Abstract
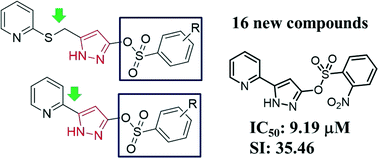
Hepatitis B virus (HBV) is an infectious disease, which can cause acute and chronic infections. Every year, over 7.5 million persons die due to HBV. No effective drug exists for the treatment of HBV. Thus, we designed and synthesized 16 new pyridine-pyrazole-sulfonate compounds containing pyridine-SCH2-pyrazole and pyridine-pyrazole derivatives. Their structures were characterized by 1H-NMR, 13C-NMR, IR, mass spectroscopy, and high performance liquid chromatography. All the compounds were evaluated for their anti-HBV activities and a structure–activity relationship (SAR) in HepG2 2.2.15 cells was established. We found that the pyridine-pyrazole derivatives could inhibit the HBV gene expression and viral DNA replication. Among these compounds, 2-[3-(2-nitrophenylsulfonyl)oxy-5-pyrazol-yl]pyridine 19d has shown the most potent inhibitory activity with an IC50 value of 9.19 μM and high selectivity index, SI (TC50/IC50) of 35.46. Hence, we believe our compounds could serve as a potential lead compounds for anti-HBV drug development.

 Please wait while we load your content...
Please wait while we load your content...