Designing hyperbranched polymers for gene delivery†
Quanbing
Mou‡
,
Yuan
Ma‡
,
Xin
Jin
* and
Xinyuan
Zhu
*
School of Chemistry and Chemical Engineering, State Key Laboratory of Metal Matrix Composites, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240, PR China. E-mail: xyzhu@sjtu.edu.cn; jinxin@iccas.ac.cn
First published on 7th March 2016
Abstract
Gene therapy through delivery of nucleic acids to the affected cells is a promising option for the treatment of various diseases. However, the delivery process is hampered by a series of barriers including gene packaging, in vivo and intracellular barriers. To overcome these obstacles, numerous vectors have been developed to achieve improved safety and enhanced gene transfection efficiency. Among these vectors, cationic hyperbranched polymers (HBPs) have attracted much attention due to their unique properties (e.g. low viscosity, good solubility, and multi-functionality) and three-dimensional globular structures. Thanks to the flexibility of HBPs, these properties can be tailored to overcome the above-mentioned barriers and develop an optimal gene vector. For example, HBPs with adjustable charge density can tightly condense nucleic acids and maintain polyplexes in solution. Besides, biocompatible HBP-based polyplexes can survive in the blood stream and penetrate the blood vessel wall and surrounding tissue. Furthermore, the stimuli-responsive HBPs release their genes at an appropriate point in the delivery process after endolysosomal escape. All of these properties are important in designing novel vectors for efficient gene delivery. Till now, many works focusing on tailoring cationic HBPs' properties for efficient gene delivery have been reported. This review briefly summarizes the main barriers of gene delivery, how to control the corresponding properties and recent progress of HBPs for gene delivery. After understanding the obstacles deeply, we hope to motivate the delicate design of cationic HBPs for clinical gene delivery applications.
1. Introduction
Gene therapy, which utilizes genetic materials to treat human diseases, has gained great attention over the past two decades as a promising option for the treatment of disorders such as immunodeficiency,1 cystic fibrosis,2 cardiovascular disease,3 cancer4 and other diseases. To achieve optimal therapeutic effects, gene therapy requires efficient delivery of nucleic acids (e.g. DNA, RNA and oligonucleotides) to the affected cells; however, this process is hampered by its low stability against nucleases and a series of physiological barriers. Hence, recent research efforts focus on the development of effective gene delivery vehicles to compact, protect and transport genes to the disease site. To achieve these goals, ideal gene delivery vectors should be endowed with excellent gene packaging capacity, high in vivo stability, potential to involve targeting ligands, and ability to assist intracellular delivery. Current gene vectors can be divided into two categories: viral and non-viral vectors. Viral carriers, such as adenoviruses and retroviruses, exhibit high transfection efficiency, but their clinical application is mainly hindered by safety concerns and cost-effectiveness. To overcome these problems, researchers have exploited alternatives, non-viral synthetic vectors, including cationic polymers,5 liposomes,6 peptides,7 proteins and exosomes,8 achieving improved safety and potential for large-scale production. Among these vectors, cationic polymers have attracted much attention due to their diversity in chemical compositions, topological structures and biological properties.9 Besides, cationic polymers employ facile synthetic procedures and possess potential for modification to functional ligands. In addition, some of them are biodegradable and lack immunogenicity in vivo. Hence, cationic polymers are ideal candidates for gene vectors with numerous research studies reported in the past two decades.To date, linear cationic polymers have still been broadly used for gene delivery since their first observance several decades ago. Hyperbranched polymers (HBPs), which possess a highly branched three-dimensional dendritic architecture, were just considered as gene vectors several years ago but now have become particularly attractive to act as gene delivery vehicles. HBPs exhibit significant superiority in properties and functions due to their unique structures compared with linear polymers and dendrimers. Compared with linear polymers, HBPs possess low solution or melt viscosity, high solubility and large quantities of terminal functional groups and interior cavities.10 Besides, in comparison to dendrimers, HBPs not only retain the advantages derived from their dendritic structure, but also employ convenient synthetic procedures and possess high cost-effectiveness.11 Especially, HBPs are some of the most important materials for biomaterials with excellent properties including controllable charge density, biocompatibility, biodegradability and stimuli-responsiveness. These outstanding advantages promote HBPs to be promising candidates for gene delivery vehicles. In the past few years, swift growth was observed in the field of HBP-based gene vectors. However, a systematic review has not yet been published in this field.
In this review, we provide a summary of recent research progress in HBPs for gene delivery. In the following sections, we will firstly illustrate the main barriers of gene delivery and present some current strategies to overcome these obstacles. Subsequently, the properties of HBPs are introduced, which facilitate abolition of these barriers and promotion of their application in gene delivery. Finally, we describe in detail various kinds of HBP vectors, including covalent, noncovalent and multifunctional HBP-based gene delivery systems. We hope to inspire continuous endeavors in this promising area and pave the way to the development of various cationic HBPs for gene delivery.
2. Barriers of gene delivery
Naked nucleic acids may suffer from many obstacles in the transportation process, which ultimately results in super low transfection efficiency. Hence, a successful gene delivery system is urgently needed to overcome all the barriers and enhance the transfection efficiency. A typical strategy is to firstly condense DNA tightly, then maintain the stability in a physiological environment, and finally decompose at the determined site after cell internalization. Based on reported works, the barriers of gene delivery, both extracellular and intracellular, can be summarized as follows.2.1 Gene packaging
Generally, DNA is condensed into compact structures via electrostatic interactions between cationic gene vectors and the negatively charged phosphate backbone of DNA. Such complexation of cationic polymers and negative DNA in aqueous solution will generate micelle-like structures, which are called polyplexes. The structural properties of polyplexes, such as size and morphology, are mainly affected by the polymer architecture, the preparation conditions (including concentration, pH, buffer type, and so on), and the number of cationic moieties.12 In addition, the stability of polyplexes is related to the structural parameters of cationic polymers, such as amino types, charge density and charge distribution.9 Besides, the stability of polyplexes is also influenced by the number of polymer nitrogen (N) and DNA phosphorus atoms (P).13 Generally speaking, high N/P ratios are widely used to form stable and compact polyplex particles, which can protect nucleic acids from extracellular as well as intracellular degradation (serum nuclease action and lysosomal digestion). However, it is notable that strong DNA condensation is not the sole requirement for high transfection efficacy. A perfect vector should balance the DNA binding ability with release properties, which means setting the DNA free at the determined site by responsive release or by competitive binding with intracellular substances (such as genomic DNA, cytosolic proteins or anionic membrane lipids).92.2 In vivo barriers
The systemic delivery of genes is hindered by a series of barriers, such as enzymatic degradation, rapid clearance and non-specific transport. Gene vectors can solve these problems with improved stability in the blood stream and excellent targeting ability to specific cells. Firstly, gene-delivery systems should achieve both chemical and physical stability during systemic circulation. Chemically, nucleic acids may undergo enzymatic degradation in blood and tissues and lose their chemical stabilities; hence, gene vectors are vital in defending genes against nucleases through steric effects.14 Physically, polyplexes may be aggregated resulting from the physiological salt conditions and adsorption of serum proteins (such as serum albumin and other negatively charged proteins). Unexpected aggregation usually results in rapid clearance by phagocytic cells and the reticuloendothelial system (RES)15 or even vascular blockage. To solve these two problems, polyplexes can be adorned with hydrophilic moieties such as polyethylene glycol (PEG),16 sugars,17 and proteins18 in the periphery to achieve ignored particle–protein interactions and enhanced stability. Moreover, in some diseases like cancer, targeting ligands are usually conjugated on gene vectors to increase the therapeutic efficiency with high targeting specificity. Fortunately, due to their large numbers of terminal functional groups, it is feasible to attach targeted ligands on HBP-based gene vectors, including membrane-bound receptor proteins, glycosidic derivatives, and some small molecules (such as folate). In addition, one strategy to enhance the targeting efficiency is fabricating surface-electrically neutral polyplexes, such as PEG, to avoid nonspecific electrostatic binding to the cell surface.192.3 Intracellular barriers
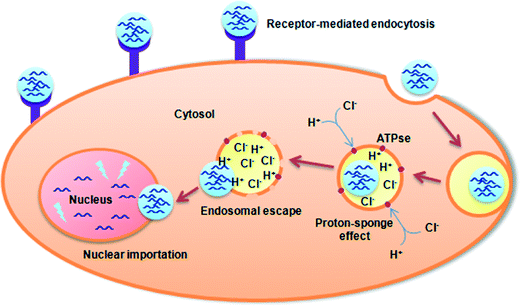
After the polyplexes overcome various in vivo barriers, they firstly attach to the cell surface via different mechanisms. To facilitate cell internalization, different strategies are used. Typically, cationic polyplexes can attach to cell membranes through electrostatic interactions with anionic cell surface proteoglycans, followed by internalization via adsorptive pinocytosis.20 For instance, Baldeschwieler demonstrated that gene transfection was inhibited after treatment with the inhibitor of proteoglycan sulfation (e.g. sodium chlorate).20 For targeted polyplexes, intracellular entry may be facilitated by receptor-mediated endocytosis when targeting ligands including antibodies, peptides and small molecules bind to their receptors in the cell surface. In both cases, polyplexes reside in endocytic vesicles (early or late endosomes) after successful cell uptake, and are subsequently trafficked into lysosomes, all of which represent hostile acidic environments and contain various degradative enzymes (such as DNAse or RNAse) (Fig. 1). Therefore, polyplexes should escape from the endolysosome to the cytosol as soon as possible, which can be realized through several ways. For example, the incorporation of chloroquine, a lysosomotropic agent raising the lysosomal pH and then inhibiting the degradative enzymes, may increase the gene transfection efficiency.21 Besides, synthetic peptide-conjugated polymers can also facilitate endosomal escape by disrupting the endolysosomal membrane.22 In addition, certain polymers possessing amine groups with low pKa values, including PEI and polyamidoamine (PAMAM), can induce a “proton-sponge” effect. These polymers exhibit a buffering ability in the endosomal vesicle, which induces the endosome to swell and lyse. Subsequently, polyplexes are released into the cytoplasm.After endosomal escape, the polyplexes still face multiple barriers to transport DNA through the cytoplasm to the nucleus. For instance, the mobility of polyplexes in the cytosol is hindered by cytoskeletal elements due to their large size.23 In addition, DNA may also degrade in the presence of cytosolic nucleases in the cytosol. Finally, after the residual DNA approaches the nuclear surface, they should wait for cell division and rely on nuclear membrane breakdown to enter the nucleus.24 Meanwhile, for non-dividing cells, nuclear entry may be facilitated by conjugation of short nuclear localization signal (NLS) peptides to polyplexes for targeted transport through the nuclear pore complex.25 In addition, the cationic polymer vectors may induce a nuclear-localizing effect via electrostatic interactions due to the positive charge.
To summarize, there are many obstacles in the gene delivery process such as gene packaging, in vivo delivery, and intracellular transport. Rational design of vectors with tunable charge density, biocompatibility and stimuli-responsive ability can overcome these barriers. Fortunately, cationic HBP-based gene delivery systems are flexible to be designed. For instance, some cationic HBP-based gene delivery systems have high in vivo delivery efficiency for the inclusion of shielding and targeting moieties. Besides, the appropriate modification of HBPs may accelerate the endosomal escape and nuclear importation of polyplexes. As is reviewed in the following sections, the properties of HBPs such as charge density controllability, biocompatibility and stimuli-responsiveness provide a good starting point for designing effective gene transfection agents.
3. Properties
As is mentioned above, non-viral vectors used for gene delivery need elaborate design to abolish these complicated barriers. Among various non-viral vectors, HBPs have unique advantages of low viscosity, good solubility, and multi-functionality, which play important roles in efficient gene binding and subsequent transfection. The flexibility of HBPs can be used to tailor the properties toward the development of an optimal gene vector. To face the physico-chemical challenges, DNA or RNA must be protected by vectors in solution, which requires HBPs with adjustable charge density to complex genes. Then, biocompatible HBPs can circulate in the blood stream and penetrate the blood vessel wall and surrounding tissue. In the cells of interest, HBPs must release their genes at some point in the delivery process after endolysosomal escape. Thus, stimuli-responsive HBPs can lead to controllable gene release and increased gene expression. These properties including charge density controllability, biocompatibility and stimuli-responsiveness ensure the rapid development of HBPs as gene vectors.3.1 Charge density controllability
DNA and RNA are negatively charged biomacromolecules, which can be degraded by nuclease enzymes within a few minutes. After binding to the positive HBPs through electrostatic interactions, the polyplexes protect DNA or RNA by sterically blocking the corresponding enzymes. However, cationic HBPs have to fulfill several requirements including protection of genes, endolysosomal escape and cytoplasmic release. The charge density can dramatically influence the cellular uptake and cytotoxic effects of HBPs apart from gene packaging. For example, the remaining positive charge after condensation of genes will lead to apoptosis and necrosis of cells. Therefore, a suitable charge density may reduce cytotoxic effects and enhance transfection efficiency.Till now, many published papers have focused on how to control the charge density of HBPs for efficient gene delivery. These methods for control include: (1) surface modification, (2) molecular weight adjustment, (3) use of different molecular structures, (4) use of different reaction processes, and (5) supramolecular recognition (Table 1). Firstly, for surface modification, Shi and coworkers modified the amine groups of PEI with acetyl, carboxyl and hydroxyl groups, and PEG chains to modulate the PEI surface charge to be positive, negative, or neutral.26 The end-groups in hyperbranched polysiloxysilane (HBPS) were modified with other hydrophilic molecules such as carboxylic acid (COOH) and quaternary ammonium groups (N+(CH3)3I−) with different surface charge densities. The zeta potential was altered from −40 mV to +64 mV by blending HBPS–COOH and HBPS–N+(CH3)3I−.27 Apart from modification using these small molecular weight molecules, the charge density of HBPs can also be modified with polymers as reported by Tian and coworkers.28 They grafted hydrophobic polyalanine to PEI (named PPAs) to form a series of amphiphilic copolymers. These synthesized cationic copolymers exhibited improved nucleic acid condensation properties, showing higher transfection efficiency compared to PEI. Secondly, adjusting the molecular weights of HBPs can also affect the charge density. Ma and coworkers synthesized hyperbranched polyglycerol amines (HPG amines) with different amine densities, corresponding to different charge densities of HPG amines, through adjusting the molecular weights of HPGs.29 The amine groups per molecule in correlation with the molecular weights showed that the cellular uptake was improved with increasing effective charge density. However, HBPs suffer from the high charge density and show agglomeration effects, which will reduce the efficiency dramatically. Thus, HBPs with neither a high nor a low charge density are suitable for cellular uptake and transfection. Thirdly, different structural properties such as the degree of branching (DB) of HBPs leads to different gene transfection efficiencies. Zhu and coworkers prepared a series of cationic PAMAMs with different branched architectures (DBs from 0.44 to 0.04) but similar compositions and molecular weights.30 With the increase in DB, the cationic polymers become more and more compact, accompanied by the enhancement of primary and tertiary amino groups. Therefore, the buffering capacities of cationic PAMAMs are strengthened. Fourthly, HBPs obtained using different reactants with similar structures have different condensation abilities and buffering capacities. A series of cationic HBPs were designed and synthesized by ring-opening polymerization between diepoxides and several polyamines. Polymers with branched or cyclic polyamines exhibited higher buffering capacity and tighter DNA affinity.31 Last but not the least, different from the above covalent-related modification methods, the charge density of HBPs can be further adjusted through supramolecular recognition. Zhu and coworkers prepared a novel class of charge-tunable supramolecular dendritic polymers (SDPs) via the host–guest interaction between two different cationic β-cyclodextrin (β-CD) derivative hosts and an adamantane-modified HPG (HPG-AD) guest (Fig. 2).32 The preparation of SDPs provides a facile approach for adjusting the charge density of supramolecular polycations by using noncovalent interactions.
| Charge-tuning methods | Examples | Ref. |
|---|---|---|
| Note: Hyperbranched poly(ethylene glycol)s (HPEGs); poly(phenylene ethynylene)s (PPEs); tris(2-aminoethyl)amine-attached β-cyclodextrin-centered hyperbranched polyglycerol (CD-HPG-TAEA). | ||
| Surface modification | PPAs | 28 |
| HBPS | 27 | |
| PEI derivatives | 26 | |
| PAMAMs | 33 | |
| Molecular weight adjustment | HPG amines | 29 |
| CD-HPG-TAEA | 34 | |
| HPG amines | 35 | |
| Different molecular structures | PAMAMs | 30 |
| Different reaction processes | HPEGs | 36 |
| PPEs | 37 | |
| Supramolecular recognition | SDPs | 32 |
 | ||
| Fig. 2 (A) Schematic representation of charge-tunable SDPs constructed via β-CD/AD host–guest interactions; (B) agarose gel electrophoresis retardation of pDNA by sample IC1 (a), sample IC2 (b), sample IC3 (c), and sample IC5 (d) at various N/P ratios (all samples have 1/1 molar ratio of β-CD/AD). AFM images of the pDNA (e) and pDNA polyplexes with IC1 (f), IC2 (g), IC3 (h), IC4 (i), and IC5 (j) at N/P = 5. Reproduced with permission.32 Copyright 2011, Royal Society of Chemistry. | ||
3.2 Biocompatibility
Polycationic polymers are widely used in gene delivery systems because they can bind to negatively charged DNA or RNA through electrostatic interactions to form polyplexes. These polyplexes protect the DNA or RNA from being degraded by nucleolytic enzymes. However, during blood circulation, these positively charged vectors might be unstable under physiological salt conditions or adsorb serum proteins to form polyplex aggregates. For example, PEI, which keeps its carried genes tightly condensed and protected, has been considered as the gold standard for polymer-based gene carriers because of its polyplexes' relatively high transfection efficacy.38 However, their biodistribution profiles upon systemic delivery and toxicity greatly limit their wide application. These limitations can be addressed by suitable modification of PEI. Among polycationic polymers, cationic HBPs can be modified similar to PEI to promote their further application in vivo.In order to obtain these biocompatible cationic HBPs, many strategies including post-modification of HBPs, preparation of alternative biocompatible HBPs, formation of nano-structures of HBPs, and cross-linking of cationic HBPs have been explored. For post-modification of HBPs, a lot of biocompatible small molecules, such as glutamic acid,39 maltose,40 maltotriose,40 oligomaltose,41 and β-CD,42 are introduced to modify cationic HBPs. The modified nanoparticles are of low cytotoxicity and high gene transfection efficiency in comparison with the original ones. Apart from modification using these small molecules, numerous polymers (e.g., PEG,26,43,44 polyalanine,28,45 HPEG,46 polyphenylalanine,45 polyglycerol,47 polylysine,48 PAMAM,49 poly(γ-benzyl-L-glutamate)50,51 and poly[N-(2-hydroxyethyl)-L-glutamine]52) are also employed. Surprisingly, Zhu and coworkers found that even HPEGs prepared via a one-pot Michael addition reaction of 2,20-(ethylenedioxy)bis-(ethylamine) (EOBEA) and poly(ethylene glycol) diacrylate (PEGDA) further end-capped with diethylamine with low charge density still exhibited high transfection efficiency in a COS-7 cell line in the absence and presence of serum.36 The modified HPEGs showed low cytotoxicity. That is to say, to obtain biocompatible HBPs with high transfection efficiency, focus should be on not only the modification of high charge density HBPs, but also some low charge density HBPs after post-modification, which can achieve the same effect. Moreover, beyond these modification methods through covalent interactions, Narain and Ahmed fabricated ternary complexes of PEI with anionic polymers and plasmid DNA through electrostatic interactions. These anionic glycopolymers were complexed with branched PEI at an optimal weight-by-weight ratio to reduce the toxicity of PEI. Different from the above-mentioned complicated modification process, many biocompatible HBPs with high transfection efficiency are reported to deliver genes directly without further modification. These biocompatible HBPs include polyspermine,53 hyperbranched cationic glycogen derivatives,54 HPAMAM,33,55 hyperbranched poly(ester amine),56 hyperbranched poly(sulfone amine),57 hyperbranched cationic polymers obtained by ring-opening polymerization between diepoxides and several polyamines,31 statistical copolymers of N-(2-hydroxypropyl)methacrylamide (HPMA) and the dendritic methacrylic monomer 2-(3-(bis(2-(diethylamino)ethyl)amino)propanamido)ethyl methacrylate (poly-(TEDETAMA-stat-HPMA)),58 hyperbranched cationic polysaccharide derivatives,59 and hyperbranched cationic amylopectin derivatives (Fig. 3).60 In addition, the self-assembly of amphiphilic cationic copolymers61,62 and co-assembly of PEI with PG6-PLA63 have been used to fabricate nano-complexes for biocompatible gene delivery. The biocompatible nano-complexes can be endowed with responsive abilities.64 Finally, the toxicity of cationic HBPs can be reduced through simple cross-linking. Both tris[2-(acryloyloxy)ethyl] isocyanurate65 and methyl acrylate66 are employed to cross-link PEI. These cross-linked PEIs exhibit lower cytotoxicity and rather high gene transfection efficiency compared to their corresponding PEIs.
3.3 Stimuli-responsiveness
Cationic HBPs with suitable charge density and biocompatibility can tightly condense genes and be stable in the blood stream without the risk of forming polyplex aggregates. However, during intracellular transport, to release the genes in a controllable manner is a great challenge. Thanks to the differentiated pathological environments and external stimuli such as pH, temperature, redox potential, light, ionic strength, magnetic and electric fields, ultrasound, etc., these characteristics are very useful to trigger the controllable release of genes in the responsive systems. Stimuli-responsive HBP systems can change their volume, structure or properties with respect to the corresponding environmental stimuli. The stimuli-responsive properties of cationic HBPs can effectively tailor the release behaviors of polyplexes and improve the gene transfection efficiency.Redox-sensitive systems are some of the most studied stimuli-responsive cationic HBPs for gene delivery. Due to the existence of a large difference in the redox potential between the mildly oxidizing extracellular milieu and the reducing intracellular fluids, reduction-sensitive HBP systems have received great attention for intracellular drug delivery. The disulfide bond can be cleaved in the presence of reducing agents such as glutathione (GSH) which is found in the blood plasma of humans in micromolar concentrations, whereas its concentration in the cytosol is around 10 mM.67 Interestingly, the cytosolic GSH concentration in some tumor cells is found to be about several times higher than the level in normal cells. Therefore, polyplexes based on the disulfide bond can facilitate the intracellular release of noncovalently encapsulated genes by cleavage of this bond. A series of disulfide-containing reactants such as N,N′-cystaminebisacrylamide,33,68–71 1,2-bis(2-(3-methylbuta-1,3-dien-2-yloxy)ethyl) disulfane,72 (propargylcarbamate)ethyldisulfide ethyl-1-carbamide-imidazole (PPA-cyst-CI),73 cystamine (CYST)74 and N,N′-dimethylcystamine (DMC)74,75 have been used to prepare novel redox-sensitive HBPs by Michael addition polymerization (Fig. 4). In some cases, these redox-sensitive polyplexes can significantly increase the transfection activity compared with non-reducible controls. Apart from the above backbone redox-sensitive HBPs, Thurecht and coworkers reported the covalent attachment of small RNA to the end-groups of a nonviral vector using a disulfide linkage.76 Moreover, to accelerate the release of genes from polyplexes, Liu and coworkers synthesized hyperbranched self-immolative polymers (hSIPs) with redox-cleavable capping moieties. The polyplexes underwent endosomal escape into the cytosol after 8 h of incubation, and then disassembled in the cytosol after being subjected to GSH-triggered hSIP depolymerization (Fig. 5).77
 | ||
| Fig. 5 (A) Schematic structures of reductive milieu-triggered hyperbranched self-immolative polymers (hSIPs); (B) schematic illustration of the formation of electrostatic polyplexes between pDNA and hSIP5 bearing multiple cationic PDMAEMA segments at the periphery; the subsequent hSIP depolymerization and pDNA release were triggered by intracellular reductive milieu (e.g., GSH). Adapted with permission.77 Copyright 2015, American Chemical Society. | ||
pH-responsive systems have attracted more and more attention because of the wide presence of pH variations within the body. The pH in the blood and normal tissues is approximately 7.4. In tumor sites and inflammatory tissues, extracellular pH (pH = 6.5–7.2) becomes acidic, mainly caused by the anaerobic respiration and subsequent glycolysis together with the tumor phenotype.78,79 The pH values of various intracellular compartments are quite different. The pH in the cytosol is about 7.4, while both the endosome (pH = 5.0–6.5) and lysosome (pH = 4.5–5.0) have a more acidic environment. The reversible covalent bonds can be tunably formed and broken under mild conditions, thus they are suitable for the construction of environment-responsive systems. Among the known reversible covalent bonds, the acylhydrazone bond, stable under neutral and alkaline conditions but hydrolyzed under acidic conditions, is particularly of interest in view of the thermodynamic stability, wide structural variability, and applications in both biomedicine and materials sciences. As one of the pH-reversible linkages, phenylboronate linking can be achieved through the reaction between phenylboronic acid-tethered hyperbranched oligoethylenimine (OEI-PBA) and 1,3-diol-rich HPG.64,80 The special buildup core–corona nanoconstruction offered significant advantages over the parent OEI-PBA, including strengthened affinity to siRNA and acidity-responsive release of payloads.80 Acid-labile β-thiopropionate can be slowly degraded under acidic conditions (pH = 5.0) to form a carboxylic acid, which was also used by Pan and coworkers to construct a pH-responsive gene delivery system.81 Apart from the acid-labile linkage, Feng and coworkers fabricated a pH-responsive vesicle via β-CD-centered HPG and linear AD-terminated octadecane spontaneous interlinkage.34 The responsive ability can be attributed to the weakened cohesion within the vesicle membrane caused by the increased hydrophilicity and strengthened charge repulsion among the protonated amine groups.34
Except for the above-mentioned differentiated pathological environments including redox potential and pH, some enzymes have differential expression between normal tissue and cancer tissue. For example, matrix metalloproteinase (MMP) is expressed in high levels in tumor cells. However, MMP transcript levels are low in normal cells. Thus, an MMP-substrate peptide is used to make a tumor-specific cleavable gene delivery system.82 Besides, different from using differential enzymatic expression to control the gene release, some cationic HBPs undergoing non-specific proteolytic degradation have also attracted attention from a few researchers. Klok and coworkers prepared an attractive hyperbranched polylysine (HBPL). Enzymatic degradation by trypsin is complete after 8 h. The degradation of HBPL cannot go to completion but will generate fragments of primarily ε-amide-linked lysine oligomers.83 Furthermore, Chen and coworkers developed an amphiphilic cationic hyperbranched poly(ethylene glycol)–polyethylenimine–poly(γ-benzyl L-glutamate) (PEG–PEI–PBLG) using trypsin degradation for gene delivery.84 The enzymatic degradability may help to minimize the cumulative cytotoxicity and also improve effective gene transfection.
While pH-, redox-, and enzyme-sensitive systems can respond to intracellular stimuli quickly, they require obvious changes in the chemical environment which might be not suitable for biological milieu. In contrast, light is an external stimulus that does not require any additional substances and chemical environmental changes. In addition, light stimulus shows unique advantages including a broad range of tunable parameters, relative biocompatibility and controllability in both spatial and temporal perspectives. However, only a few research works focus on photo-responsive HBP gene delivery systems. For example, Haag and coworkers reported two photo-responsive core/shell nanoparticles based on HPG for controlled release of DNA.85 The nanoparticles were composed of either bis(3-aminopropyl)methylamine (AMPA) or pentaethylenehexamine (PEHA) derivatives attached to the HPG core with a photo-responsive o-nitrobenzyl linker. The photolytic cleavage process was completed within only 2 min upon irradiation at 350 nm with a 100 W mercury lamp (Fig. 6).85
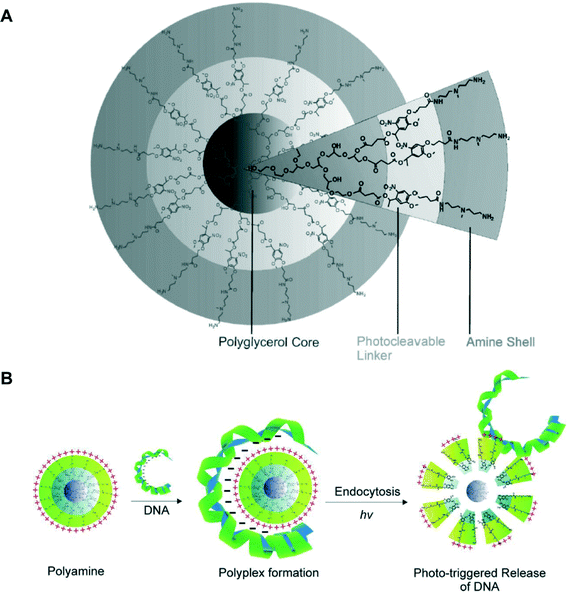 | ||
| Fig. 6 (A) The core/shell architecture based on HPG bearing an amine shell connected through an o-nitrobenzyl linker; (B) complexation of DNA by the dendritic core/shell architecture and photo-triggered release of DNA. Reproduced with permission.85 Copyright 2011, Wiley-VCH. | ||
Apart from the above-mentioned responsive HBPs for gene delivery, HBPs with other internal and external stimuli, such as temperature, salt, glucose, CO2, magnetic and electric fields, and ultrasound, can also be used for the design and application of responsive gene delivery systems. Besides, multi-responsive HBP systems can respond to more than one stimulus and fulfill better the manifold requirements of a delivery system in a complex biological environment. The combination of different responsiveness gives various functionalities, allowing the optimization of gene delivery with superior biocompatibility and enhanced transfection efficiency. However, up to now, there have been only a few works focusing on these delivery systems. For example, Pan and coworkers synthesized the pH-responsive and GSH-responsive hyperbranched star copolymers HP(DMAESP-co-BS2MOE) (methacryloyloxy-3-thiahexanoyl-N,N-dimethyl acetate (DMAESP); 2-(2′-bromoisobutyryloxy) ethyl 2′′-methacryloyl oxyethyl disulfide (BS2MOE)).81 HP(DMAESP-co-BS2MOE) exhibited a pH-responsive charge conversion behavior for the methacrylate unit and tertiary amine group of DMAESP linked by an acid-labile β-thiopropionate connection. Besides, HP(DMAESP-co-BS2MOE) containing disulfide bonds could be further degraded in the presence of GSH. Thus, after internalization of the corresponding polyplexes, the pH-responsive charge conversion behavior could promote the efficient release of the loaded DNA and the micelles would be cleaved by GSH to reduce the cytotoxicity. Therefore, multi-responsive HBP systems show a promising future in gene delivery.
4. Applications to gene delivery
Among various cationic polymer-based gene vectors, cationic HBPs are particularly interesting because of their advantages, such as charge density controllability, biocompatibility, stimuli-responsiveness, high safety, low/no immunogenicity, and convenience of synthesis and modification. Therefore, cationic HBPs used as gene delivery materials have attracted more and more attention, which greatly facilitates the polyplexes to go through various barriers, including gene packaging, in vivo barriers and intracellular barriers, and finally work in the nucleus of cells.4.1 Covalent polymer-based gene delivery systems
Among all synthetic cationic HBPs for gene delivery, hyperbranched PEI (HPEI) is regarded as the gold standard of gene transfection with the highest positive charge density. HPEI has strong DNA condensation capability and buffering capacity for the existence of numerous terminal primary amines. The above-mentioned proton-sponge effect has been widely adopted to explain the extremely high transfection efficiency of HPEI in living cells. Behr and coworkers found that HPEI caught the unidimensional DNA more efficiently than a linear polycation, such as polylysine, a conventional linear polycationic gene vector.86 However, the high cytotoxicity and non-biodegradability of HPEI greatly limit its in vivo application.To accelerate the in vivo application of gene delivery systems, various biocompatible or degradable cationic HBPs have been developed. As one of the most biocompatible cationic HBPs, HPG was modified to prepare efficient gene vectors. After incorporation of amino groups onto HPG, the effective charge density can be adjusted using the hydroxyl-to-amine group ratio in varying molecular weights. The HPG with moderate charge density has higher potential for effective DNA delivery compared to the high/low charged ones independent of their size, but the final efficiency can be optimized by the molecular weight.29 Besides, biocompatible PEG or HPEG was used to modify the toxic HPEI or HPAMAM to improve gene transfection efficiency and biocompatibility.43,44,46,62,84 The HBPS with a positive surface charge was found to be a suitable vehicle for non-viral gene delivery.27 Apart from these biocompatible systems, biodegradable hyperbranched polycations with hydrolytically labile groups such as ester and amide have been developed. For instance, biodegradable hyperbranched poly(ester amine)s,56,87,88 PAMAMs,33,71,75 poly(amino ester)s,89,90 and poly(amino acid)s83,91 were successfully synthesized. In addition to these hydrolytically degradable polycations, reducible hyperbranched polycations containing disulfide bonds,71,73,75,77,81 photo-responsive HPGs85 with oligoamine shells and pH-responsive hyperbranched architectures with pH-reversible groups64,80,81 have also been investigated. These responsive HBPs are stable in the blood, but easily cleavable in a stimulating environment such as photo-stimulation. For the biocompatibility and biodegradability of cationic HBPs, some cationic HBPs even show better transfection efficiency than HPEI.
Natural biomacromolecules/biopolymers with low cytotoxicity and good biocompatibility such as amylopectin and glycogen have been used as gene vectors, but their transfection efficiency is frequently disappointing. Through the amination of polysaccharides, various cationized polysaccharides have been designed and synthesized as gene delivery vectors.54,59,60 For example, a series of hyperbranched cationic amylopectin derivatives conjugated with 1,2-ethylenediamine, diethylenetriamine and 3-(dimethylamino)-1-propylamine residues were synthesized. The amylopectin derivatives could bind pDNA and Forkhead box O1 (FoxO1) to form polyplexes that showed high gene delivery capability and transfection efficiency in 293 T cells (Fig. 7).60 The cationic natural biomacromolecules/biopolymers synthesized through post-modification are attractive for developing safe and efficient gene vectors.
 | ||
| Fig. 7 (A) Synthesis of hyperbranched cationic amylopectin derivatives conjugated with oligoamine residues: chemical reaction and proposed structures of amylopectin and its hyperbranched cationic derivatives; (B) fluorescence micrographs and light-inverted micrographs of 293 T cells and A549 cells transfected with four amylopectin derivative/pDNA polyplexes (w/w = 10): (a) 1,2-ethylenediamine–amylopectin/pDNA, (b) diethylenetriamine–amylopectin/pDNA, (c) 3-(dimethylamino)-1-propylamine–amylopectin/pDNA, and (d) HPEI/pDNA polyplexes (N/P = 10) (×100). Reproduced with permission.60 Copyright 2012, Elsevier. | ||
4.2 Noncovalent polymer-based gene delivery systems
As is discussed above, the gene transfection efficiency is greatly related to the structural parameters of cationic polymers such as charge density, biocompatibility and stimuli-responsiveness.92 However, optimization of these structural parameters generally requires a large number of covalent polymerizations or modifications, which makes the preparation of optimal polycations tedious and raises the cost of preparation. Due to their dynamic-tunable properties and potential use in biomedical applications, supramolecular polymers based on noncovalent interactions have attracted tremendous interest.67,93 For example, Zhu and coworkers prepared a series of supramolecular dendritic polymers with different amines obtained by simply mixing HPG-AD with two cationic β-CD derivatives in water.32 These supramolecular polycations showed low cytotoxicity and high transfection efficiency comparable to those of HPEI. Significantly, the transfection efficiency could be easily improved by only adjusting the ratios of various cationic β-CD derivatives. Similarly, Feng and coworkers fabricated nano-sized vesicles via self-engineering on the basis of host–guest interactions between β-CD-centered HPG and AD-terminated octadecane for the combined achievement of drug encapsulation and DNA delivery (Fig. 8).34 This co-delivery vehicle presents a good example of rational design of cationic supramolecular vesicles for stimuli-responsive drug/DNA transport. | ||
| Fig. 8 Illustration of nanovesicle self-engineering driven by host–guest interactions between AD-C18 and CD-HPG-TAEA for gene delivery and drug encapsulation. Reproduced with permission.34 Copyright 2015, American Chemical Society. | ||
4.3 Multifunctional gene delivery systems
Monofunctional systems can only overcome specific biological barriers during drug delivery, dramatically limiting their therapeutic efficacy. Correspondingly, multifunctional gene delivery systems, which combine targeting, imaging and gene transfection together, hold tremendous promise in disease therapy. Due to this multifunctional combination, the diagnosis, targeted gene delivery, and real-time monitoring and judgment of therapeutic progress can be integrated, which offers new possibilities toward the development of personalized medicine. The unique properties of HBPs such as charge density controllability and the existence of many end-groups make them an ideal platform for the construction of multifunctional gene delivery systems. Zhu and coworkers synthesized HPAMAMs containing different amounts of β-CD (HPAMAM-CDs) through the Michael addition copolymerization of N,N′-methylenebisacrylamide, 1-(2-aminoethyl)piperazine, and mono-6-deoxy-6-ethylenediamino-β-CD (Fig. 9).42 In comparison with pure HPAMAM, HPAMAM-CDs showed lower cytotoxicity and significantly enhanced photoluminescence. HPAMAM-CDs could condense pDNA very efficiently. The cell internalization of the HPAMAM-CD/pDNA polyplexes was studied through flow cytometry and confocal laser scanning microscopy (CLSM) by detecting the fluorescence of HPAMAM-CD itself, which avoided the fluorescent labeling process. It demonstrated that the cellular uptake of HPAMAM-CD/pDNA polyplexes was very fast and HPAMAM-CDs mainly located in the cytoplasm of the cells during the gene transportation process. At the same time, the inner cavities of β-CDs in HPAMAM-CDs could be used as drug containers. Thus, HPAMAM-CDs may have potential applications as delivery materials in the combination of bioimaging, chemotherapy and gene therapy. Compared with fluorescence imaging, in which the scattering in tissues limits the spatial resolution with increasing depth, magnetic resonance imaging has numerous advantages including its excellent imaging penetration depth and safety, and excellent contrast between various soft tissues. Thus, to realize real-time monitoring and judge the therapeutic progress in vivo, Thurecht and coworkers prepared MRI-based HBP theranostics, which acted as an imaging agent for 19F MRI while at the same time carrying specific therapeutic genes (such as small interfering RNA) to a site of interest.76 Similarly, Shuai and coworkers developed PEI-g-PCL carrying other MRI contrast agents (superparamagnetic iron oxide nanoparticles) for combined application in gene delivery and MRI diagnosis.94 | ||
| Fig. 9 (A) Synthetic procedure for HPAMAM-CDs; (B) Flow cytometry histogram profiles of COS-7 cells incubated with HPAMAM-CD/pDNA polyplexes; (C) CLSM images of COS-7 cells incubated with HPAMAM-CD/pDNA polyplexes (the green fluorescence is from HPAMAM-CD and the blue one is from the nuclei (cell nuclei were stained with DAPI)). Reproduced with permission.42 Copyright 2011, American Chemical Society. | ||
5. Conclusion and perspectives
Over the past decades, HBPs have shown promising potential for gene delivery, and great efforts have been put into this research area. In this review, we summarize the tremendous progress in the exploitation of HBPs, including the properties and various kinds of HBPs for gene delivery applications. The rapid growth of HBP-based gene delivery systems is mainly attributed to the unique advantages of HBPs. Firstly, HBPs employ convenient synthetic procedures, such as simple one-pot fabrication, which is beneficial for large-scale production and industrial transformation. Secondly, HBPs possess tunable three-dimensional branched structures, which may be optimized to meet the design principles for gene delivery vectors. For instance, their charge density, biocompatibility and stimuli-responsiveness can be facilely adjusted in order to develop stable gene polyplexes and facilitate their endosomal escape. Thirdly, HBPs have large numbers of terminal functional groups and internal cavities, which provide opportunities for integration of targeting ligands, imaging probes, and other therapeutic agents to form multifunctional gene delivery platforms.Despite achieving enormous progress, this area still faces several key challenges. Firstly, one of the main current drawbacks of HBPs is their ill-defined structure. Due to their broad polydispersity, HBPs exhibit heterogenicity and possess complexities which make their biological evaluation difficult. Therefore, controllable batch-to-batch quality of HBPs is an urgent need, which can facilitate the translation of HBPs from labs to clinics. Moreover, till now, although numerous HBP-based gene delivery systems have been achieved, only a few of them exhibit a high targeting ability to the region of interest. The efficient systemic delivery may be hampered by self-aggregation, protein adsorption, RES uptake, and non-specific internalization. These obstacles can be overcome by controlling the surface charge, introducing stealth corona and conjugating targeting ligands. Besides, after cell internalization, ideal gene vectors should also overcome a series of intracellular barriers, including endosomal escape and nuclear translocation, to achieve high transfection efficiency. However, current HBP-based gene delivery systems possess relatively low gene transfer efficiency, which remains orders of magnitude poorer than that of viral vectors. Hence, further efforts have to be put into optimization of the properties and functions of HBPs to facilitate the transfection process. Furthermore, there are multiple barriers during systemic delivery and intracellular transportation; however, single functionalization of HBPs can't overcome all of these barriers. Thus, more multifunctionalized HBP vectors should be developed to optimize their performance in both blood circulation and intracellular processes. In addition, multiple stimuli-responsive HBPs are urgently needed, for they can change their structure or properties under different environmental stimuli to satisfy the requirements of gene transfection in different stages.
As a final remark, with a growing understanding of barriers in gene transfection, the design principle of HBP-based gene delivery systems will be explored further. We believe that with continued refinement, HBP vectors will have great impact on gene delivery and become an important tool for gene therapy.
Acknowledgements
The authors thank the National Basic Research Program (2015CB931801) and the National Natural Science Foundation of China (51473093).Notes and references
- M. Cavazzana-Calvo, S. Hacein-Bey, G. De Saint Basile, F. Gross, E. Yvon, P. Nusbaum, F. Selz, C. Hue, S. Certain, J. L. Casanova, P. Bousso, F. Le Deist and A. Fischer, Science, 2000, 288, 669–672 CrossRef CAS PubMed.
- S. Ferrari, D. M. Geddes and E. W. F. W. Alton, Adv. Drug Delivery Rev., 2002, 54, 1373–1393 CrossRef CAS PubMed.
- V. J. Dzau, K. Beatt, G. Pompilio and K. Smith, Am. J. Cardiol., 2003, 92, 18N–23N CrossRef CAS PubMed.
- Z. R. Yang, H. F. Wang, J. Zhao, Y. Y. Peng, J. Wang, B. A. Guinn and L. Q. Huang, Cancer Gene Ther., 2007, 14, 599–615 CrossRef CAS PubMed.
- J. Zhou, J. Liu, C. J. Cheng, T. R. Patel, C. E. Weller, J. M. Piepmeier, Z. Jiang and W. M. Saltzman, Nat. Mater., 2012, 11, 82–90 CrossRef CAS PubMed.
- C. A. Alabi, K. T. Love, G. Sahay, H. Yin, K. M. Luly, R. Langer and D. G. Anderson, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 12881–12886 CrossRef CAS PubMed.
- V. Gopal, J. Controlled Release, 2013, 167, 323–332 CrossRef CAS PubMed.
- L. Alvarez-Erviti, Y. Seow, H. Yin, C. Betts, S. Lakhal and M. J. A. Wood, Nat. Biotechnol., 2011, 29, 341–345 CrossRef CAS PubMed.
- D. W. Pack, A. S. Hoffman, S. Pun and P. S. Stayton, Nat. Rev. Drug Discovery, 2005, 4, 581–593 CrossRef CAS PubMed.
- Y. Zhou, W. Huang, J. Liu, X. Zhu and D. Yan, Adv. Mater., 2010, 22, 4567–4590 CrossRef CAS PubMed.
- C. Gao and D. Yan, Prog. Polym. Sci., 2004, 29, 183–275 CrossRef CAS.
- M. A. Mintzer and E. E. Simanek, Chem. Rev., 2009, 109, 259–302 CrossRef CAS PubMed.
- J. Yang, Q. Zhang, H. Chang and Y. Cheng, Chem. Rev., 2015, 115, 5274–5300 CrossRef CAS PubMed.
- X. Lu, T. H. Tran, F. Jia, X. Tan, S. Davis, S. Krishnan, M. M. Amiji and K. Zhang, J. Am. Chem. Soc., 2015, 137, 12466–12469 CrossRef CAS PubMed.
- P. R. Dash, M. L. Read, L. B. Barrett, M. A. Wolfert and L. W. Seymour, Gene Ther., 1999, 6, 643–650 CrossRef CAS PubMed.
- M. Ogris, S. Brunner, S. Schüller, R. Kircheis and E. Wagner, Gene Ther., 1999, 6, 595–605 CrossRef CAS PubMed.
- V. Toncheva, M. A. Wolfert, P. R. Dash, D. Oupicky, K. Ulbrich, L. W. Seymour and E. H. Schacht, Biochim. Biophys. Acta, Gen. Subj., 1998, 1380, 354–368 CrossRef CAS.
- R. Kircheis, L. Wightman, A. Schreiber, B. Robitza, V. Rössler, M. Kursa and E. Wagner, Gene Ther., 2001, 8, 28–40 CrossRef CAS PubMed.
- S. Mishra, P. Webster and M. E. Davis, Eur. J. Cell Biol., 2004, 83, 97–111 CrossRef CAS PubMed.
- K. A. Mislick and J. D. Baldeschwieler, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 12349–12354 CrossRef CAS.
- P. Erbacher, A. C. Roche, M. Monsigny and P. Midoux, Exp. Cell Res., 1996, 225, 186–194 CrossRef CAS PubMed.
- H. Lee, J. H. Jeong and T. G. Park, J. Controlled Release, 2001, 76, 183–192 CrossRef CAS PubMed.
- K. Luby-Phelps, P. E. Castle, D. L. Taylor and F. Lanni, Proc. Natl. Acad. Sci. U. S. A., 1987, 84, 4910–4913 CrossRef CAS.
- S. Brunner, T. Sauer, S. Carotta, M. Cotten, M. Saltik and E. Wagner, Gene Ther., 2000, 7, 401–407 CrossRef CAS PubMed.
- M. A. E. M. van der Aa, G. A. Koning, C. d'Oliveira, R. S. Oosting, K. J. Wilschut, W. E. Hennink and D. J. A. Crommelin, J. Gene Med., 2005, 7, 208–217 CrossRef CAS PubMed.
- S. Wen, F. Zheng, M. Shen and X. Shi, J. Appl. Polym. Sci., 2013, 128, 3807–3813 CrossRef CAS.
- W. J. Kim, A. C. Bonoiu, K. S. Lee, T. Hayakawa, C. Xia, M. A. Kakimoto, H. E. Pudavar and P. N. Prasad, Proceedings of SPIE - The International Society for Optical Engineering, San Diego, CA, 2009 Search PubMed.
- Z. Guo, H. Tian, L. Lin, J. Chen, C. He, Z. Tang and X. Chen, Macromol. Biosci., 2014, 14, 1406–1414 CrossRef CAS PubMed.
- M. Hellmund, K. Achazi, F. Neumann, B. N. S. Thota, N. Ma and R. Haag, Biomater. Sci., 2015, 3, 1459–1465 RSC.
- R. Wang, L. Zhou, Y. Zhou, G. Li, X. Zhu, H. Gu, X. Jiang, H. Li, J. Wu and L. He, Biomacromolecules, 2010, 11, 489–495 CrossRef CAS PubMed.
- Q. F. Zhang, Q. Y. Yu, Y. Geng, J. Zhang, W. X. Wu, G. Wang, Z. Gu and X. Q. Yu, ACS Appl. Mater. Interfaces, 2014, 6, 15733–15742 CAS.
- R. Dong, L. Zhou, J. Wu, C. Tu, Y. Su, B. Zhu, H. Gu, D. Yan and X. Zhu, Chem. Commun., 2011, 47, 5473–5475 RSC.
- Y. Ping, D. Wu, J. N. Kumar, W. Cheng, C. L. Lay and Y. Liu, Biomacromolecules, 2013, 14, 2083–2094 CrossRef CAS PubMed.
- B. Yang, X. Dong, Q. Lei, R. Zhuo, J. Feng and X. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 22084–22094 CAS.
- A. M. Staedtler, M. Hellmund, F. Sheikhi Mehrabadi, B. N. S. Thota, T. M. Zollner, M. Koch, R. Haag and N. Schmidt, J. Mater. Chem. B, 2015, 3, 8993–9000 RSC.
- C. Tu, N. Li, L. Zhu, L. Zhou, Y. Su, P. Li and X. Zhu, Polym. Chem., 2013, 4, 393–401 RSC.
- Y. Q. Huang, R. Zhang, C. X. Song, R. C. Jiang, X. F. Liu, G. W. Zhang, Q. L. Fan, L. H. Wang and W. Huang, Polymer, 2015, 59, 93–101 CrossRef CAS.
- M. Breunig, U. Lungwitz, R. Liebl and A. Goepferich, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 14454–14459 CrossRef CAS PubMed.
- M. Hemmati, B. Kazemi, F. Najafi, A. Zarebkohan and R. Shirkoohi, J. Drug Targeting, 2015 DOI:10.3109/1061186X.2015.1078338.
- S. Höbel, A. Loos, D. Appelhans, S. Schwarz, J. Seidel, B. Voit and A. Aigner, J. Controlled Release, 2011, 149, 146–158 CrossRef PubMed.
- D. Gutsch, D. Appelhans, S. Höbel, B. Voit and A. Aigner, Mol. Pharmaceutics, 2013, 10, 4666–4675 CrossRef CAS PubMed.
- Y. Chen, L. Zhou, Y. Pang, W. Huang, F. Qiu, X. Jiang, X. Zhu, D. Yan and Q. Chen, Bioconjugate Chem., 2011, 22, 1162–1170 CrossRef PubMed.
- B. Liang, M. L. He, Z. P. Xiao, Y. Li, C. Y. Chan, H. F. Kung, X. T. Shuai and Y. Peng, Biochem. Biophys. Res. Commun., 2008, 367, 874–880 CrossRef CAS PubMed.
- Y. Sun, Y. Jiao, Y. Wang, D. Lu and W. Yang, Int. J. Pharm., 2014, 465, 112–119 CrossRef CAS PubMed.
- J. Xia, L. Chen, J. Chen, H. Tian, F. Li, X. Zhu, G. Li and X. Chen, Macromol. Biosci., 2011, 11, 211–218 CrossRef CAS PubMed.
- P. Banerjee, R. Weissleder and A. Bogdanov Jr, Bioconjugate Chem., 2006, 17, 125–131 CrossRef CAS PubMed.
- L. Zhang, C. H. Hu, S. X. Cheng and R. X. Zhuo, Colloids Surf., B, 2010, 76, 427–433 CrossRef CAS PubMed.
- H. Tian, L. Lin, Z. Jiao, Z. Guo, J. Chen, S. Gao, X. Zhu and X. Chen, J. Controlled Release, 2013, 172, 410–418 CrossRef CAS PubMed.
- T. H. Kim, H. W. Seo, J. Han, K. S. Ko and J. S. Choi, Macromol. Res., 2014, 22, 757–764 CrossRef CAS.
- H. Tian, W. Xiong, J. Wei, Y. Wang, X. Chen, X. Jing and Q. Zhu, Biomaterials, 2007, 28, 2899–2907 CrossRef CAS PubMed.
- J. Chen, H. Tian, Z. Guo, J. Xia, A. Kano, A. Maruyama, X. Jing and X. Chen, Macromol. Biosci., 2009, 9, 1247–1253 CrossRef CAS PubMed.
- J. Chen, H. Tian, Z. Guo, L. Lin, X. Dong, X. Zhu and X. Chen, J. Appl. Polym. Sci., 2012, 123, 2257–2265 CrossRef CAS.
- R. L. Xie, Y. J. Jang, L. Xing, B. F. Zhang, F. Z. Wang, P. F. Cui, M. H. Cho and H. L. Jiang, Int. J. Pharm., 2015, 478, 19–30 CrossRef CAS PubMed.
- X. Liang, X. Ren, Z. Liu, Y. Liu, J. Wang, J. Wang, L. M. Zhang, D. Y. B. Deng, D. Quan and L. Yang, Int. J. Nanosci., 2014, 9, 419–435 Search PubMed.
- K. Zhu, C. Guo, H. Lai, W. Yang, Y. Xia, D. Zhao and C. Wang, J. Mater. Sci.: Mater. Med., 2011, 22, 2477–2485 CrossRef CAS PubMed.
- Z. Zhong, Y. Song, J. F. J. Engbersen, M. C. Lok, W. E. Hennink and J. Feijen, J. Controlled Release, 2005, 109, 317–329 CrossRef CAS PubMed.
- S. Y. Chen, G. M. Sun, L. Jiang, N. Li, P. Y. Li and X. Y. Zhu, Gaodeng Xuexiao Huaxue Xuebao, 2009, 30, 825–829 CAS.
- J. A. Redondo, E. Martínez-Campos, R. Navarro, H. Reinecke, C. Elvira, J. L. López-Lacomba and A. Gallardo, Eur. J. Pharm. Biopharm., 2015, 93, 303–310 CrossRef CAS PubMed.
- Z. Liu, H. Gong, R. Zeng, X. Liang, L. M. Zhang, L. Yang and Y. Lan, Int. J. Nanosci., 2015, 10, 2735–2749 CAS.
- Y. Zhou, B. Yang, X. Ren, Z. Liu, Z. Deng, L. Chen, Y. Deng, L. M. Zhang and L. Yang, Biomaterials, 2012, 33, 4731–4740 CrossRef CAS PubMed.
- Q. Lv, K. Wang, D. Xu, M. Liu, Q. Wan, H. Huang, S. Liang, X. Zhang and Y. Wei, Macromol. Biosci., 2016, 16, 223–230 CrossRef CAS PubMed.
- X. Shuai, T. Merdan, F. Unger, M. Wittmar and T. Kissel, Macromolecules, 2003, 36, 5751–5759 CrossRef CAS.
- L. Zhang, C. Hu, Y. Fan and Y. Wu, J. Mater. Chem. B, 2013, 1, 6271–6282 RSC.
- H. Z. Jia, W. Zhang, X. L. Wang, B. Yang, W. H. Chen, S. Chen, G. Chen, Y. F. Zhao, R. X. Zhuo, J. Feng and X. Z. Zhang, Biomater. Sci., 2015, 3, 1066–1077 RSC.
- M. Wang, B. Wu, J. D. Tucker, P. Lu, C. Cloer and Q. L. Lu, Hum. Gene Ther., 2014, 25, 419–427 CrossRef CAS PubMed.
- T. H. Kim, H. Choi, G. S. Yu, J. Lee and J. S. Choi, Macromol. Res., 2013, 21, 1097–1104 CrossRef CAS.
- R. Dong, Y. Zhou, X. Huang, X. Zhu, Y. Lu and J. Shen, Adv. Mater., 2015, 27, 498–526 CrossRef CAS PubMed.
- W. Yang, C. Y. Pan, X. Q. Liu and J. Wang, Biomacromolecules, 2011, 12, 1523–1531 CrossRef CAS PubMed.
- C. J. Chen, Z. X. Zhao, J. C. Wang, E. Y. Zhao, L. Y. Gao, S. F. Zhou, X. Y. Liu, W. L. Lu and Q. Zhang, Int. J. Nanosci., 2012, 7, 3837–3849 CAS.
- Y. Z. You, Z. Q. Yu, M. M. Cui and C. Y. Hong, Angew. Chem., Int. Ed., 2010, 49, 1099–1102 CrossRef CAS PubMed.
- J. Chen, C. Wu and D. Oupický, Biomacromolecules, 2009, 10, 2921–2927 CrossRef CAS PubMed.
- L. Tao, W. C. Chou, B. H. Tan and T. P. Davis, Macromol. Biosci., 2010, 10, 632–637 CrossRef CAS PubMed.
- X. Jiang, J. Liu, L. Xu and R. Zhuo, Macromol. Chem. Phys., 2011, 212, 64–71 CrossRef CAS.
- F. Martello, M. Piest, J. F. J. Engbersen and P. Ferruti, J. Controlled Release, 2012, 164, 372–379 CrossRef CAS PubMed.
- U. L. Rahbek, A. F. Nielsen, M. Dong, Y. You, A. Chauchereau, D. Oupicky, F. Besenbacher, J. Kjems and K. A. Howard, J. Drug Targeting, 2010, 18, 812–820 CrossRef CAS PubMed.
- A. Ardana, A. K. Whittaker and K. J. Thurecht, Macromolecules, 2014, 47, 5211–5219 CrossRef CAS.
- G. Liu, G. Zhang, J. Hu, X. Wang, M. Zhu and S. Liu, J. Am. Chem. Soc., 2015, 137, 11645–11655 CrossRef CAS PubMed.
- R. A. Gatenby and R. J. Gillies, Nat. Rev. Cancer, 2004, 4, 891–899 CrossRef CAS PubMed.
- D. Neri and C. T. Supuran, Nat. Rev. Drug Discovery, 2011, 10, 767–777 CrossRef CAS PubMed.
- H. Z. Jia, W. Zhang, J. Y. Zhu, B. Yang, S. Chen, G. Chen, Y. F. Zhao, J. Feng and X. Z. Zhang, J. Controlled Release, 2015, 216, 9–17 CrossRef CAS PubMed.
- Q. Liu, C. Y. Hong and C. Y. Pan, Gaofenzi Xuebao, 2015, 15–24, DOI:10.11777/j.issn1000-3304.2015.14165.
- H. Hatakeyama, H. Akita, K. Kogure, M. Oishi, Y. Nagasaki, Y. Kihira, M. Ueno, H. Kobayashi, H. Kikuchi and H. Harashima, Gene Ther., 2007, 14, 68–77 CrossRef CAS PubMed.
- Z. Kadlecova, Y. Rajendra, M. Matasci, D. Hacker, L. Baldi, F. M. Wurm and H. A. Klok, Macromol. Biosci., 2012, 12, 794–804 CrossRef CAS PubMed.
- H. Y. Tian, C. Deng, H. Lin, J. Sun, M. Deng, X. Chen and X. Jing, Biomaterials, 2005, 26, 4209–4217 CrossRef CAS PubMed.
- W. Fischer, M. A. Quadir, A. Barnard, D. K. Smith and R. Haag, Macromol. Biosci., 2011, 11, 1736–1746 CrossRef CAS PubMed.
- O. Boussif, F. Lezoualc'h, M. A. Zanta, M. D. Mergny, D. Scherman, B. Demeneix and J.-P. Behr, Proc. Natl. Acad. Sci. U. S. A., 1995, 92, 7297–7301 CrossRef CAS.
- R. B. Arote, E. S. Lee, H. L. Jiang, Y. K. Kim, Y. J. Choi, M. H. Cho and C. S. Cho, Bioconjugate Chem., 2009, 20, 2231–2241 CrossRef CAS PubMed.
- T. H. Kim, S. E. Cook, R. B. Arote, M. H. Cho, J. W. Nah, Y. J. Choi and S. C. Chong, Macromol. Biosci., 2007, 7, 611–619 CrossRef CAS PubMed.
- D. Wu, Y. Liu, X. Jiang, L. Chen, C. He, S. H. Goh and K. W. Leong, Biomacromolecules, 2005, 6, 3166–3173 CrossRef CAS PubMed.
- D. Wu, Y. Liu, X. Jiang, C. He, S. H. Goh and K. W. Leong, Biomacromolecules, 2006, 7, 1879–1883 CrossRef CAS PubMed.
- Q. Peng, J. Zhu, Y. Yu, L. Hoffman and X. Yang, J. Biomater. Sci., Polym. Ed., 2015, 26, 1163–1177 CrossRef CAS PubMed.
- X. Zhu, Y. Zhou and D. Yan, J. Polym. Sci., Part B: Polym. Phys., 2011, 49, 1277–1286 CrossRef CAS.
- R. Dong, Y. Zhou and X. Zhu, Acc. Chem. Res., 2014, 47, 2006–2016 CrossRef CAS PubMed.
- R. Yuan, Y. Zuo, Z. Xiao, W. Huang and X. Shuai, Gaofenzi Xuebao, 2009, 104–110 CAS.
Footnotes |
| † The authors declare no competing financial interests. |
| ‡ Both authors contributed equally to this manuscript. |
| This journal is © The Royal Society of Chemistry 2016 |