Curcumin inspired synthesis of unsymmetrical diarylpentanoids with highly potent anti-parasitic activities: in silico studies and DFT-based stereochemical calculation†‡
Abstract
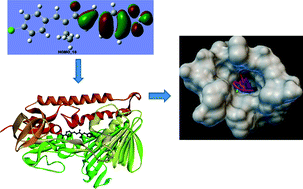
Unsymmetrical diarylpentanoid analogues of curcumin have been synthesized by Claisen–Schmidt condensation of different aldehydes and ketones. Anisaldehyde treated with 2-butanone and chlorobenzaldehyde with 2-pentanone resulted in intermediate compounds 1 and 2. Intermediate compounds 1 and 2 yield compounds 3–21 by treating them with different aldehydes having electron donating (methoxyl) and electron withdrawing (halogens and nitro) moieties. All compounds were evaluated for antiproliferative activity, particularly for the promastigote form of L. amazonensis and the epimastigote and trypomastigote forms of T. cruzi. The results revealed that these curcuminoid analogues have potency against parasites. Among 21 compounds, six were more potent than benznidazole for the epimastigote form, while 14 compounds were more potent for the trypomastigote form of T. cruzi. The binding interactions of all compounds were confirmed through molecular docking computational studies. Potent compounds showed very good interactions with the enzyme trypanothione reductase (PDB code 1BZL). DFT calculation helped us to understand the best possible conformation and optimized the structure. Our synthesized compounds can further be studied to develop lead compounds to treat parasitic diseases.

 Please wait while we load your content...
Please wait while we load your content...