In vitro and in vivo activity of ML302F: a thioenolate inhibitor of VIM-subfamily metallo β-lactamases
Jonathan W.
Betts
a,
Lynette M.
Phee
ab,
Muhd H. F.
Abdul Momin
a,
Klaus-Daniel
Umland
c,
Jurgen
Brem
c,
Christopher J.
Schofield
c and
David W.
Wareham
*ab
aAntimicrobial Research Group, Barts & The London School of Medicine and Dentistry, Queen Mary University of London, London, E1 2AT, UK. E-mail: d.w.wareham@qmul.ac.uk
bDivision of Infection, Barts Health NHS Trust, London, E1 1BB, UK
cDepartment of Chemistry, University of Oxford, Oxford, OX1 3TA, UK
First published on 16th November 2015
Abstract
The thioenol ML302F was recently identified as an inhibitor of class B metallo-β-lactamases (MBLs). We assessed the activity of ML302F when combined with meropenem (MEM) against 31 carbapenem resistant Gram-negative clinical isolates. Minimum inhibitory concentrations of MEM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ML302F were determined at fixed ratios of 1
ML302F were determined at fixed ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1
4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 using strains producing variants of the clinically relevant VIM-like MBL. Toxicity and efficacy in vivo was assessed in a Galleria mellonella invertebrate model against strains producing VIM-1, VIM-2 and VIM-4 variants. At a fixed MEM
8 using strains producing variants of the clinically relevant VIM-like MBL. Toxicity and efficacy in vivo was assessed in a Galleria mellonella invertebrate model against strains producing VIM-1, VIM-2 and VIM-4 variants. At a fixed MEM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ML302F ratio of 1
ML302F ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, 22/31 isolates were rendered either susceptible (MIC ≤ 2 mg L−1), or intermediate (MIC 4–8 mg L−1) to MEM. ML302F alone was not toxic at up to 80 mg kg−1 in G. mellonella and treatment with MEM 0.6 mg kg−1
8, 22/31 isolates were rendered either susceptible (MIC ≤ 2 mg L−1), or intermediate (MIC 4–8 mg L−1) to MEM. ML302F alone was not toxic at up to 80 mg kg−1 in G. mellonella and treatment with MEM 0.6 mg kg−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ML302F 4.8 mg kg−1 significantly improved the survival of infected larvae. As ML302F was able to successfully restore susceptibility to resistant strains both in vitro and in vivo it represents a structurally interesting inhibitor in the search for new MBL inhibitors.
ML302F 4.8 mg kg−1 significantly improved the survival of infected larvae. As ML302F was able to successfully restore susceptibility to resistant strains both in vitro and in vivo it represents a structurally interesting inhibitor in the search for new MBL inhibitors.
Introduction
The global dissemination of multi-drug resistant Gram-negative bacteria has seriously limited antibiotic treatment options. As few novel synthetic antimicrobials are in development the challenge is to increase and protect the lifespan and spectrum of existing compounds for as long as possible. This is most important in the case of β-lactams (penicillins, cephalosporins, carbapenems) especially when resistance occurs due to the acquisition and production of β-lactamases that catalyse β-lactam hydrolysis.1 There are two distinct mechanistic classes of β-lactamase – the nucleophilic serine enzymes and the metallo β-lactamases (MBLs). Inhibitors of the serine enzymes (clavulanic acid/tazobactam/avibactam) have been developed and already applied clinically.2 However, no such compounds have yet been developed to target MBLs. The use of carbapenems such as ertapenem, imipenem or meropenem (MEM) in combination with an effective metallo-β-lactamase inhibitor, could prolong their lifespan as well as increase their spectrum of use to carbapenemase producing bacteria. Thus, there are ongoing efforts to develop MBL inhibitors of suitable potency and breadth of selectivity for clinical use.3 In the latter regard the ability to inhibit the different subtypes of clinically relevant MBLs and apparently rapidly emerging variants will likely be important.The rhodanine ML302 was initially identified as a MBL inhibitor in a high throughput screen and subsequently characterised as a non-competitive inhibitor of class B (IMP, VIM, NDM) metallo-β-lactamases in vitro.4 Recently the mechanism behind the apparent inhibition by ML302 was investigated in crystallographic, NMR, and kinetic studies. These, unexpectedly revealed that ML302 undergoes hydrolysis, to generate the thioenol ML302F (Fig. 1). The thiol of ML302F chelates the two zinc ions present in the active site of most MBLs in a manner mimicking an intermediate in β-lactam hydrolysis.5 In some cases the observed inhibition of MBLs by ML302/ML302F combinations may involve ternary complexes. In some instances ML302F is able to inhibit the carbapenemase activity of MBLs at low μM concentrations (IC50 VIM-2 0.30 μM) sufficient to restore the activity of meropenem against MBL-producing strains.5
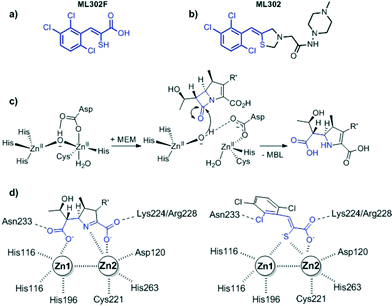 | ||
| Fig. 1 Structures of (a) the thioenol ML302F, (b) the ‘parent’ rhodanine ML302, (c) outline mechanism for MBL catalysed β-lactam hydrolysis as exemplified with meropenem (PDB ID: 4EYL), and (d) cartoons based on a crystal structure of ML302F (PDB ID: 4PVO) complexed with MBLs showing how the inhibitor mimics an enzyme-intermediate/product complex. | ||
Neither ML302 nor ML302F have significant cytotoxicity towards eukaryotic cells in tissue culture (>100 μM) but have not yet been assessed in any animal model of antimicrobial therapeutics. The wax moth caterpillar Galleria mellonella is increasingly being used as a simple invertebrate model for pre-clinical assessment of the efficacy, toxicity and pharmacokinetics of antimicrobials.6–9 Here we report studies on the activity of ML302F in combination with MEM against a collection of carbapenem resistant clinical isolates (Pseudomonas, Klebsiella, Enterobacter, Escherichia coli, Providencia) producing clinically relevant variants of the VIM (VIM-1/2/4) MBL. Preferred ratios of MEM to ML302F required for synergy in vitro were determined and then used at a simulated humanized pharmacokinetic dose in the treatment of G. mellonella infected with carbapenem resistant P. aeruginosa, K. pneumoniae and E. coli.
Results and discussion
The antimicrobial activities of MEM and ML302F alone and in combination were assessed in vitro against 31 VIM-producing clinical isolates. Synergy between MEM and ML302F was first confirmed in checkerboard assays against 7/8 isolates tested (FICI ≤ 0.5). An MEM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ML302F ratio of 1
ML302F ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1
4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 was found to be optimal resulting in a ≥4 fold reduction in the MIC of MEM.
8 was found to be optimal resulting in a ≥4 fold reduction in the MIC of MEM.
Thirty strains were either resistant (MIC > 8 mg L−1) or had reduced susceptibility (MIC ≥ 4 mg L−1) to MEM alone as judged by the European Committee on Antimicrobial Susceptibility (EUCAST) breakpoint criteria. One K. pneumoniae isolate harbouring blaVIM-1 was deemed susceptible (MIC 1 mg L−1). ML302F had no antimicrobial activity alone at concentrations up to 512 mg L−1.
Using a fixed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 ratio of MEM
8 ratio of MEM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ML302F in vitro susceptibility to carbapenems was increased in 22/31 (70%) of the isolates with 11 rendered susceptible (MIC ≤ 2 mg L−1) or intermediate (MIC 4–8 mg L−1). For 9 isolates, the MIC of MEM remained >8 mg L−1 (Table 1).
ML302F in vitro susceptibility to carbapenems was increased in 22/31 (70%) of the isolates with 11 rendered susceptible (MIC ≤ 2 mg L−1) or intermediate (MIC 4–8 mg L−1). For 9 isolates, the MIC of MEM remained >8 mg L−1 (Table 1).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) versus multidrug-resistant Gram-negative bacteria producing VIM carbapenemases
8) versus multidrug-resistant Gram-negative bacteria producing VIM carbapenemases
| MBL | Isolate | MIC (mg L−1) | FICI | |
|---|---|---|---|---|
| MEM | MEM + ML302F | |||
| VIM-1 | K. pneumoniae GR54 | 128 | 4 | 0.09 |
| VIM-1 | K. pneumoniae 177 | 1 | 1 | 1.02 |
| VIM-1 | P. stuartii 67 | 8 | 1 | 0.14 |
| VIM-1 | P. stuartii 70 | 8 | 1 | 0.14 |
| VIM-2 | P. aeruginosa 30 | 64 | 8 | 0.25 |
| VIM-2 | P. aeruginosa 47 | 32 | 1 | 0.05 |
| VIM-2 | P. aeruginosa 50 | 32 | 8 | 0.38 |
| VIM-2 | P. aeruginosa GR57 | 32 | 4 | 0.19 |
| VIM-2 | P. aeruginosa GR58 | 32 | 8 | 0.38 |
| VIM-2 | P. aeruginosa GR62 | 32 | 8 | 0.38 |
| VIM-2 | P. aeruginosa GR64 | 32 | 4 | 0.19 |
| VIM-2 | P. aeruginosa 3 (13) | 64 | 8 | 0.25 |
| VIM-2 | P. aeruginosa 8 (13) | 128 | 16 | 0.38 |
| VIM-2 | P. aeruginosa 9 (13) | 128 | 16 | 0.38 |
| VIM-2 | P. aeruginosa 11 (13) | 32 | 2 | 0.09 |
| VIM-2 | P. aeruginosa 6 (14) | 128 | 16 | 0.38 |
| VIM-2 | P. aeruginosa 11 (14) | 128 | 32 | 0.75 |
| VIM-2 | P. aeruginosa 12 (14) | 128 | 32 | 0.75 |
| VIM-2 | P. aeruginosa 13 (14) | 32 | 16 | 0.75 |
| VIM-2 | P. aeruginosa 14 (14) | 64 | 2 | 0.06 |
| VIM-2 | P. aeruginosa 15 (14) | 64 | 16 | 0.5 |
| VIM-2 | P. aeruginosa 16 (14) | 64 | 16 | 0.5 |
| VIM-2 | P. aeruginosa 27 (14) | 64 | 16 | 0.5 |
| VIM-4 | E. cloacae 102 | 32 | 4 | 0.19 |
| VIM-4 | E. coli 98 | 16 | 2 | 0.16 |
| VIM-4 | K. oxytoca 95 | 32 | 8 | 0.38 |
| VIM-4 | K. oxytoca 126 | 32 | 4 | 0.19 |
| VIM-4 | K. pneumoniae 101 | 32 | 2 | 0.09 |
| VIM-4 | K. pneumoniae 120 | 16 | 2 | 0.25 |
| VIM-4 | K. pneumoniae 128 | 4 | 1 | 0.27 |
| VIM-4 | K. pneumoniae 196 | 16 | 1 | 0.08 |
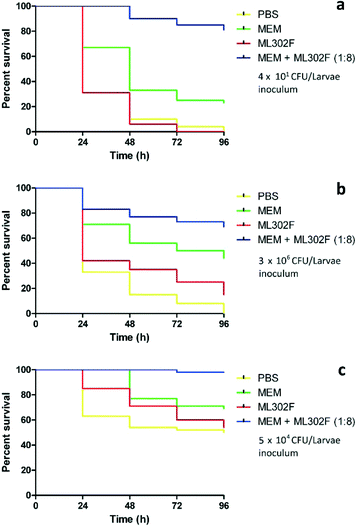
ML302F was non-toxic when administered to G. mellonella by injection at doses up to 80 mg kg−1 (100% survival of all larvae at 96 h). The inoculum required for staggered killing of >50% of larvae by carbapenem resistant strains over 96 h varied by species. The LD50 was <101, 105 and 104 CFU per larvae for P. aeruginosa GR57, E. coli 98 and K. pneumoniae respectively.
In treatment assays, survival of ML302F (4.8 mg kg−1) treated larvae was similar to those given PBS for the treatment of PA GR57, EC 98 and KP 120 infections. Monotherapy with MEM (0.6 mg kg−1) was superior to either PBS and ML302F (P < 0.0001), but the % survival was only 44%, 23% and 69% versus strains EC 98, PA GR57 and KP 120, respectively. The addition of ML302F to MEM significantly improved the survival of infected larva (P < 0.0001) compared to MEM alone, with survival rates of 69% (EC 98), 81% (PA GR57) and 98% (KP 120) at 96 (Fig. 2) and a relative risk for MEM-ML302F versus MEM of 0.64, 0.28 and 0.70 for these isolates.
Experimental
Materials
Bacterial strains were from the collection of clinical isolates held at Queen Mary University London. Species identification was by analysis of MADLI-TOF mass spectrometry profiles (Bruker) and MBL detection by PCR and sequencing of the blaVIM gene Meropenem trihydrate was purchased from Ranbaxy (UK) Limited (London, UK). ML302F was produced within the Chemical Research Laboratories, Oxford University (Oxford, UK). All media were purchased from SigmaAldrich (Dorset, UK).Minimum inhibitory concentrations
Minimum inhibitory concentrations (MICs) of MEM (0–64 mg L−1) and ML302F (0–32 mg L−1) were determined by broth microtiter dilution (BMD) in Mueller-Hinton 2 cation-adjusted broth with a bacterial inocula of 105 colony forming units (CFU) per well in accordance with Clinical Laboratory Standards Institute (CLSI) guidance. Initial screening assays were performed using eight Gram-negative isolates including P. aeruginosa (VIM-2), Escherichia coli (VIM-4), Klebsiella pneumoniae (VIM-4) and Providencia stuartii (VIM-1). K. pneumoniae NCTC 9633 and P. aeruginosa ATCC 27853 were used as MEM susceptible type strains to control BMD MIC determinations.The activity of ML302F in combination with MEM was assessed in checkerboard assays with fractional inhibitory concentration index (FICI) and MEM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ML302F ratio used to quantify interactions.10 A FICI (MIC of MEM/ML302F + MIC ML302F/MEM) of ≤0.5 was defined as synergy, a FICI of 0.5 to 4.0 as indifferent or additive, and values of >4.0 as antagonistic. MICs for the MEM/ML302F combination strains studied were determined at fixed MEM
ML302F ratio used to quantify interactions.10 A FICI (MIC of MEM/ML302F + MIC ML302F/MEM) of ≤0.5 was defined as synergy, a FICI of 0.5 to 4.0 as indifferent or additive, and values of >4.0 as antagonistic. MICs for the MEM/ML302F combination strains studied were determined at fixed MEM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ML302F ratios of 1
ML302F ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1
4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 by BMD.
8 by BMD.
Toxicity assays
The in vivo toxicity of ML302F was assessed in the Galleria mellonella wax moth larvae model using a simple live dead ranking.11 Increasing doubling concentrations of ML302F (0 to 80 mg kg−1) were administered in 10 μl injections directly into the hemocoel of ten larvae before incubation at 37 °C for 96 h. Larvae were scored for mellonisation and movement every 24 h, as live or dead. Larvae for all G. mellonella assays were purchased from Livefood UK Ltd (Somerset, UK).G. mellonella virulence assay
The bacterial inocula required for staggered killing (50% lethal dose [LD50]) of G. mellonella by VIM producing isolates over 96 h was determined using 10 larvae inoculated with overnight cultures of E. coli EC98, K. pneumoniae KP120 and P. aeruginosa PA GR57. Tenfold serial dilutions were made from washed cultures, diluted in PBS and administered in 10 μl injections at final concentrations of 101–106 CFU per larvae and incubated at 37 °C for 96 h. Larvae were scored as live or dead every 24 h.MEM/ML302F treatment assays
The activity of MEM and ML302F alone and in combination were assessed using 16 G. mellonella larva as previously described.12 Larvae were inoculated with P. aeruginosa GR57, E. coli EC98 and K. pneumoniae KP120. In combination therapies, a fixed ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) of MEM
8) of MEM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ML302F containing 0.6 mg kg−1 of MEM8 and 4.8 mg kg−1 of ML302F was administered in 10 μL injections to infected larvae. PBS injections (10 μL) were used as both controls for no antimicrobial therapy and inoculation injury.
ML302F containing 0.6 mg kg−1 of MEM8 and 4.8 mg kg−1 of ML302F was administered in 10 μL injections to infected larvae. PBS injections (10 μL) were used as both controls for no antimicrobial therapy and inoculation injury.
Larvae were scored for survival over 96 h and kill curves analysed by the log-rank test for trend. Relative risk (RR) of survival between the different treatment arms was calculated using Fisher's exact test with, two-tailed P-values of <0.05 considered statistically significant.
Conclusions
In combination with MEM at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
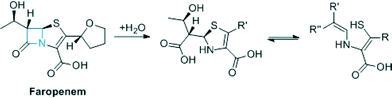
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 ratio ML302F was able to restore susceptibility to MEM amongst VIM-producing carbapenem resistant Enterobacteriaceae (CRE) and was active in vivo in the G. mellonella larva invertebrate model of CRE infection. ML302F was active against several clinically relevant VIM variants. The combined results reveal that ML302F is a structurally interesting MBL inhibitor active in both cellular and animal models and should stimulate further work on the synthesis and testing of ML302F analogues. ML302F was derived from a rhodanine by hydrolysis. We are well aware that as a class the rhodanines have a somewhat chequered history with respect to their use as (potential) pharmaceuticals.13,14 Furthermore, one may anticipate that the development of thioenols as pharmaceuticals might be difficult due to their reactivity (e.g. due to oxidation). However, as is well known the structures of many antibiotics do not follow recent ‘normal’ paradigms for drug structures.15 This is beautifully exemplified by the Class A serine β-lactamase inhibitors (clavulanic acid, tazobactam, sulbactam) that are widely used to extend penicillin and cephalosporin use. These are themselves β-lactams which act as ‘mechanism based’ inhibitors that undergo fragmentation on their target β-lactamases to produce ester linked species that are stable with respect to hydrolysis.15 The fact that rhodanines can undergo hydrolysis (whether enzyme mediated or not in the case of ML302/ML302F) to produce potent thioenol inhibitors raises the question as to whether mechanism based MBL inhibitors that are β-lactams which produce thioenol inhibitors can be generated. In this regard the development of modified carbapenems (one carbapenem, faropenem is in clinical use)16 (Fig. 3) which have the potential to undergo hydrolysis to produce thioenols that are MBL inhibitors may be of interest.
8 ratio ML302F was able to restore susceptibility to MEM amongst VIM-producing carbapenem resistant Enterobacteriaceae (CRE) and was active in vivo in the G. mellonella larva invertebrate model of CRE infection. ML302F was active against several clinically relevant VIM variants. The combined results reveal that ML302F is a structurally interesting MBL inhibitor active in both cellular and animal models and should stimulate further work on the synthesis and testing of ML302F analogues. ML302F was derived from a rhodanine by hydrolysis. We are well aware that as a class the rhodanines have a somewhat chequered history with respect to their use as (potential) pharmaceuticals.13,14 Furthermore, one may anticipate that the development of thioenols as pharmaceuticals might be difficult due to their reactivity (e.g. due to oxidation). However, as is well known the structures of many antibiotics do not follow recent ‘normal’ paradigms for drug structures.15 This is beautifully exemplified by the Class A serine β-lactamase inhibitors (clavulanic acid, tazobactam, sulbactam) that are widely used to extend penicillin and cephalosporin use. These are themselves β-lactams which act as ‘mechanism based’ inhibitors that undergo fragmentation on their target β-lactamases to produce ester linked species that are stable with respect to hydrolysis.15 The fact that rhodanines can undergo hydrolysis (whether enzyme mediated or not in the case of ML302/ML302F) to produce potent thioenol inhibitors raises the question as to whether mechanism based MBL inhibitors that are β-lactams which produce thioenol inhibitors can be generated. In this regard the development of modified carbapenems (one carbapenem, faropenem is in clinical use)16 (Fig. 3) which have the potential to undergo hydrolysis to produce thioenols that are MBL inhibitors may be of interest.
Acknowledgements
We would like to gratefully acknowledge Public Health England (Colindale, UK) and the General Hospital of Chest Diseases “Sotiria” (Athens, Greece) for supplying a number of antimicrobial-resistant isolates. We thank the Medical Research Council MR/L007665/1 grant for support of J. B. and C. J. S.Notes and references
- K. Bush, Ann. N.Y. Acad. Sci., 2013, 1277, 84–90 CrossRef CAS PubMed.
- A. M. Drawz, K. M. Papp-Wallace and R. A. Bonomo, Antimicrob. Agents Chemother., 2014, 58(4), 1835–1846 CrossRef PubMed.
- W. Fast and L. D. Sutton, Biochim. Biophys. Acta, 2013, 1834(8), 1648–1659 CrossRef CAS PubMed.
- T. Spicer, D. Minond, I. Enogieru, S. A. Saldanha, C. Allais, Q. Liu, B. A. Mercer, W. R. Roush and P. Hodder, Probe Reports from the NIH Molecular Libraries Program (US National Center for Biotechnology Information), 2010 Search PubMed.
- J. Brem, S. S. van Berkel, W. S. Aik, A. M. Rydzik, M. B. Avison, I. Pettinati, K. D. Umland, A. Kawamura, J. Spencer, T. D. W. Claridge, M. A. McDonough and C. J. Schofield, Nat. Chem., 2015, 6, 1084–1090 CrossRef PubMed.
- H. Ciesielczuk, J. Betts, L. Phee, M. Doumith, R. Hope, N. Woodford and D. W. Wareham, Virulence, 2015, 6, 145–151 CrossRef PubMed.
- J. W. Betts, L. M. Phee, M. Hornsey, N. Woodford and D. W. Wareham, Antimicrob. Agents Chemother., 2014, 58, 3541–3546 CrossRef PubMed.
- L. Hill, N. Veli and P. J. Coote, Int. J. Antimicrob. Agents, 2014, 52, 254–261 CrossRef PubMed.
- A. Y. Peleg, S. Jara, D. Monga, G. M. Eliopoulos, R. C. Moellering and E. Mylonakis, Antimicrob. Agents Chemother., 2009, 53, 2605–2609 CrossRef CAS PubMed.
- M. J. Hall, R. F. Middleton and D. Westmacott, J. Antimicrob. Chemother., 1983, 11, 427–433 CrossRef CAS PubMed.
- A. P. Desbois and P. J. Coote, J. Antimicrob. Chemother., 2012, 66, 1785–1790 CrossRef PubMed.
- M. Hornsey and D. W. Wareham, Antimicrob. Agents Chemother., 2011, 55, 3534–3537 CrossRef CAS PubMed.
- T. Mendgen, C. Steuer and C. D. Klein, J. Med. Chem., 2012, 55, 743–753 CrossRef CAS PubMed.
- M. A. T. Blaskovich, J. Zuegg, A. G. Elliot and M. A. Cooper, ACS Infect. Dis., 2015, 1, 285–287 CrossRef CAS.
- C. Bebrone, P. Lassaux, L. Vercheval, J. S. Sohier, A. Jehaes, E. Sauvage and M. Galleni, Drugs, 2010, 70, 651–679 CrossRef CAS PubMed.
- S. Hazra, S. G. Kurz, K. Wolff, L. Nguyen, R. A. Bonomo and J. S. Blanchard, Biochemistry, 2015, 54, 5657–5664 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |