Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Design and microwave assisted synthesis of novel 2-phenyl/2-phenylethynyl-3-aroyl thiophenes as potent antiproliferative agents
Rupinder Kaur
Gill
abc,
Ramandeep
Kaur
a,
Virender
Kumar
d,
Vivek
Gupta
e,
Gagandeep
Singh
f and
Jitender
Bariwal
*ag
aDepartment of Pharmaceutical Chemistry, ISF College of Pharmacy, Moga-142001, Punjab, India
bI. K. Gujral Punjab Technical University, Kapurthala, Jalandhar-144 601, Punjab, India
cDepartment of Pharmaceutical Sciences, Guru Nanak Dev University, Amritsar-143 005, Punjab, India
dDepartment of Pharmaceutical Sciences, University of Nebraska Medical Center, Omaha, Nebraska, 68198 USA
ePost-Graduate Department of Physics & Electronics, University of Jammu, Jammu Tawi-180 006, India
fBio-Organic and Photochemistry Laboratory, Department of Pharmaceutical Sciences, Guru Nanak Dev University, Amritsar-143 005, Punjab, India
gSatiate Research & Anatech Pvt. Ltd., HSIIDC, Barwala, Panchkula-134118, Haryana, India. E-mail: jitender.bariwal@gmail.com; Fax: +91 1636 239515; Tel: +91 1636 324200
First published on 21st October 2016
Abstract
Correction for ‘Design and microwave assisted synthesis of novel 2-phenyl/2-phenylethynyl-3-aroyl thiophenes as potent antiproliferative agents’ by Rupinder Kaur Gill et al., Med. Chem. Commun., 2016, 7, 1966–1972.
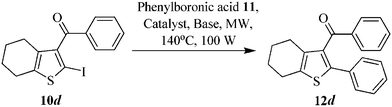
The authors regret the following errors in their paper: (1) The compound numbers shown below the structures in Table 1 should be corrected to show 10d and 12d, instead of 10a and 12a.
(2) On page 1967, section 2.1, first paragraph, when referring to optimization studies shown in Table 1 10a should be replaced by 10d and 12a should be replaced by 12d, as follows:
Earlier, we had reported the efficient formation of biaryl moiety via Suzuki–Miyaura cross-coupling reaction under microwave irradiation conditions; using tetrakis(triphenylphosphane)palladium(0) [Pd(PPh3)4] as a catalyst and Cs2CO3 as a base under MWI at 140 °C and 100 W for 10 min,29 therefore, we have synthesized our targeted compound (4,5,6,7-tetrahydro-2-phenylbenzo[b]thiophen-3-yl)(phenyl)methanone 12d by coupling of 2-iodo thiophene derivative 10d with phenylboronic acid 11via Suzuki–Miyaura cross-coupling reaction (Scheme 1) by following the same protocol as employed earlier; however, the yield obtained was very low (Table 1, entry 1). Further, the optimization of this reaction under MW irradiation was carried out using Pd(OAc)2 and [Pd(PPh3)4] as catalysts and Cs2CO3 and K2CO3 as bases in order to increase the yield of target compounds. It was observed that the use of 5 mol% of Pd(PPh3)4 as a catalyst and 3.0 eq. of K2CO3 in DMF–H2O under microwave irradiation (100 W) for 20 min at 140 °C furnished the desired compound 12d in 91% yield (Table 1, entry 3).
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2016 |