chem., 2011, 54, 1943–1947 CrossRef PubMed.
- P. Vincent, K. Jasna, C. David, D. C. Dennis, P. S. Jeffrey, R. Karen, B. C. Fabienne and V. Delphine, J. Med. Chem., 2006, 49, 3857–3871 CrossRef PubMed.
- S. Hyeseung, L. Yun Suk, B. EunJooRoh, S. Jae Hong, O. Kwang-Seok, H. L. Byung, H. K. Hogyu and S. Jung, Bioorg. Med. Chem. Lett., 2012, 22, 5668–5674 CrossRef PubMed.
- B. L. Slomiany, J. Piotrowski, A. Slomiany and J. Scand, Gastroenterology, 1997, 32, 638–642 CAS.
- C. L. Jung, S. D. Jeng, S. C. Chuan, C. H. Wen, S. H. Shyh, H. S. Pei and J. H. Guang, Complementary Altern. Cardiovasc. Med., 2012, 1–12 Search PubMed.
- H. Saqlain, A. Mohammad Sarwar, H. Hinna, D. Abhijeet, U. Sadiq, S. Y. Mohammad, B. Sameena, N. Syed, A. Yakub and K. Chetna, Med. Chem. Res., 2014, 23, 4250–4268 CrossRef.
- H. Shivaji, P. C. Shelke, M. N. Mhaske, N. Sachin, M. W. Namdeo and D. B. Vivek, Bioorg. Med. Chem. Lett., 2012, 22, 6373–6376 CrossRef PubMed.
- E. Mogedda, S. S. Haiba, R. S. AbdElKarim, M. I. Gouhar and S. A. El-Zahar, Med. Chem. Res., 2014, 23, 3418–3435 CrossRef.
- M. M. Alam, A. Hussain, S. M. Hasan and A. T. Suruchi, Eurasian J. Med., 2009, 44, 2636–2642 CrossRef CAS PubMed.
- Y. Ali, M. S. Alam, H. Hamid, A. Husain, S. Bano, A. Dhulap, C. Kharbanda, S. Nazreen and S. Haider, Eurasian J. Med., 2014, 92, 490–500 CrossRef PubMed.
- G. D. Diana, J. J. Nitz, EP 566199, 1993 Search PubMed.
- Xcalibur CCD System, Oxford Diffraction Ltd, Abingdon, Oxfordshire, England, 2007 Search PubMed.
- R. C. Clark and J. S. Reid, Acta Crystallogr., Sect. A: Found. Crystallogr., 1995, 51, 887–897 CrossRef.
- CrysAlisPro(versions1.171.34.49), Oxford Diffraction Ltd, Abingdon, Oxfordshire, England.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
- L. J. Farrugia, J. Appl. Crystallogr., 2012, 45, 849–854 CrossRef CAS.
- D. P. Bhoot, R. C. Khunt, V. K. Shankhavara and H. H. Parekh, J. Sci., Islamic Repub. Iran, 2006, 17, 23–25 Search PubMed.
- M. D. Eldridge, C. W. Murray, T. R. Auton, G. V. Paolini and R. P. Mee, J. Comput.-Aided Mol. Des., 1997, 11, 425–445 CrossRef CAS PubMed.
- C. Kharbanda, M. S. Alam, H. Hamid, K. Javed, A. Dhulap, S. Bano, Y. Ali, S. Nazreen and S. Haider, Bioorg. Med. Chem. Lett., 2014, 22, 5804–5812 CrossRef CAS PubMed.
- C. A. Winter, E. A. Risley and G. W. Nuss, Proc. Soc. Exp. Biol. Med., 1962, 111, 544–546 CrossRef CAS PubMed.
- H. Z. Saba, M. Y. Kamaruddin, M. Suzana and M. Y. Yasmin, PLoS One, 2013, 8, 1–8 Search PubMed.
- R. Koster, M. Anderson and E. J. Beer, Fed. Proc., 1959, 18, 412–416 Search PubMed.
- H. G. Vogel and H. W. Vogel, Drug Discovery and Evaluation: Pharmacological Assays, Springer-Verlag, Berlin Heidelberg, 1997, p. 1231 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1058214. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5nj00078e |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2016 |