Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A molecular assembly that crawls on a solid substrate with a metabolic-like process†
Masato
Nakada
,
Yukihiro
Fujikami
,
Masaharu
Kawaguchi
,
Daigo
Yamamoto
and
Akihisa
Shioi
*
Department of Chemical Engineering & Materials Science, Doshisha University, 1-3 Tatara Miyakodani, Kyotanabe, Kyoto 610-0321, Japan. E-mail: ashioi@mail.doshisha.ac.jp
First published on 12th May 2016
Abstract
A vesicular aggregate filled with lipid molecules exhibited crawling motion over a glass surface as a result of chemical reactions. The vesicular aggregate was composed of didodecyldimethylammonium bromide (DDAB) and sodium oleate with calcium ions. The crawling motion was induced by the chemical reaction between DDAB and iodide ions, and it caused discharge of the inner lipids. This was responsible for the size reduction of the aggregate. However, it engulfed the neighboring smaller vesicles, which were taken up into the vesicular aggregate in the dehydrated state and became a constituent of the aggregate. The size of the vesicular aggregate recovered and could translate in a sustainable manner. This is probably the first example of an amphiphilic molecular assembly that exhibits crawling motion as a result of chemical reactions without size reduction. This may be regarded as the cell-like behavior of an abiotic molecular assembly with a metabolic-like process.
Design, System, ApplicationThe development of chemical systems that mimic the behavior of living matter results in the determination of characteristics that impart a semblance of life. A chemical reaction driven vesicle can be designed with metabolic-like characteristics, taking in surrounding material to reconstruct its body and obtain energy for motion. Here, amphiphilic molecular aggregates exhibit crawling motion on a glass surface, sustained by “eating” the other aggregates and discharging waste. This biomimetic motion is achieved by cheap simple chemicals, showing that it is possible to discover intriguing effects in apparently simple systems. The chemical reactions that form precipitates are controlled by the coexisting lipids. This process drives the aggregates. The vesicle internal structure is optimised by altering the chemical constituents to give the dense packing of lipids that enables long distance sustainable motion. This scenario may be general irrespective of the used chemicals, and hence self-healing, self-duplication and communication functions may be achieved based on this study. The system may be developed for self-patterning and drug delivery: we may be able to control the aggregate motion along a line composed of eaten objects, and then, the waste may draw a micropattern. If biocompatible chemicals can be used, the aggregates may become a scavenger that removes lipid aggregates on the wall of blood vessels. |
Introduction
Living organisms maintain their lives as open and dynamic systems. Their dynamic features are maintained by metabolism. Organisms derive nutrition from and release waste to the surroundings. Through metabolism, living organisms replace old parts of the body with new ones. This process serves numerous biological functions. Because the metabolic process is associated with the self-healing and self-duplication of living organisms,1 the study of active soft matter2 exhibiting metabolic-like behavior may lead to the design of colloidal systems with these characteristics.Such colloidal systems will be possible only if they are open systems under a non-equilibrium state. Thus, a study of self-moving colloids under a strongly non-equilibrium state may provide a basis for the design of these systems. Until now, liquid droplets and catalytic particles have been employed in numerous studies of self-moving colloids. The results showed that some characteristics contained in biological motions, such as vectorial motion in an isotropic field3,4 and chemotaxis,5,6 can be mimicked by colloidal objects. The energy of self-motion is provided by chemical reactions7 and adsorption/desorption processes.8 These characteristics are inherent in biological motion.1 However, these colloidal systems do not contain metabolic-like processes, except in some rare cases.9 Here, a metabolic-like process refers to the spontaneous replacement of an old part of the body with a new one. This situation is the same for a study of self-moving vesicles (liposomes) that are formed with a bilayer membrane and used for cytomimetic research. A vesicle with self-propulsion that contained the protein ActA, which polymerizes actin filaments, was reported.10 The compression force caused by polymerization drove the vesicle. This study was inspired by the motion mechanism of Listeria monocytogenes11 and provides an example of vesicle propulsion without a decrease in its size. Recently, the authors' group has reported the cyclic motion of a vesicle under a pH gradient without size reduction.12 On the other hand, in many cases, vesicles that exhibit spontaneous motion or deformation decrease in size because chemical reactions and physical stimuli for vesicle motion cause damage to the vesicle.13
Self-moving colloids with metabolic-like characteristics have not been fully investigated. To provide this characteristic to an abiotic colloid, vesicles would be most appropriate because they are formed by amphiphilic molecules that can coexist in a solution where the vesicles move. If a vesicle takes the surrounding amphiphiles to reconstruct its body and obtain the energy for motion, it may possess self-healing, self-duplication and communication functions. The self-duplication and communication functions of vesicles (liposomes) have been studied.14 The constituents of the vesicle with self-duplication are dissolved in the vesicular solution and transformed into the membrane constituent by chemical reactions. These studies focused on the self-duplication process and were interesting from the viewpoints of the origins of life.15 The addition of biomimetic motion for the ingestion of a constituent may be the subject of future studies where the mechanism for many active biological functions will be revealed. The formation of a vesicle driven by chemical reaction that possesses metabolic-like characteristics may become the basis for this purpose.
The authors' group reported a self-moving vesicle with size reduction.16 In that study, the vesicle composed of the cationic surfactant didodecyldimethylammonium bromide (DDAB) migrated in water via ion exchange with iodide ions. The motion was accompanied by size reduction of the vesicle because DDAB and iodide ions formed a precipitate that was eliminated from the vesicle membrane. In the present study, sodium oleate and calcium ions were mixed with a DDAB based vesicle. The mixture formed an aggregate with a complicated inner structure. This vesicular aggregate (VA) was adsorbed on a glass surface. During the diffusion of iodide ions, the VA moved over the glass surface, leaving behind a thin film on the glass surface. This film was the reaction product of the inner lipid molecules. As a result, the inner lipids were discharged from the VA. This resulted in the size reduction of the VA. However, the VA took other smaller vesicles into the body and recovered its size. Without this size recovery, VA could not show sustainable self-motion. A VA with these characteristics may be regarded as a model molecular assembly that exhibits cell like behavior with a metabolic-like process. The sustained translational motion may be applied to an active transport carrier of useful chemicals and particles, for patterning in materials design and smart delivery in microchannels.
Experimental
Chemicals
Sodium oleate (>97%) and didodecyldimethylammonium bromide (>98%) were purchased from Tokyo Chemical Industry Co., Ltd. The other chemicals used were of reagent grade and obtained from Wako Pure Chemical Industries, Ltd. All chemicals were used without further purification.Vesicle preparation
The drying–rehydration method was used for vesicle preparation.17 Sodium oleate and DDAB were dissolved in acetone with sonication. Calcium chloride and a fluorescent probe (perylene) were also added to the solution. The resulting solution was poured into a glass vial, and the acetone was evaporated under nitrogen gas and then completely under vacuum. After evaporation, a thin film remained on the wall of the vial. A bicine buffer solution (pH ≈ 8.4) was then poured into the vial and maintained at 50°C for 1 h. The concentration of bicine was 70 mM, and its pH was adjusted to the desired value using a small amount of NaOH. The vesicle suspension was obtained by gentle shaking of the solution. The concentrations of each solute were 3.2 mM (DDAB), 2.3 mM (sodium oleate), and 10 mM (CaCl2) in the vesicle suspension. These concentrations were chosen because the motion of VA was observed with sufficient reproducibility. A trace amount of perylene was used.Vesicle observation
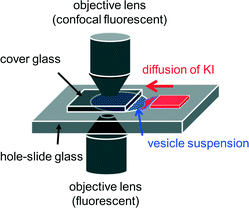
The vesicle suspension was poured into a hole-slide glass and covered with a cover glass. As shown in Fig. 1, a small gap was formed beside the cover glass, and an aqueous solution containing 40 mM KI (without bicine) was diffused from the gap into the vesicle suspension. The volume ratio of the KI solution to the vesicle suspension was approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and the final KI concentration after complete diffusion was estimated to be 10 mM. After the convection disappeared, the vesicles were observed by fluorescence microscopy (Olympus IX-71). An Olympus BX-51 confocal microscope was also used. Image analysis was performed using Move-tr/2D (Library Co., Ltd.), ImageJ (Wayne Rasband, NIH), and Movie Ruler ver. 2 (Photoron Ltd.). All experiments were performed at room temperature, which was controlled by an air conditioner within approximately 18–25 °C.
3, and the final KI concentration after complete diffusion was estimated to be 10 mM. After the convection disappeared, the vesicles were observed by fluorescence microscopy (Olympus IX-71). An Olympus BX-51 confocal microscope was also used. Image analysis was performed using Move-tr/2D (Library Co., Ltd.), ImageJ (Wayne Rasband, NIH), and Movie Ruler ver. 2 (Photoron Ltd.). All experiments were performed at room temperature, which was controlled by an air conditioner within approximately 18–25 °C.
Results and discussion
Structure of the vesicular assembly
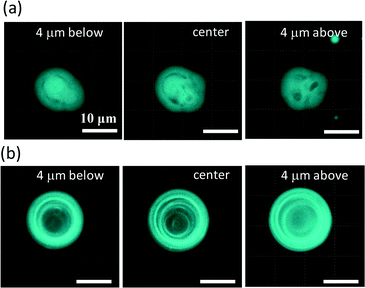
Fig. 2 shows fluorescence confocal microscopy images of the amphiphilic molecular assemblies formed by the present experiment. Three cross sections at different depths are shown. Perylene, a hydrophobic molecule, was used as the fluorescence probe. Fluorescence microscopy showed that the molecular assemblies can be classified into two types, as shown in Fig. 2a and b.Fig. 2a shows the first type. Tiny vesicles appear to be packed, but the structure is complicated and obscure. This type of structure will be discussed later and is denoted as VA (vesicular aggregate). The second type shown in Fig. 2b is an onion-like vesicle. A multi-membrane structure can be observed, which is denoted as OV. A much simpler vesicle that was composed of a single water pool surrounded by an amphiphilic molecular layer was also observed.
To examine the structure of VA, water was added to the solution. In this experiment, the water permeates into the aggregate. Fig. 3 shows confocal fluorescence microscopy images of a VA during water permeation. Fig. 3a shows the time course of a VA that swells due to water permeation. The images at t = 11.0 and 43.0 s were taken by fluorescence confocal microscopy, whereas at t = 29.2 s, weak incident light was introduced into the fluorescence microscope to clarify the image. Immediately after the addition of water (t = 0), fluorescence was emitted uniformly from the VA (t = 11.0). As the water permeated, the volume increased, and the fluorescence was concentrated on the restricted portions (t = 43.0). The non-fluorescent part is considered to be the water pool. The image at t = 43.0 s indicates that a VA swollen by water is comprised of numerous small water pools surrounded by amphiphilic molecules. Other examples of swollen VAs are shown in Fig. 3b. In these results, the water pools were rather uniform and spherical. These results indicate that a VA before water addition is composed of dehydrated molecular assemblies. The dehydrated vesicles packed tightly in a VA is one possible interpretation. Fig. 3c exemplifies a vesicle formed in [DDAB]/[oleate] = 2 without Ca2+ (i.e. more DDAB-rich compared to the standard conditions for sample preparation). Numerous smaller vesicles aggregated under these conditions. This suggests that an aggregate with nearly homogeneous fluorescence in the presence of Ca2+ may be composed of densely packed dehydrated vesicles. However, reconstruction of an inner structure may occur by water addition, and the fluorescent part that surrounds the internal water compartments was too thick to be a bilayer structure. The inner structure before water addition may be more complicated such as a cubic phase. The three dimensional view of the VA, which was constructed from the cross sectional images, showed that VAs are adsorbed under the cover glass (on the glass surface) (see ESI† Movie S1).
The populations of the two types of vesicles (VA and OV) depend on the compositions of the vesicular solution. When DDAB, oleate and Ca2+ coexist, most of the molecular assemblies are the VA type. Confocal fluorescence microscopy of a VA with perylene showed almost uniform emission from them, as shown in Fig. 2a and 3a (11.0 s). This indicates the dense packing of lipid molecules. When DDAB molecules are the only constituent of the vesicles, they form simple vesicles.18 The addition of Ca2+ transforms the simple vesicles into a more complicated structure, as shown in Fig. S1a,† where the vesicles appear to be intermediate between OV and VA: spherical shells can be seen, but the inner structure is complicated. When oleate was added to DDAB without Ca2+, numerous OV type vesicles appeared (see Fig. S1b†). According to these results, the mixture of DDAB and oleate formed numerous VAs in the presence of Ca2+, but OVs may contain less Ca2+. Ca2+ and oleate generally form a precipitate in water. We did not see any macroscopic precipitates in the DDAB–oleate solution with Ca2+ at the present chemical concentrations. Thus, the Ca2+ and oleate compounds dissolved in the solution with the help of DDAB, resulting in the formation of VAs. Mixtures of single-tailed anionic and cationic surfactants often precipitate in water. However, they can form vesicles at appropriate mixing ratios.19 The stability of the present VAs may be discussed in a similar manner to that of the spontaneous vesicles.
Crawl motion of the vesicular aggregate
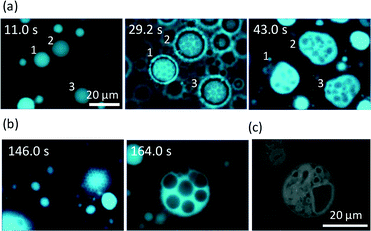
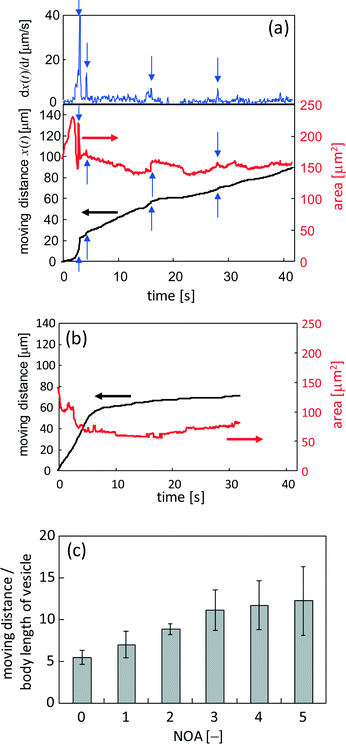
When KI diffuses toward the VAs, they deform and crawl as they are adsorbed on the glass surface. OVs do not exhibit any long distance motion, and they sometimes collapse at their places. This suggests that the dense packing of lipid molecules inside a VA is essential for sustainable motion under KI diffusion. Fig. 4 shows the fluorescence microscopy image of the crawl motion of a VA (see ESI† Movie S2). The VA with a red mark in Fig. 4 exhibits translational motions and absorbs the surrounding smaller vesicles, some of which are indicated by the white arrows. Fig. 5a and b show the moving distance of the center and the area occupied by the moving VA. Two types of experimental results are shown. The first one is when the VA absorbs smaller vesicles for its motion (Fig. 5a), and the second one is when it does not (Fig. 5b). The former type of motion is observed when numerous smaller vesicles surround the VA. In this case, the area of the VA remains relatively constant, as shown in Fig. 5a. The initial increase in area results from a change in the adsorbed state of the VA onto the glass. The volume of the VA is probably constant, but the area projected onto the glass increases. In contrast, the VA that does not absorb the surrounding vesicles collapses monotonically.The moving distance of the VA that absorbs the surrounding vesicles increases monotonically over a period of more than 40 s, while the motion of the VA without absorption stops within a few seconds. The gradual increase after approximately 6 s, as shown in Fig. 5b, is the result of vibrational fluctuations without translation. The time when the observable absorption events occurred is indicated by the blue arrows in Fig. 5a. The VA size was recovered by absorption. The speed of VA motion, which is shown in the upper panel of Fig. 5a, became faster at each absorption. This suggests that absorption provides not only the constituent of the VA body but also the motility of the VA. The same experiments were performed, and the relationship between the mean moving distance and the number of observable absorptions (NOA) was determined. The moving distance from the start position to the final one is shown. (The VA no longer moves at the final position.) The result is shown in Fig. 5c. The ordinate shows the moving distance that is scaled by the body length of the VA. When the NOA is less than three, the distance increases linearly with increasing NOA. However, the rate of the increase decreases when the NOA exceeds four.
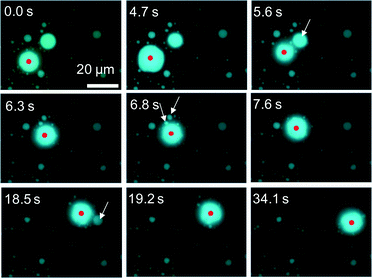
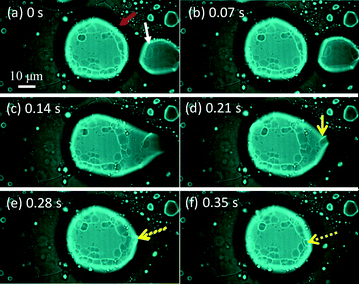
Fig. 6 shows snapshots of a moving VA that absorbs a smaller vesicle. They are indicated by a red arrow and a white arrow in Fig. 6a (see ESI† S3 (slideshow)). The structure of the smaller one appears to be much simpler than that of the VA. This shows that the smaller one is almost a simple vesicle in structure. Immediately after their contact, the VA forms a tip and absorbs the smaller vesicle through the tip. Fig. 6a–f and ESI† S3 show that the size of the smaller vesicle decreases drastically as a result of the absorption. This suggests that the water contained in the smaller vesicle is drained rapidly during absorption, and the resulting dehydrated vesicle becomes part of the VA.
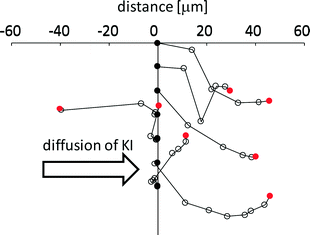
Fig. 7 shows the trajectory of seven VAs that exhibit long distance motion. KI diffuses from left to right. The VA tends to go down along the gradient of the KI concentration, even though randomness is included in each trajectory. For this tendency, a moving VA may be able to meet smaller vesicles that have not reacted with KI. If a VA went up along the gradient, the smaller vesicles that the VA could encounter would be exposed to the concentrated KI solution. In this case, the smaller vesicles would already have reacted and cannot provide the motility of the VA. Therefore, this weak chemotactic nature may be required so that the VA meets fresh smaller vesicles. However, because randomness is included in the trajectory, the probability of the VA colliding with fresh vesicles decreases with time. Hence, the VA cannot maintain its motility. Therefore, the total traveling distance shown in Fig. 5c does not increase as much when NOA is larger than 3.
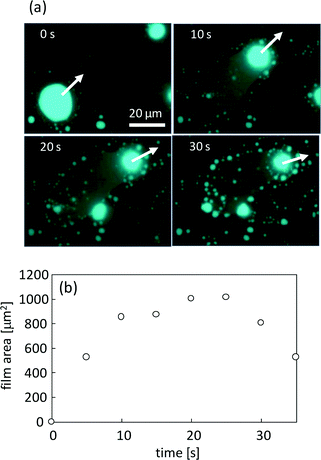
Fig. 8a shows the film that is formed by VA motion (see ESI† Movie S4). The fluorescence of perylene is emitted from the film spread over the surface, and the VA moves leaving the film behind. For this characteristic, once the formed film surrounds the VA, it no longer moves. After film formation, numerous small aggregates form at the periphery of the film, as shown in Fig. 8a (30 s). Fig. 8b shows the time dependency of the film area. The area increases with the exclusion of DDAB and oleate molecules from the VA but decreases shortly through the transformation into the aggregates. After this transformation, the glass surface is free from the film and may be able to serve as a place for VA motion. The film and the resulting aggregates are the waste of the VA, which is the reaction product with KI.
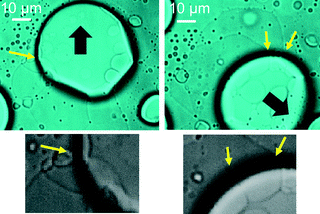
Fig. 9 shows that the film is formed from smaller vesicles contained in the VA. An inner smaller vesicle appears to be a simple vesicle, where a simple vesicle means that it has a single water pool surrounded by an amphiphilic membrane. The inner vesicle and the film are connected to each other; the conjunction points are indicated by the yellow arrows. The direction of VA motion is indicated by the black arrow. This result shows that the film formed behind the moving VA is generated by the collapse of vesicles contained in the VA. This collapse generates the motility of the VA. The inner vesicle may have been present as a dehydrated vesicle before the addition of the KI solution or may be formed by water permeation after the addition.
When two VAs collide, they behave as a prey–predator system. One VA absorbs the other, through which the VA achieves motility. The relationship appears to be determined by the relative size of the VA. The larger VA filled with inner lipid molecules plays the role of the predator. The larger VA absorbs smaller vesicles to obtain a part of the body and its motility. The VA with complicated inner structures formed in the presence of Ca2+. Thus, the concentration of Ca2+ is probably lower in the simpler vesicle (prey). We may consider that a Ca-rich aggregate could move to engulf Ca-poor aggregates. Based on this postulate, the Ca2+ concentration in the VAs would decrease by eating the simpler vesicles. This may explain why the motion of the VA stops after several collisions with simpler vesicles.
The chemical conditions required for the prey–predator behavior were examined by changing the cation species, concentrations, and pH. At least four aggregates were selected to observe the response to the addition of KI. Here, the aggregate refers to an object with homogeneous fluorescence. The concentrations of DDAB, oleate, and Ca2+ were double their standard concentrations because a larger number of aggregates were required for these experiments. However, the KI concentration was fixed at 40 mM. These results are summarized in the table in the ESI.† A pH above 8.0 was required to induce the prey–predator behavior. This suggests that the dissociation of oleic acid to form oleate is necessary. When the Ca2+ concentration was above 40 mM, the aggregate translation was less common. The oleate anion and Ca2+ formed a water-insoluble compound. The higher Ca2+ concentration typically formed precipitates to eliminate oleate molecules from the VAs. When the Ca2+ concentration was less than 10 mM, the prey–predator behavior was less common because most of the aggregates were OV-type. When the Ca2+ concentration was approximately 20 mM, the Ca2+ and oleate compounds did not precipitate in the presence of DDAB. Moreover, most of the aggregates were VA-type. This indicates that the compound was dissolved in the VA membranes. DDAB molecules prevented precipitation of the calcium salt of oleate, and the complicated inner structure formed. The 20 mM Ca2+ concentration corresponded to 10 mM under the standard experimental conditions.
Additional experiments were performed using Mg2+, Sr2+, and Co2+ instead of Ca2+. The motion observed for Mg2+ was almost identical to that of Ca2+. In contrast, aggregates containing Sr2+ and Co2+ did not show the prey–predator behavior but showed a similar response to the Ca2+-free experiments of the original system. This suggests that the solubility of M(RCOO)2 in the DDAB membrane was strongly affected by the cation type (M2+).
The mixing ratio of DDAB and oleate changed in the presence of 10 mM Ca2+. Most of the aggregates were similar to the OV type when less oleate was used (oleate: 1.5 mM, DDAB: 4.1 mM, Fig. S2a†). This indicates that OV-type aggregates formed in the absence of oleate or Ca2+. Thus, the complicated inner structure of the VA was formed by the interaction of Ca2+ and oleate in the presence of DDAB. After KI diffusion, the chemical reaction proceeded and the OVs were transformed into simple vesicles (Fig. S2b†). The inner vesicles collapsed due to the reaction and appeared to be adsorbed on the outer membrane. There were fewer aggregates when less DDAB was used (oleate: 3.3 mM, DDAB: 2.3 mM). An irregular change in the aggregates was observed due to KI diffusion, but a meaningful observation was difficult. However, this result suggests that the major constituents of VA and OV are DDAB molecules, and the main chemical reaction for the VA motion is that of DDAB and KI.
A vesicle composed of DDAB migrates three dimensionally in water when KI is added.16 The vesicle moved in a KI-containing solution three dimensionally as its size decreased monotonically. DDAB and KI formed a precipitate by ion exchange between Br− and I−. The precipitate formation proceeded at the outer surface of a vesicle. The precipitates gathered at a single point on the vesicle surface. This point was chosen occasionally. Once the precipitation point had been selected, all precipitates gathered at the point. This point was positioned at the tail of the moving vesicle. The gathering of the precipitates caused surface flow of the precipitates toward the tail of a vesicle. This surface flow induced solvent flow around the vesicle, and the vesicle propelled itself by momentum conservation. In the present system, the reaction of DDA+ and I− is considered to form a film on the glass surface in the presence of oleate and Ca2+. The film formation may play a similar role to precipitate formation in the previous system.16
In this discussion, the decrease in the solubility of DDA+ plays an essential role in the crawling motion. In general, the solubility of DDA+ in water decreases with increasing ionic strength. This suggests that the same crawling motion may occur under concentrated KBr, which was actually observed (ESI† Movie S5). When concentrated KBr (1 M) is injected, the VAs exhibit crawling motion. However, this was not observed when the concentration was 40 mM, which was used for the KI diffusion experiment. Iodide ions decrease the solubility of DDA+ more effectively than Br−.
Elucidation of the mechanism of crawling motion would be difficult without understanding the detailed chemistry of the VA and the film. In the ESI,† however, we attempted to propose a possible mechanism for vesicle crawling based on the assumption that VA is composed of numerous dehydrated vesicles.
Summary and perspective
A vesicular aggregate (VA) composed of didodecyldimethylammonium bromide (DDAB) and oleate exhibited translational motion by a chemical reaction with KI. The reaction generated the driving force for motion and decreased the size of the VA. However, the VA recovered its size by absorbing the surrounding small and simpler vesicles that had not reacted. The VA showed sustainable motion as a result of this ‘eating’ behavior. The VA had a complicated inner structure containing lipid molecules. The chemical reaction between I− and DDA+ inside the VA formed a film that spread over the glass surface. This film formation drove the VA. The VA then absorbed smaller vesicles to obtain its motility. Water that had been contained in the smaller vesicle was excluded by this absorption process. As a result, the dehydrated smaller vesicle became a constituent of the moving VA. This is probably the first example of an abiotic molecular assembly crawling over a solid substrate via ‘eating’ and waste discharge.In the future, movement of vesicle-type aggregates can be exploited by means of a kind of chemical control. If the aggregate is placed on the solid surface where the smaller ones are placed in line, the aggregate moves along the line, leaving the film behind it. The onset of this motion is controlled by a chemical trigger (e.g. KI). This may be used for self-patterning; the film may form the pattern. As another possibility, the vesicle-type aggregate may be used as a scavenger that removes lipid aggregates on the solid surface. A similar system has already been reported with a liquid droplet.20 If a similar system can be realized with biocompatible chemicals, it may be applied to clean the wall of blood vessels. The present study may become a basis for further intriguing ideas.
Acknowledgements
The authors thank Mr. Shohei Siraishi for help in the experiments with the calcium-free system in the early stage of this study. A.S. gratefully acknowledges the financial support from JSPS KAKENHI (Grant Numbers 16H04189 and 25630351) and the MEXT-Supported Program for the Strategic Research Foundation at Private Universities.Notes and references
- R. Phillips, J. Kondev and J. Theriot, Physical Biology of the Cell, Garland Science, Taylor & Francis Group, LLC, 2009 Search PubMed.
- F. C. MacKintosh and M. E. Cates, Soft Matter, 2011, 7, 3050 RSC.
- Y. Sumino, N. Magome, T. Hamada and K. Yoshikawa, Phys. Rev. Lett., 2006, 94, 068301 CrossRef PubMed.
- D. Yamamoto, T. Takada, M. Tachibana, Y. Iijima, A. Shioi and K. Yoshikawa, Nanoscale, 2015, 7, 13186 RSC.
- I. Lagzi, S. Soh, P. J. Wesson, K. P. Browne and B. A. Grzybowski, J. Am. Chem. Soc., 2010, 132, 1198 CrossRef CAS PubMed.
- Y. Hong, N. M. K. Blackman, N. D. Kopp, A. Sen and D. Velegol, Phys. Rev. Lett., 2007, 99, 178103 CrossRef PubMed.
- W. F. Paxton, K. C. Kistler, C. C. Olmeda, A. Sen, S. K. St. Angelo, Y. Cao, T. E. Mallouk, P. E. Lammert and V. H. Crespi, J. Am. Chem. Soc., 2004, 126, 13424 CrossRef CAS PubMed.
- A. Shioi, K. Katano and Y. Onodera, J. Colloid Interface Sci., 2003, 266, 415 CrossRef CAS PubMed.
- T. Banno and T. Toyota, Langmuir, 2015, 31, 6943 CrossRef CAS PubMed; M. M. Hanczyc, T. Toyota, T. Ikegami, N. Packard and T. Sugawara, J. Am. Chem. Soc., 2007, 129, 9386 CrossRef PubMed; P. L. Luisi, M. Allegretti, T. P. de Souza, F. Steiniger, A. Fahr and P. Stano, ChemBioChem, 2010, 11, 1989 CrossRef PubMed.
- P. A. Giardini, D. A. Fletcher and J. A. Theriot, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 6493 CrossRef CAS PubMed.
- F. Gerbal, P. Chaikin, Y. Rabin and J. Prost, Biophys. J., 2000, 79, 2259 CrossRef CAS PubMed.
- E. Nawa, D. Yamamoto and A. Shioi, Soft Matter, 2013, 9, 7832 RSC; E. Nawa, D. Yamamoto and A. Shioi, Bull. Chem. Soc. Jpn., 2015, 88, 1536 CrossRef CAS; E. Nawa, D. Sakashita, K. Owaki, D. Yamamoto and A. Shioi, Colloids Interface Sci. Commun., 2015, 8, 10 CrossRef.
- F. Brochard-Wyart, P. G. de Gennes and O. Sandre, Phys. A, 2000, 278, 32 CrossRef CAS; F. Nomura, M. Nagata, T. Inaba, H. Hiramatsu, H. Hotani and K. Takiguchi, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 2340 CrossRef PubMed; T. Hamada, Y. Hirabayashi, T. Ohta and M. Takagi, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2009, 80, 051921 CrossRef PubMed.
- I. Chen and P. Walde, Cold Spring Harbor Perspect. Biol., 2010, 2, a002170 Search PubMed; K. Kurihara, M. Tamura, K. Shohda, T. Toyota, K. Suzuki and T. Sugawara, Nat. Chem., 2011, 3, 775 CrossRef CAS PubMed; P. Carrara, P. Stano and P. L. Luisi, ChemBioChem, 2012, 13, 1497 CrossRef PubMed; K. Kurihara, Y. Okura, M. Matsuo, T. Toyota, K. Suzuki and T. Sugawara, Nat. Commun., 2015, 6, 8352 CrossRef PubMed.
- P. L. Luisi, The Emergence of Life – From Chemical Origins to Synthetic Biology, Cambridge University Press, 2006 Search PubMed.
- T. Miura, H. Oosawa, M. Sakai, Y. Syundou, T. Ban and A. Shioi, Langmuir, 2010, 26, 1610 CrossRef CAS PubMed.
- S. Manley and V. D. Gordon, Current Protocols in Cell Biology, 2008, vol. 40, p. 24.3 Search PubMed.
- F. M. Menger and K. D. Gabrielson, Angew. Chem., Int. Ed. Engl., 1995, 34, 2091 CrossRef CAS.
- E. W. Kaler, A. K. Murthy, B. E. Rodriguez and J. A. Zasadzinski, Science, 1989, 245, 1371 CAS.
- Y. Goto, M. Kanda, D. Yamamoto and A. Shioi, Sci. Rep., 2015, 5, 14348 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Fluorescence confocal microscopy images of vesicles; tabulated dynamic aggregate behaviors; movies of the molecular assemblies, crawling motion of the vesicular aggregates, absorption of smaller vesicles, and film formation. See DOI: 10.1039/c5me00012b |
| This journal is © The Royal Society of Chemistry 2016 |