Capture and immobilisation of iodine (I2) utilising polymer-based ZIF-8 nanocomposite membranes†
E. M.
Mahdi
,
Abhijeet K.
Chaudhuri
and
Jin-Chong
Tan
*
Multifunctional Materials & Composites (MMC) Laboratory, Department of Engineering Science, University of Oxford, Parks Road, OX1 3PJ, UK. E-mail: jin-chong.tan@eng.ox.ac.uk
First published on 24th February 2016
Abstract
Polymer nanocomposites made up of nanoporous metal–organic frameworks (MOFs) are fast becoming a staple of next generation hybrid composites, and are currently being intensely developed for gas capture and separation. This work reports the first attempt to capture and retain iodine (I2) using polymer-MOF (ZIF-8) nanocomposites. Membranes of ZIF-8-based nanocomposites (comprising either a glassy Matrimid or a rubbery polyurethane (PU) matrix) were prepared via a colloidal-mixing approach and their viability for I2 capture and retention effects was determined through absorption experiments, nanoindentation mechanical measurements, and thermogravimetric (TGA) analysis. The absorption experiments demonstrated that I2 capture and retention is possible in all of the nanocomposite membranes, although the PU/ZIF-8 30 wt% nanocomposite exhibited higher affinity for I2 absorption (>32 wt%). It is reasoned that the molecular affinity and attraction between I2, (2-methylimidazolate organic ligands of) ZIF-8 nanoparticles, and polymer matrices (Matrimid and PU) will catalyse the formation of weak secondary bonds, resulting in the ‘capture’ and ‘retention’ of I2 within molecular segments of the polymers and inside the pores of ZIF-8. The enhancement of the Young's modulus (E) of the PU/ZIF-8 30 wt% nanocomposite (E increased by ∼6%) is postulated to be due to I2 rigidification, while TGA analysis proved that I2 retention within both free volume of the polymer and ZIF-8 sodalite cages remained intact up till the points of structural degradation, at ∼200 °C for the PU-based nanocomposites, and at ∼300 °C for the Matrimid-based nanocomposites. We propose that the affinity of the organic ligands in ZIF-8 and the formation of free volume in the nanocomposites from the presence of ZIF-8 attracted I2, and the formation of secondary bonds between these constituents (H-bonds) strengthened not only the nanocomposite, but also kept I2 from being released despite the larger pore size and gate-opening dynamics of ZIF-8. It was therefore concluded that a combination of nanoparticles of porous MOFs and a rubbery polymer is promising for further development to enable I2 capture and retention applications.
1. Introduction
Nuclear technology is prominent in two sectors that are intrinsic parts of our lives: power generation1 and medicine.2 Nuclear power plants (NPP) are regarded as a source of clean, renewable, and cost-effective energy,3 while nuclear medicine forms the core of many diagnostic techniques and treatments for illnesses plaguing mankind.4 Nuclear-related processes produce many by-products such as isotopes of iodine (129I and 131I), 135Cs and 99Tc, some of which emit alpha (α), beta (β), gamma (γ), and neutron (n), all of which are hazardous to humans and the environment. Due to their extensive half-lives (t1/2), some ranging to millions and billions of years, a permanent and effective disposal approach for these by-products, aptly called nuclear waste products, is necessary.5,6Some common radionuclides that are produced as by-products of power generation are isotopes of iodine: 129I, with a t1/2 of 1.5 × 107 years, which is detrimental to the environment, and 131I, with a t1/2 of 8.02 days, which is detrimental to human metabolic processes.7 As such, many research works have focused on the successful capture and confinement of iodine.8,9 Common industrial practice in capturing and storing iodine is dominated by activated carbon10,11 and zeolite12 filters and scrubbers; however, recent research works are oriented towards metal–organic frameworks (MOFs)13,14 or variants of composites,9,15,16 to varying degrees of success.
MOFs are an emerging class of hybrid materials17–19 formed by coordinating multifunctional organic linkers to metallic ions or clusters, giving rise to a plethora of 3-D porous networks containing periodic nanosized pores or extended metal–organic channels.20–22 The presence of metallic cores and organic linkers introduces tunable physico-chemical properties that are absent from purely metallic or purely organic materials.23 Many MOFs are extremely porous (typically >1000 m2 g−1)24 to accommodate guest encapsulation or host–guest confinement interactions,25 while certain MOF structures feature coordinatively unsaturated metal sites26 that are highly accessible for molecular adsorption. From the mechanical standpoint, the open frameworks of MOFs are relatively more flexible (lower stiffness)27,28 and also softer compared to their constituents or inorganic structural analogues.29 A notable example of MOFs that embody these properties is the subclass called Zeolitic Imidazolate Frameworks (ZIFs),30 which are structural analogues of inorganic zeolites, established catalysts in the petrochemical industry. Ag-impregnated zeolites (Ag-MORs)31 have been used to capture iodine, and filters to that effect are available commercially. ZIFs exhibit a tunable accessible surface area30 and a respectably high thermal stability (up to 500 °C),32 making them ideal candidates for many potential applications.33–35 Recent reports reveal that the terahertz lattice dynamics and soft modes of ZIFs36 may also play a role in guest sorption, retention and delivery.
ZIF-8 [Zn(mIm)2; mIm = 2-methylimidazolate], which is a cubic ZIF material adopting a sodalite architecture (surface area up to 1950 m2 g−1),32 has been shown to be effective in the capture and retention of iodine in both the powdered form and extruded pellets.7 Despite its reported effectiveness, the deliverability of ZIF-8 remains an open question. Depositing loose powders of ZIF-8 into aqueous environments is clearly not a practical approach for industrial practices.37 Therefore, recent work has focused on the formation of MOFs, and by extension, ZIF-based composites38 and membrane systems39,40 to improve robustness and enhance deliverability. There are plenty of examples of MOFs and ZIF-based composites,41,42 encompassing polymer-based and ceramic-based matrices. However, MOF-polymer composites are especially favoured, due to the affinity between the organic ligands of MOFs and the polymer molecules/chains, which results in the formation of strong and continuous structures that retained all of the advantages of MOFs without compromising the structural features associated with polymers. The flexibility and ease of processing of polymers also make them easily deployable and impregnated with MOF nanoparticles at controlled loadings.43,44 It should also be pointed out that recently reported work suggested that the deployment of composites,15,16 when exploiting the advantages of both the matrix and fillers, is more effective in capturing and storing iodine compared to either constituents acting on their own.
Previous attempts have been made to capture and immobilise iodine within ZIF-8/HKUST-1, Ag, and glass powders (EG2992 and EG2998), forming a glass-composite material (GCM), which turned out to be a success, as reported by Sava et al.45 Despite the effectiveness of this reported approach, its energy intensiveness and the requirement to employ a mixture of multiple constituents within a single system might prove to be over demanding for practical engineering solutions.
The current study aims to combine the ease of material processability and adaptability of polymers and the large surface area and regular pores offered by MOFs to create a composite membrane system, which is structurally resilient, easily deliverable, and mechanically robust to capture and retain iodine. This work aims to develop, assess and characterise the viability of ZIF-8/polymer nanocomposites as an iodine capture and retention model system. Herein, one of the objectives is to also minimise the energy expenditure associated with the capture and retention process, and thus to rely upon the molecular interactions and affinity between iodine, ZIF-8 nanoparticles, and polymer matrices (either glassy or rubbery) to expedite the uptake of iodine into the thin composite membranes. The introduction of this so-called passive polymer-based system represents an equally effective alternative that is cost effective and highly deliverable, the concept of which is potentially viable for development to enable industrial applications in the long run.
2. Materials and methods
2.1 Synthesis of ZIF-8 nanoparticles and processing of ZIF-8/Matrimid and PU/ZIF-8 nanocomposite membranes
The methods involved in synthesising ZIF-8 nanoparticles (Fig. 1) are reported elsewhere46 and are replicated in this work. Polyurethane (PU) beads were purchased from Sigma Aldrich, while Matrimid®5218 flakes were supplied by Huntsman Materials (the polymeric structure for the polymers and ZIF-8 are presented in the ESI† in Fig. S2). Both polymers were used as is without any further alterations. A measured amount of PU, based on eqn (1), was dissolved in tetrahydrofuran (THF), while the same was done for Matrimid flakes in chloroform (CHCl3), so as to achieve the targeted ZIF-8 nanoparticle loading of 30 wt%.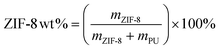 | (1) |
 | ||
| Fig. 1 Scanning electron micrograph showing ZIF-8 nanoparticles prior to being embedded in polymer-MOF nanocomposite. The mean diameter of the nanoparticles is ca. 150 nm. | ||
Both were magnetically stirred for 24 hours, forming a clear and transparent viscous solution for PU, and a clear yellowish viscous solution for Matrimid. Two vials of ZIF-8 nanoparticles were prepared (∼2 g each), dispersed in CHCl3 and THF, and were added to the Matrimid and PU solutions at a measured rate, respectively. The combined PU/ZIF-8 and ZIF-8/Matrimid solutions were again magnetically stirred for 24 hours, forming opaque and viscous solutions. The PU/ZIF-8 solution was casted onto a PTFE substrate, while the ZIF-8/Matrimid solution was casted onto a glass substrate using an automated doctor blade machine at a constant speed of 15 mm−1, with the thickness of the doctor blade set to 150 μm. After casting, the membranes were left to slowly cure in a glove bag saturated with THF vapour (PU/ZIF-8) and CHCl3 vapour (ZIF-8/Matrimid) for 6 hours, after which they were removed and placed in a vacuum oven and dried for 24 hours at 85 °C to remove any residual THF/CHCl3 within the PU, Matrimid, or ZIF-8 nanoparticles. After drying, the membranes were removed from the vacuum oven and carefully dislodged from the substrate, had their thicknesses measured, and stored for further tests. The methodology described above resembles that of our recent report to fabricate thin mixed-matrix membranes of MOF/polymer nanocomposites.44
2.2 Iodine (I2) capture
A solution of 10 mM iodine (henceforth called I2) was prepared with cyclohexane (C6H12) as its base; both were purchased from Sigma Aldrich and used as is without further modifications or purification. The previously synthesised PU/ZIF-8 and ZIF-8/Matrimid nanocomposites were dried in a vacuum oven for 24 hours at 85 °C prior to the I2 uptake experiments to remove any moisture or solvents remaining on the surface of or within the membranes. These nanocomposites were then carefully weighed, with the weight recorded for every sample. The nanocomposites were then placed in a 50 mL glass vial, and 1 mL of the aforementioned I2 solution was sampled and placed into the vials and hermetically sealed. The vials were placed away from direct exposure to sunlight in order to prevent UV degradation or from influencing I2 capture. Images of the vials were taken at designated intervals of 0, 1, 2, 4, 6, 24, 48, 72 and 96 hours. At the end of the uptake experiments, the membranes were removed from the solution, washed, and weighed.The samples exhibiting the highest level of capture and retention of iodine were used for desorption experiments, where the sample was submerged in ethanol (EtOH) and allowed to desorb for a set amount of time. Images of the desorption experiments were taken to monitor the level of iodine released from the samples. The I2-absorbing polymers and nanocomposites were subjected to multiple characterisation techniques to confirm their absorptive and retentive capabilities.
2.3 Scanning electron microscopy (SEM)
Matrimid, PU, and their corresponding ZIF-8 nanocomposites, as is, were submerged in liquid nitrogen (LN2) and then fractured at the cross sections to produce a clear view of their through-thickness internal morphology. The fractured samples were mounted on an SEM stub and secured using carbon tapes at an angle of 90°. The samples were then sputter-coated with gold for 30 seconds, and were then loaded into the field emission gun SEM (TESCAN LYRA3 FEG-SEM/FIB apparatus) for imaging. Imaging was conducted under high vacuum (HV mode), at 5–10 kV, employing a magnification of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–30
000–30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×.
000×.
2.4 Thermogravimetric analysis (TGA)
The influence of I2 uptake on the thermal and structural modifications of the pristine nanocomposite membranes was studied using the TGA method. The device used in this work is a TGA-Q50 (TA instruments), which comes with its own induction-heating chamber and platinum (Pt) sample holder. A portion of the nanocomposites was carefully sectioned from the main sample (∼1.5 mg), and placed onto the Pt sample holder. Prior to the experiments, the sample holder was calibrated to zero weight in order to prevent any foreign particles or objects from influencing the readings. The samples were then subjected to the heat treatment outlined and programmed into the software: a heating rate of 10 °C min−1, an initial temperature of 50 °C and a final temperature of 800 °C. These parameters and temperatures were selected in order to guarantee that the samples (which include Matrimid, PU, and ZIF-8) undergo the total range of thermal phase changes and decomposition. The weight percent (wt%) variation of the samples was recorded at every temperature interval, and these data were then used to construct thermal decomposition plots of the polymer matrices and their corresponding nanocomposites, with and without I2 guest molecules.2.5 Nanoindentation mechanical characterisation
The blank and I2-loaded nanocomposites were secured onto an aluminium stub using a mounting wax, and their quasi-static nanomechanical properties, specifically the corresponding Young's modulus (E) and nanohardness (H), were determined using the nanoindentation method via an MTS NanoIndenter XP (Agilent Technologies, USA), equipped with a Berkovich three-sided pyramid diamond tip. A collection of 20 indents (at a depth of 2000 nm) were made per sample at random locations on the membrane surface, as per the method described by Mahdi et al.443. Results and discussion
3.1 Iodine uptake, immobilisation and release rates
Fig. 2 shows the cross-sectional images of the membranes. It is clear from the SEM images that the dispersion of the ZIF-8 nanoparticles throughout the polymer matrices is uniform. It should also be pointed out that the ZIF-8 nanoparticles embedded in Matrimid are encapsulated by Matrimid, exhibiting spherically coated surfaces,44 while the ZIF-8 nanoparticles embedded onto PU seem to retain their original rhombic dodecahedron crystal configuration,47 further increasing the accessibility to the ZIF-8 within the nanocomposite, which is important vis-à-vis I2 capture and retention. The presence of ZIF-8 was also confirmed via X-ray diffraction performed on the membranes (see XRD in ESI,† Fig. S1).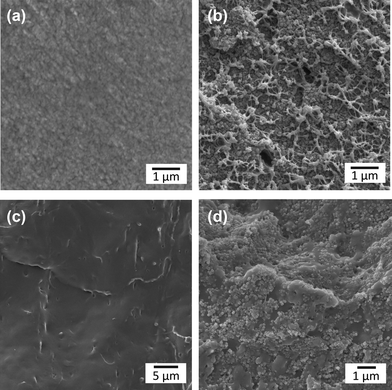 | ||
| Fig. 2 Cross-sectional SEM images of the polymer and polymer-MOF nanocomposites, with (a) Matrimid, (b) ZIF-8/Matrimid 30 wt%, (c) PU, and (d) PU/ZIF-8 30 wt%. | ||
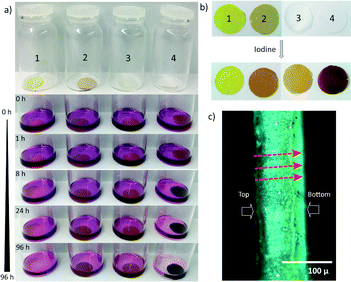
Fig. 3 shows the vials containing the Matrimid and PU-based ZIF-8 membranes, filled with I2, at designated time intervals (0–96 h), while Fig. 4 shows the quantitative uptake capacity of the membranes, as measured by the wt% of absorbed I2.
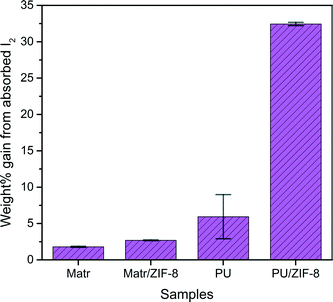
The physical changes in the PU and PU-based nanocomposite are rather pronounced, with PU exhibiting a yellowish tinge, while the PU/ZIF-8 30 wt% membrane show a dark purple tinge (Fig. 3b). The colour changes are attributed to the absorbed I2. Data in Fig. 4 shows that the PU/ZIF-8 30 wt% nanocomposite exhibiting a substantial weight gain of 32.4 wt%, while PU shows a much smaller increase of 5.9 wt%. It can be seen that higher concentrations of absorbed I2 will result in the nanocomposites exhibiting a similar colour to that of I2, while lower absorbed concentrations will result in a light brownish tinge. Due to the relatively small amount of absorbed I2 in the Matrimid-based membranes (1.8 wt% and 2.7 wt% for Matrimid and Matrimid/ZIF-8 30 wt%, respectively), their corresponding colours post-absorption remained the same (Fig. 3b). It was evident that the uptake and retention of I2 resulted in the change of colours of the polymers and their corresponding nanocomposites.
To confirm I2 capture within the nanocomposite as opposed to mere surface coverage of I2, we visually assessed the cross-section of the I2-loaded composite (Fig. 3c). Optical images taken of the cross-section of the nanocomposite capturing the highest amount of I2, i.e. PU/ZIF-8 30 wt%, exhibited features suggestive of I2 transport across the nanocomposite interior. The colour gradient in Fig. 3c indicates the direction of the flow of I2, proving that I2 was indeed absorbed by the nanocomposite as opposed to only being present on the surface layer of the composite. Notably, the top and bottom surfaces of the nanocomposite could be easily distinguished. It can be seen that the top surface that was exposed to I2 exhibited a darker shade of purple, as opposed to the bottom surface of the membrane sample. This differential colouration phenomenon elucidates that a concentration gradient is present in the composite, thus indicating that I2 has infiltrated the nanocomposite but remained entrapped within as opposed to being filtered out of the membrane.
We examined the I2 release characteristics by monitoring the change in colour of the solution, where the I2-loaded PU/ZIF-8 30 wt% nanocomposite was immersed and hermetically sealed (Fig. 5). The polymer matrices, as discussed previously, decrease the accessibility to the high surface area of ZIF-8 nanoparticles, which extended the I2 release time (beyond 6.5 h), relative to the case of (loose) MOF powders, where a substantial release of captured I2 was reported after only two hours.48 Furthermore, the hydrophobicity of the polymer matrices prevented access of polar solvents to the nanopores of the ZIF-8 nanoparticles, allowing the nanocomposites to retain I2 within their microstructures. This was proven by immersing the I2-loaded PU/ZIF-8 30 wt% composite into water, and after a period of 10 days (see Fig. S3 in the ESI†), there were no colour changes detected in the surrounding water, signifying that I2 remained immobilised. Importantly, this result shows that despite the affinity of I2 towards polar solvents (e.g. water), the desorption of I2 can be suppressed by encapsulating it within a polymer/MOF nanocomposite where the matrix is an intrinsically hydrophobic phase. We reasoned that the complex microstructure of the nanocomposite (Fig. 2) further prevented open access to the adsorption sites located inside the sodalite cages of ZIF-8,7 such that the absorbed I2 can remain immobilised within the membrane.
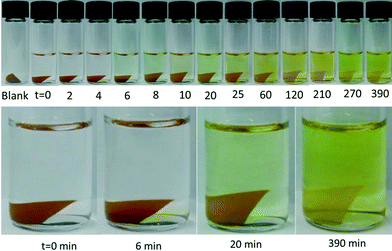 | ||
| Fig. 5 Iodine release was investigated by monitoring the change in the colour of ethanolic solution in I2-loaded PU/ZIF-8 30 wt% at different time points. Since the polymer matrix in the nanocomposite decreased the accessibility to the high surface area of ZIF-8 pores, I2 release in ethanol was observed to slow down and remain virtually unchanged beyond 6.5 h (see ESI† Fig. S3 for the sample after 10 days of immersion time). | ||
3.2 Nanoindentation properties
The nanoindentation technique was used to characterise the nanomechanical properties of the polymers and their corresponding nanocomposites pre- and post-I2 absorption. The results are summarised in Fig. 6.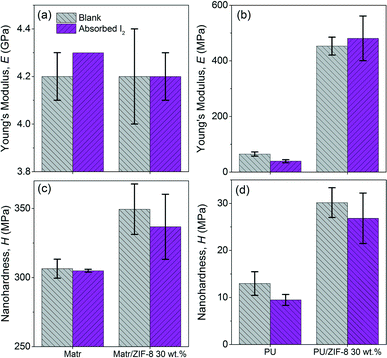 | ||
| Fig. 6 Nanoindentation results of the Matrimid and PU-based nanocomposite membranes. (a & b) Young's modulus of Matrimid-based and PU-based membranes, respectively. (c & d) Nanohardness of Matrimid-based and PU-based membranes, respectively. Note that the glassy Matrimid matrix (whose Tg > room temperature, RT) is significantly stiffer and harder than the rubbery matrix of PU (Tg < RT). Specifically for the blank samples, we note that the decrease in the Young's modulus of Matrimid/ZIF-8 30 wt% compared with that of pristine Matrimid is associated with the incorporation of the ZIF-8 fillers (EZIF-8 ∼3 GPa),28 which are more compliant than the Matrimid phase; this effect has been explained in detail in recent reports.44,50 The nanoindentation data shown here were averaged from a surface penetration depth ranging from 1 to 2 μm. | ||
The application of the nanoindentation technique is a practical choice: bulk-testing approaches such as uniaxial tensile tests of the membranes will result in the bulk properties (in this case, the matrix of the nanocomposites) being determined. The influence of the nanoparticles (ZIF-8) will subsequently be overlooked. The nanoindentation technique makes measured indents onto minute areas of the surface of the samples at controlled depths (up to ∼2 μm),44,49 enabling it to investigate the delicate surface regions where the ZIF-8 nanoparticles, the polymer matrices, and incidentally, the absorbed I2 are actually interacting. For the polymer matrices, this translates into probing the surface where the polymeric chains and I2 are interacting. This method will allow us to measure relatively small mechanical property differences, more precisely capturing the change in the Young's modulus (elastic stiffness) and hardness (resistance against plastic deformation) of the ZIF-8 mixed-matrix membranes due to their absorption of I2.
Fig. 6(a) and (c) show that the nanomechanical properties of the Matrimid-based membranes are only slightly affected by the absorption of I2. This is reflected by the fact that the Young's Modulus (E) differs by ∼2% for Matrimid and no change was detected for its nanocomposite sample. While for the hardness (H) values, the error bars for both samples are within the range of each other, implying that the amount of I2 absorbed by the Matrimid-based membranes are relatively small (all changes fall under the ∼5% range) vis-à-vis the nanomechanical properties. The glassy nature of the Matrimid matrix at room temperature (Matrimid glass transition temperature, Tg ∼345 °C)51 is important and may account for this outcome. It is envisaged that the molecular packing and rigidity underpinning the polymeric chains of Matrimid form an interconnected network that is sufficiently dense, thereby preventing the infiltration and uptake of I2 guest molecules. We found that the introduction of ZIF-8 nanoparticles (fillers) into Matrimid, despite their expected disruption to the molecular packing of Matrimid,30,31 did little to boost the overall I2 absorptive capabilities, thus its marginal impact on the nanomechanical properties of Matrimid-based membranes.
However, a different trend was observed in the PU-based membranes (Fig. 6b and d). It is seen that the Young's modulus (E) and nanohardness (H) values changed by a discernible amount for neat PU (−25.5 MPa, corresponding to a ∼40% drop for E, and −3.5 MPa for H, corresponding to a drop of ∼27%) and PU/ZIF-8 30 wt% (28 MPa for E, corresponding to an increase of ∼6%, and 3 MPa for H, corresponding to a decrease of ∼11%) post-I2 absorption. It should be pointed out that unlike in neat Matrimid, the nanomechanical properties in the neat PU sample displayed a contradictory behaviour where E and H values declined with absorption of I2. This softening effect is speculated to be due to the pervasiveness of I2 in the hard and soft segments of PU (see ESI† Fig. S2), which increased the interatomic distance between the polymeric chains, thus weakening the inter- and intra-molecular bonding interactions affecting their structural integrity.34 We also propose that the looser molecular packing of the rubbery PU polymeric chain (Tg < RT) and the nominal molecular attachment of the ZIF-8 to the PU matrix accounted for the increased absorption of I2 into the PU/ZIF-8 30 wt% nanocomposite, due to increased accessibility to ZIF-8 nanoparticles. The infiltration and I2 uptake within the ZIF-8 nanoparticles will be assisted by the size of the porous cages of ZIF-8 (ref. 52) (11.4 Å against the size of the iodine molecule of 5.6 Å), if the (loosely packed) rubbery polymer chains will provide an open pathway to reach the ZIF-8 pores, thereby boosting the total I2 uptake (see Fig. 4). Our results show that, for the PU/ZIF-8 30 wt% nanocomposite, upon I2 absorption there is no major variation detected in E and H, both of which appear to lie within the respective statistical errors.
3.3 Thermogravimetric analyses (TGA) of the I2-absorbing nanocomposite membranes
Fig. 7 shows the TGA profiles of the absorbing nanocomposite membranes and their blank counterparts. It is seen from Fig. 7(a)–(b) that the absorption by the Matrimid-based nanocomposites is almost negligible, as the profile of the absorbing sample is almost identical to the blank sample, with almost minute variations throughout the thermal decomposition profile. This is supported by the weight uptake differential data in Fig. 4, where the wt% changes post-I2 absorption for the Matrimid-based nanocomposites remained under 3%.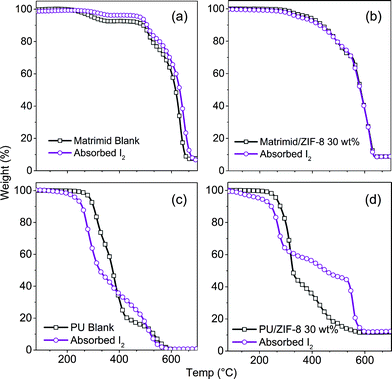 | ||
| Fig. 7 TGA plots of (a) Matrimid, (b) ZIF-8/Matrimid 30 wt%, (c) PU, and (d) PU/ZIF-8 30 wt%, with their respective blank counterparts. For an overall combined plot of TGA, including the ZIF-8 nanoparticles, refer to Fig. S5 in the ESI.† | ||
On the contrary, as per Fig. 4, there is a marked rise in weight change (+32.4%) in the PU/ZIF-8 30 wt% composite, which is also reflected in the thermal decomposition profile of PU and PU-based nanocomposites. For PU, at ∼210 °C, the I2-loaded composite began to decompose. This is speculated to be due to the initiation of thermal degradation of organics, and with PU molecular structure degradation, the I2 bonded to the soft segment of the PU polymeric chain is being released. It should also be pointed out that the flash point of I2 is at 184.3 °C; however, I2 only began to evaporate from PU at 210 °C, meaning that the host–guest interactions shield the I2 entrapped in PU from evaporating at its characteristically lower temperature stated above. We proposed that only when the chain integrity of PU was compromised that I2 was exposed to the surrounding thermal conditions, thus triggering its evaporation process.
The thermal decomposition of the I2-loaded sample was more rapid compared to its blank counterpart. The decomposition of the hard segment of PU appeared to begin at 310 °C for the I2-loaded sample, which is earlier than the blank sample, where it began to decompose at 405 °C. The reason for this is that the I2-loaded sample has formed an intrinsic part of the microstructure of PU, and once I2 began to be liberated from the hard segments (ESI† Fig. S2) due to structural degradation from the soft segments, the structural integrity of the hard segment begins degrading also, in conjunction with release of I2. A similar trend was observed in the PU/ZIF-8 30 wt% nanocomposite, where thermal decomposition of the loaded composite began a lot sooner than the blank nanocomposite; however, it is unique in that I2 is released from the decomposed soft segment of PU and decomposing ZIF-8 (∼350 °C, see ESI† Fig. S5). The results in Fig. 3 suggest that I2 is absorbed into ZIF-8 cages as well as the soft and hard segments of PU. Upon thermal decomposition of PU, ZIF-8 nanoparticles are exposed, and the organic ligands forming bonds with I2 and the hard segment of PU will began to decompose, releasing I2 and breaking the chemical and physical interactions formed with PU.
The phenomenon surrounding the release of I2 from ZIF-8 cages is obvious in Fig. 7d, where from 300–550 °C, the I2-loaded sample decomposes at a rate that is slower than its blank counterpart. This can be attributed to the entrapped I2 in the ZIF-8 cages being released as the ZIF-8 cages collapse because of thermal decomposition.53 At 550 °C, when I2 was completely removed, a sharp drop was observed in the wt% of the nanocomposite. Subsequently at 600 °C, its thermal decomposition profile matched that of its blank counterpart, showing that all the entrapped I2 was indeed removed from both PU and ZIF-8. What is important to establish here is that, by comparing Fig. 7c and d, there is strong evidence supporting the notion that thermal stability of the I2-absorbing PU/ZIF-8 nanocomposite has significantly improved as a consequence of guest immobilisation (section 3.4).
3.4 Proposed mechanism responsible for iodine absorption and immobilisation in MOF-polymer nanocomposite membranes
Fig. 8 illustrates the proposed absorption mechanism that explains I2 uptake and retention by the polymer-MOF nanocomposites. Based on the observed I2 uptake by the polymers and their corresponding ZIF-8 nanocomposites, we propose that the uptake of iodine will be made more efficient with the utilisation of a flexible polymeric molecular backbone. The high surface area of ZIF-8 nanoparticles (∼1650 m2 g−1)54 is expected to offer significant active sites to afford adsorption of I2,55 mostly via combined actions of chemisorption56 and physisorption;7 however, retention of the adsorbate is significantly strengthened by the polymer matrices. Both polymers (Matrimid and PU) exhibited almost negligible amounts of I2 uptake (Fig. 4), and it is presumed that the high uptake shown by the composites (especially PU/ZIF-8 30 wt%) is due to I2 entering the pores of ZIF-8 nanoparticles instead of the free volume within the polymers formed by the presence of the nanoparticles. However, the functional groups and aromatic moieties in both Matrimid and PU are potential sites for the weak molecular interactions with I2 molecules; therefore, the possibility of I2 storage within inter-polymer-MOF pockets (free volume) cannot be completely ruled out. The presence of these unoccupied pockets could also potentially serve as active molecular flexible pathways that allow for I2 to infiltrate the composites and guide the molecules towards the more active and high-surface area ZIF-8 nanoparticles cages.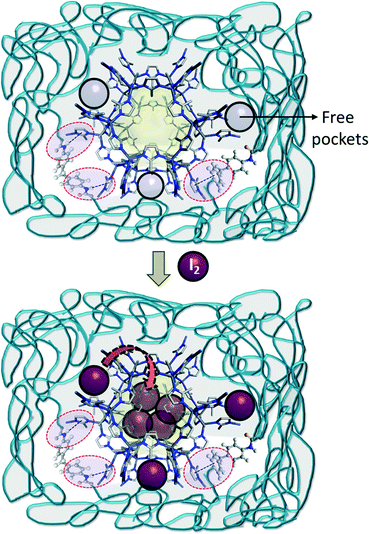 | ||
| Fig. 8 The proposed I2 absorption mechanism by the polymer-MOF nanocomposite. The introduction of ZIF-8 nanoparticles within the polymer matrix offers porosity attributed to the intrinsic sodalite cages of ZIF-8 (filler phase) and free volumes arising from the disruption of the molecular packing of the nanocomposite by the nanoparticle fillers. These voids could serve as active sites, attracting I2 molecules and forming bonds with the mIm ligands of ZIF-8, and the ‘hard’ and ‘soft’ segments of the PU (see ESI† Fig. S2) or the active coordination sites present in Matrimid.44 | ||
Fig. 8 details the plausible molecular level interactions between I2, ZIF-8, and the polymers (PU in the diagram) in the ZIF-8 nanocomposites. The light yellow spheres indicate the void formed by the presence of ZIF-8 nanoparticles, while the black circles represent the inter-polymer-ZIF-8 pockets. These free volumes could be of large and small dimensions, which is incidentally not necessary for strong interactions with I2 when compared to the continuous array of ordered active voids of the ZIF-8 nanoparticles. Smaller volume voids with higher surface area may result in stronger nanoscale confinement with incoming foreign guest species, such as I2. This effect could be investigated in greater detail by means of, for example, positron annihilation lifetime spectroscopy (PALS).54,57
Furthermore, comparing the chemical structures of the polymer matrices could provide additional insights into their potential role in the capture and retention of I2 within ZIF-8 polymer nanocomposites. PU is made up of amide linkages that are suitable for non-covalent interactions, while its flexible ether moiety could potentially provide a site for strong interaction and catalyse molecular dynamics within the matrix that improves small guest molecular mobility. However, in the case of Matrimid, its rigid aromatic backbone could potentially hinder the formation of inter-polymer-ZIF-8 pockets via its strong interchain aromatic interactions.
It is clear from our experimental data that the PU samples are more susceptible to I2 uptake compared to the Matrimid samples. We reasoned that the intrinsic porosity of PU and its ability to interact with guest species via weak interactions could explain these differences. The molecular flexibility of the PU's primary chain allows for entropic dynamics of its rubbery structure to capture I2 more rapidly. On the contrary, tightly packed structural configurations, which are especially prevalent in Matrimid (glassy phase), could result in a lower accessible surface area, rendering them less effective for I2 immobilisation. The introduction of ZIF-8 nanoparticles into both polymer matrices will create strong “anchoring sites” and increase the free volume (due to disruption of molecular packing) that will enhance the resulting nanocomposite capacity to immobilise I2. On this basis, the model we proposed in Fig. 8 represents the most probable capture and storage mechanism for the Matrimid/ZIF-8 30 wt% and PU/ZIF-8 30 wt% nanocomposites developed in this work.
4. Conclusions
This work represents the first reported attempt to capture and retain iodine within membranes made from ZIF-8/polymer nanocomposites. A few conclusions can be drawn from the body of this work, as listed below.• Iodine was indeed captured and retained within the pristine polymer membranes (albeit at small wt%), but the immobilisation of I2 in the nanocomposite is prominent particularly in the case of PU/ZIF-8 30 wt% membranes.
• We identified that the rubbery polymer matrix (e.g. PU) results in better absorptive and retention capabilities, which could be associated with the more accessible ZIF-8 pores to afford physisorption and chemisorption processes and further accompanied by physical confinement inside free volumes of the polymers.
• We projected that the mechanism that allowed iodine to be captured and retained within the polymers and nanocomposites can be attributed to the molecular interactions and affinity between the mIm (deprotonated ligand of HmIm) of ZIF-8, iodine molecules, and some of the polymeric chains of the matrices. Therefore, this passive process requires almost no injection of external energy, but relies completely on molecular and chemical affinities existing between the multiple organic–inorganic molecular constituents in the nanocomposite.
• The formation of ZIF-8/polymer nanocomposites improved the deliverability of the samples and allowed the retention of I2 at temperatures far beyond its flashpoint, thus boosting the thermal stability as evidenced from the TGA experiments.
• The initial design proposal in the context of iodine capture and retention would suggest that a combination of highly porous MOFs containing strong sorption sites (e.g. ZIFs,30 MILs,58,59 CuBTC,60 CPOs61,62etc.) and rubbery polymers, such as PU, polydimethylsiloxane (PDMS) and polyisobutylene (PIB), will be favourable for maximising iodine capture rates in the emerging class of MOF-based mixed-matrix membrane (MMM)39,63,64 nanocomposites.
• There is a wide scope for future developments in the aforementioned areas, not only to better elucidate the underpinning physico-chemical mechanisms but also to design and tune suitable combinations of membrane nanocomposites to yield bespoke guest immobilisation capacities.
Acknowledgements
E. M. Mahdi would like to thank Yayasan Khazanah (YK) for the DPhil scholarship that funded his research work at Oxford University. We acknowledge the Royal Society Research Grants (RG140296) for equipment funding. The authors thank Mr. S. Ying and Prof. A. M. Korsunsky for access to the Scanning Electron Microscope facilities (MBLEM Laboratory at Oxford, EU FP7 Project iSTRESS (604646)), and Prof. Steve Roberts and Dr. David Armstrong for usage of nanoindentation facilities at Oxford Materials. We acknowledge the provision of advanced material characterisation facilities, including TGA, by the Research Complex at Harwell (RCaH), Rutherford Appleton Laboratory (RAL), Oxfordshire. We thank Dr. Marek Jura and Dr. Gavin Stenning at R53 Materials Characterisation Laboratory in ISIS RAL, for access to the Rigaku X-ray diffractometer.References
- A. Verbruggen, E. Laes and S. Lemmens, Renewable Sustainable Energy Rev., 2014, 32, 16–28 CrossRef.
- V. S. Ramos, V. R. Crispim and L. E. Brandao, Appl. Radiat. Isot., 2013, 82, 111–118 CrossRef CAS PubMed.
- V. H. M. Visschers, C. Keller and M. Siegrist, Energy Policy, 2011, 39, 3621–3629 CrossRef.
- L. G. Carneiro, E. A. de Lucena, C. d. S. Sampaio, A. L. Dantas, W. O. Sousa, M. S. Santos and B. M. Dantas, Appl. Radiat. Isot., 2015, 100, 70–74 CrossRef CAS PubMed.
- R. P. Rechard, J. H. Lee, E. L. Hardin and C. R. Bryan, Reliab. Eng. Syst. Safe., 2014, 122, 145–164 CrossRef.
- A. Salama, M. F. El Amin and S. Sun, Prog. Nucl. Energy, 2015, 85, 747–755 CrossRef CAS.
- D. F. Sava, M. A. Rodriguez, K. W. Chapman, P. J. Chupas, J. A. Greathouse, P. S. Crozier and T. M. Nenoff, J. Am. Chem. Soc., 2011, 133, 12398–12401 CrossRef CAS PubMed.
- D. F. Sava, K. W. Chapman, M. A. Rodriguez, J. A. Greathouse, P. S. Crozier, H. Zhao, P. J. Chupas and T. M. Nenoff, Chem. Mater., 2013, 25, 2591–2596 CrossRef CAS.
- S. Ma, S. M. Islam, Y. Shim, Q. Gu, P. Wang, H. Li, G. Sun, X. Yang and M. G. Kanatzidis, Chem. Mater., 2014, 26, 7114–7123 CrossRef CAS.
- O. B. Yang, J. C. Kim, J. S. Lee and Y. G. Kim, Ind. Eng. Chem. Res., 1993, 32, 1692–1697 CrossRef CAS.
- H. Sun, P. La, Z. Zhu, W. Liang, B. Yang and A. Li, J. Mater. Sci., 2015, 50, 7326–7332 CrossRef CAS.
- K. W. Chapman, P. J. Chupas and T. M. Nenoff, J. Am. Chem. Soc., 2010, 132, 8897–8899 CrossRef CAS PubMed.
- B. Assfour, T. Assaad and A. Odeh, Chem. Phys. Lett., 2014, 610–611, 45–49 CrossRef CAS.
- C. Falaise, C. Volkringer, J. Facqueur, T. Bousquet, L. Gasnot and T. Loiseau, Chem. Commun., 2013, 49, 10320–10322 RSC.
- G. Massasso, J. Long, J. Haines, S. Devautour-Vinot, G. Maurin, A. Grandjean, B. Onida, B. Donnadieu, J. Larionova, C. Guerin and Y. Guari, Inorg. Chem., 2014, 53, 4269–4271 CrossRef CAS PubMed.
- G. Massasso, M. Rodriguez-Castillo, J. Long, A. Grandjean, B. Onida, Y. Guari, C. Guerin and J. Larionova, J. Mater. Chem. A, 2015, 3, 179–188 CAS.
- H. C. Zhou, J. R. Long and O. M. Yaghi, Chem. Rev., 2012, 112, 673–674 CrossRef CAS PubMed.
- J. C. Tan and B. Civalleri, CrystEngComm, 2015, 17, 197–198 RSC.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed.
- S. Kitagawa, R. Kitaura and S. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334–2375 CrossRef CAS PubMed.
- P. Silva, S. M. Vilela, J. P. Tome and F. A. Almeida Paz, Chem. Soc. Rev., 2015, 44, 6774–6803 RSC.
- S. Galli, N. Masciocchi, V. Colombo, A. Maspero, G. Palmisano, F. J. Lopez-Garzon, M. Domingo-Garcia, I. Fernandez-Morales, E. Barea and J. A. R. Navarro, Chem. Mater., 2010, 22, 1664–1672 CrossRef CAS.
- A. K. Cheetham, C. N. Rao and R. K. Feller, Chem. Commun., 2006, 4780–4795 RSC.
- G. Férey, Chem. Soc. Rev., 2008, 37, 191–214 RSC.
- M. D. Allendorf, M. E. Foster, F. Leonard, V. Stavila, P. L. Feng, F. P. Doty, K. Leong, E. Y. Ma, S. R. Johnston and A. A. Talin, J. Phys. Chem. Lett., 2015, 6, 1182–1195 CrossRef CAS PubMed.
- P. D. Dietzel, R. E. Johnsen, R. Blom and H. Fjellvag, Chem. – Eur. J., 2008, 14, 2389–2397 CrossRef CAS PubMed.
- J. C. Tan and A. K. Cheetham, Chem. Soc. Rev., 2011, 40, 1059–1080 RSC.
- J. C. Tan, B. Civalleri, C. C. Lin, L. Valenzano, R. Galvelis, P. F. Chen, T. D. Bennett, C. Mellot-Draznieks, C. M. Zicovich-Wilson and A. K. Cheetham, Phys. Rev. Lett., 2012, 108, 095502 CrossRef PubMed.
- J. C. Tan, T. D. Bennett and A. K. Cheetham, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 9938–9943 CrossRef CAS PubMed.
- A. Phan, C. J. Doonan, F. J. Uribe-Romo, C. B. Knobler, M. O'Keeffe and O. M. Yaghi, Acc. Chem. Res., 2010, 43, 58–67 CrossRef CAS PubMed.
- T. M. Nenoff, M. A. Rodriguez, N. R. Soelberg and K. W. Chapman, Microporous Mesoporous Mater., 2014, 200, 297–303 CrossRef CAS.
- K. S. Park, Z. Ni, A. P. Cote, J. Y. Choi, R. Huang, F. J. Uribe-Romo, H. K. Chae, M. O'Keeffe and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10186–10191 CrossRef CAS PubMed.
- S.-L. Li and Q. Xu, Energy Environ. Sci., 2013, 6, 1656 CAS.
- S. N. Wijenayake, N. P. Panapitiya, S. H. Versteeg, C. N. Nguyen, S. Goel, K. J. Balkus, I. H. Musselman and J. P. Ferraris, Ind. Eng. Chem. Res., 2013, 52, 6991–7001 CrossRef CAS.
- M. R. Ryder and J. C. Tan, Mater. Sci. Technol., 2014, 30, 1598–1612 CrossRef CAS.
- M. R. Ryder, B. Civalleri, T. D. Bennett, S. Henke, S. Rudić, G. Cinque, F. Fernandez-Alonso and J. C. Tan, Phys. Rev. Lett., 2014, 113, 215502 CrossRef PubMed.
- J. Caro, Curr. Opin. Chem. Eng., 2011, 1, 77–83 CrossRef CAS.
- N. Stock and S. Biswas, Chem. Rev., 2012, 112, 933–969 CrossRef CAS PubMed.
- B. Zornoza, C. Tellez, J. Coronas, J. Gascon and F. Kapteijn, Microporous Mesoporous Mater., 2013, 166, 67–78 CrossRef CAS.
- L. Diestel, N. Wang, B. Schwiedland, F. Steinbach, U. Giese and J. Caro, J. Membr. Sci., 2015, 492, 181–186 CrossRef CAS.
- H. B. Tanh Jeazet, C. Staudt and C. Janiak, Dalton Trans., 2012, 41, 14003–14027 RSC.
- T. Yang, G. M. Shi and T.-S. Chung, Adv. Energy Mater., 2012, 2, 1358–1367 CrossRef CAS.
- Q. Song, S. K. Nataraj, M. V. Roussenova, J. C. Tan, D. J. Hughes, W. Li, P. Bourgoin, M. A. Alam, A. K. Cheetham, S. A. Al-Muhtaseb and E. Sivaniah, Energy Environ. Sci., 2012, 5, 8359 CAS.
- E. M. Mahdi and J. C. Tan, J. Membr. Sci., 2016, 498, 276–290 CrossRef CAS.
- D. F. Sava, T. J. Garino and T. M. Nenoff, Ind. Eng. Chem. Res., 2012, 51, 614–620 CrossRef.
- J. Cravillon, S. Münzer, S.-J. Lohmeier, A. Feldhoff, K. Huber and M. Wiebcke, Chem. Mater., 2009, 21, 1410–1412 CrossRef CAS.
- Y. C. Pan, D. Heryadi, F. Zhou, L. Zhao, G. Lestari, H. B. Su and Z. P. Lai, CrystEngComm, 2011, 13, 6937–6940 RSC.
- S. Parshamoni, S. Sanda, H. S. Jena and S. Konar, Chem. – Asian J., 2015, 10, 653–660 CrossRef CAS PubMed.
- J. C. Tan, C. A. Merrill, J. B. Orton and A. K. Cheetham, Acta Mater., 2009, 57, 3481–3496 CrossRef CAS.
- N. W. Khun, E. M. Mahdi, S. Q. Ying, T. Sui, A. M. Korsunsky and J. C. Tan, APL Mater., 2014, 2, 124101 CrossRef.
- A. C. Comer, D. S. Kalika, B. W. Rowe, B. D. Freeman and D. R. Paul, Polymer, 2009, 50, 891–897 CrossRef CAS.
- S. A. Moggach, T. D. Bennett and A. K. Cheetham, Angew. Chem., Int. Ed., 2009, 48, 7087–7089 CrossRef CAS PubMed.
- J. T. Hughes, D. F. Sava, T. M. Nenoff and A. Navrotsky, J. Am. Chem. Soc., 2013, 135, 16256–16259 CrossRef CAS PubMed.
- Q. Song, S. K. Nataraj, M. V. Roussenova, J. C. Tan, D. J. Hughes, W. Li, P. Bourgoin, M. A. Alam, A. K. Cheetham, S. A. Al-Muhtaseb and E. Sivaniah, Energy Environ. Sci., 2012, 5, 8359–8369 CAS.
- T. D. Bennett, P. J. Saines, D. A. Keen, J. C. Tan and A. K. Cheetham, Chem. – Eur. J., 2013, 19, 7049–7055 CrossRef CAS PubMed.
- J. T. Hughes, D. F. Sava, T. M. Nenoff and A. Navrotsky, J. Am. Chem. Soc., 2013, 135, 16256–16259 CrossRef CAS PubMed.
- D. Cangialosi, H. Schut, A. van Veen and S. J. Picken, Macromolecules, 2003, 36, 142–147 CrossRef CAS.
- S.-H. Huo and X.-P. Yan, J. Mater. Chem., 2012, 22, 7449 RSC.
- J. O. Hsieh, K. J. Balkus, J. P. Ferraris and I. H. Musselman, Microporous Mesoporous Mater., 2014, 196, 165–174 CrossRef CAS.
- A. K. Chaudhari, I. Han and J. C. Tan, Adv. Mater., 2015, 27, 4438–4446 CrossRef CAS PubMed.
- J. Kahr, R. E. Morris and P. A. Wright, CrystEngComm, 2013, 15, 9779 RSC.
- P. D. Dietzel, P. A. Georgiev, J. Eckert, R. Blom, T. Strassle and T. Unruh, Chem. Commun., 2010, 46, 4962–4964 RSC.
- H. B. T. Jeazet, C. Staudt and C. Janiak, Dalton Trans., 2012, 41, 14003–14027 RSC.
- G. X. Dong, H. Y. Li and V. K. Chen, J. Mater. Chem. A, 2013, 1, 4610–4630 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: XRD plots of the blank nanocomposites, raw data pertaining to nanoindentation, and TGA compilation of all the nanocomposites, including ZIF-8 nanoparticles. See DOI: 10.1039/c5me00008d |
| This journal is © The Royal Society of Chemistry 2016 |