Getting there is half the battle: recent advances in delivering therapeutics
Sasha Cai
Lesher-Perez
a,
Tatiana
Segura
a and
Christopher
Moraes
*b
aDept. of Chemical and Biomolecular Engineering, University of California – Los Angeles, USA
bDept. of Chemical Engineering, McGill University, Canada. E-mail: chris.moraes@mcgill.ca
First published on 11th December 2015
Abstract
Delivery of therapeutic molecules at the right time, place and at the correct dosage is critical to improve the effectiveness of therapeutic regimens. Barriers in the body, normally needed to maintain function, often impede delivery of therapeutic payloads to their target areas. Designing innovative solutions to circumvent these environmental factors, and ensure the timely delivery of therapeutic doses, is an essential element in improving human health. Here we highlight recent studies that focus on bypassing different barriers crucial for improving therapeutic delivery, by temporarily modifying the in vivo microenvironment, re-designing therapeutic carrier vehicles to improve control characteristics and on-demand delivery, and developing convenience-based strategies to improve patient compliance and access to therapeutics.
According to certain fictional adolescent mutant reptiles, “A wise man once said: ‘Forgiveness is divine, but never pay full price for late pizza’.” Pizza delivery may seem like a simple endeavor, but cold pizza just doesn't make the cut, no matter how you slice it. Fast food businesses with contractual delivery obligations (“30 minutes or it's free!”) carefully evaluate the capacity of their transportation networks, but unexpected considerations such as inclement weather, construction, or traffic can pose severe barriers to delivery. Getting past these barriers in a timely fashion results in a successful transaction, a satisfied customer, and hopefully a decent tip. However, circumventing these environmental barriers, and ultimately improving the efficiency of the delivery system often requires a great deal of creativity: how might we improve the process of getting items where they need to go, within the required delivery window?
Recent technological advances in the consumer delivery industry aim to address these issues. For example, Amazon's ongoing development of personalized delivery services, “…using small unmanned aerial vehicles to safely get packages into customers' hands in 30 minutes or less”, is ambitious and potentially revolutionary. These enhanced carrier systems will allow Amazon to circumvent conventional barriers that prevent timely delivery of crucial goods. The costs of this new infrastructure will likely limit early adoption to niche products and consumers, but presents a viable alternative for critically time-sensitive situations. An alternative strategy made famous by Uber and other proponents of the sharing economy is to implement new delivery mechanisms using pre-existing infrastructure. This approach aims to enable mass access to private transportation systems, by leveraging technology to create a conveniently-accessible network of available vehicles and drivers, by utilizing existing cars and individual owners as the network of private drivers-for-hire. Although the legality of this disruptive ‘grassroots’ approach remains uncertain in many locales, the convenience and ability to leverage existing infrastructure has led to considerable development and usage of the Uber network.
Aspects of these innovative consumer strategies have been foundational approaches in the design of drug delivery systems, in which the need to deliver precisely targeted quantities of therapeutics within the dynamic environment of the human body presents unique challenges to timely and effective treatment. Motivated by recent advances in drug delivery technologies, we highlight recent studies in the field and focus on three main avenues of development that mirror commercial delivery innovations: (1) temporarily modifying the in vivo environment to remove or bypass barriers; (2) re-designing the carrier vehicle to improve delivery and control characteristics; and (3) developing convenience-based strategies to improve patient compliance and access to therapeutics.
The in vivo environment in the human body presents unique challenges to the delivery of therapeutics. Maintaining homeostasis is a crucial body function, and by definition this includes removing or preventing the delivery of artificial (potentially therapeutic) agents. Hence a properly-functioning body should erect barriers that limit transport of these agents. When considering the body as a fractal and hierarchically compartmentalized system of organs (DOI: 10.1007/s10439-011-0455-6), it becomes clear that non-invasive, yet precisely targeted delivery is a challenging proposition. For example, the blood–brain barrier (BBB) presents a significant hurdle to the delivery of potential chemotherapeutics and treatments for neurological diseases such as Parkinson's, Alzheimer's and Huntington's. Consisting of complex and continuous tight junctions in the microvascular brain endothelium, the BBB precisely regulates transport of agents into the brain, and this functionality cannot conventionally be altered without introducing long-term health risks. Hence, conventional drug delivery approaches which utilize environmental characteristics such as leaky vasculature in tumors, or biomolecular homing signatures in antibody-based therapies cannot be applied across the BBB.
Efforts to study the BBB itself may lead to insights in this field. Microfluidics-oriented research groups have developed several organ-on-a-chip BBB models, including a recent system by Sellgren et al., in which astrocytes in a three-dimensional hydrogel are cultured adjacent to a microvascular BBB endothelium under physiologically-relevant shear (DOI: 10.1063/1.4935594). Although these platforms may ultimately be useful in identifying molecules that assist transport across the BBB, recent approaches that use physical means to actively and temporarily disrupt the barrier for short-term drug delivery have shown strong promise. The overall concept, suggested over a decade ago, is that microscale wounds in the BBB would heal rapidly providing a temporary access window for transport into the brain. This has recently been performed by Martel and co-workers via thermal energy generated by magnetic heating of nanoparticles using a magnetic resonance imaging machine (DOI: 10.1016/j.jconrel.2015.02.027). Magnetic heating can be precisely localized to the brain region with this instrument, which can also be used to image BBB permeability, which was shown to be substantial, but reversible over time. Furthermore no inflammation or immune reaction was observed in using this technology, suggesting that the approach may be clinically viable.
Although extremely promising, the equipment required is not broadly available, therefore identifying alternative actuation techniques may be helpful. Focused ultrasound has been considered extensively, and recent studies have demonstrated that when applied in combination with microbubble contrast agents, the BBB can be effectively crossed. The microbubbles respond to the ultrasound wave, enhancing the disruptive power of the ultrasound in specific locations. Hence, ultrasound may be applied to the brain, and microbubbles injected into the blood stream focus disruptive energy at the endothelium. As reported recently in PLoS ONE, repeated application of this procedure over 20 months in primate subjects was successful without any observable long-term negative physiological or neurological effects (DOI: 10.1371/journal.pone.0125911). The success of these studies prompted a human trial of this technology by a Canadian team at Toronto's Sunnybrook Hospital (DOI: 10.1007/978-3-319-22536-4_16) who successfully tested the procedure on a human patient to treat a cancerous brain tumor. Though much work remains to be done, these studies together suggest that temporarily altering the BBB environment using these non-invasive techniques presents a broadly applicable strategy to getting drugs to where they need to go.
Rather than altering the environment directly, drug delivery vehicles may also be designed to work with the natural mechanisms present to circumvent these barriers. Encapsulation and the design of pro-drugs can be used to successfully deliver an inactive and transportable form of the drug before the drug is converted to an active state within the body. Metabolic events, environmental triggers, or temporal degradation may activate the drug, and stimuli-responsive carrier materials have recently been used as engineered smart vehicles able to release therapeutic molecules on-demand (DOI: 10.1038/nmat3776). Several recent studies focused on engineering liposomes have contributed to this foundational theme, demonstrating critical improvements in the temporal, spatial and functional activity of delivery systems.
Liposomes have been used as drug vehicles due to their ability to reduce nonspecific side-effects and toxicity of encapsulated drugs, and can be surface-modified to target specific areas. Surface modification can improve liposome targeting by using targeting moieties, as well as circulation time, creating “stealth liposomes” that circulate undetected by the immune system, until reaching the appropriate location for payload delivery. This approach is typically limited by a slow drug release profile. Recent work by Luo et al. in Biomaterials developed novel stealth liposomes comprised of porphyrin–phospholipids, which can be used to form theranostic nanoparticles. These liposomes can be ruptured under appropriate photo-activation, which enhances payload release rate in an on-demand manner, while still maintaining high storage and serum stability (DOI: 10.1016/j.biomaterials.2015.10.027). The team showed that engineering these remotely triggered drug carriers can maximize localization of drug release at the target area (Fig. 1), consequently minimizing exposure of bioavailable drugs to healthy organs. These enhanced vehicles provide unprecedented control over release timing, but are perhaps limited in that once activated, drug release is irreversible.
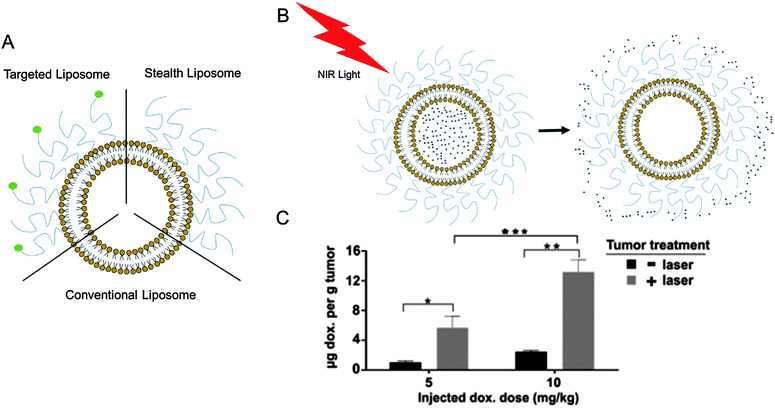 | ||
| Fig. 1 Stimuli responsive liposomes can provide enhanced on-demand drug deposition in target areas. (A) Schematic representation of conventional, stealth, targeted liposome types. (B) Schematic representation of near-infrared laser responsive release of drug payload from stealth liposomes. (C) Enhanced doxorubicin deposition from stealth porphyrin–phospholipids liposomes into tumor with laser exposure. Figure adapted with permission from Luo et al., Biomaterials, 2016, 75, 193 (DOI: 10.1016/j.biomaterials.2015.10.027). | ||
While a single release mechanism in stimuli-responsive drug carriers provides the ability of targeting a desired dosing window and location, incorporating on-and-off release mechanisms enables tunable dosing strategies and controlled pulsatile release, which may be of critical importance for certain therapies. In Advanced Healthcare Materials, Kearney et al. recently described a hydrogel carrier system for the controllable release of entrapped nanoparticles within ionically crosslinked hydrogels (DOI: 10.1002/adhm.201500254). These hydrogel carriers can remotely release their encapsulated payload through the use of on/off ultrasonic stimulation as an external switch. In a recent report in Biomaterials, Kennedy et al. utilized a similar hydrogel carrier system (DOI: 10.1016/j.biomaterials.2015.10.008) to produce differential release profiles by modifying the “strength” of the hydrogel capsules, while applying the same ultrasonic stimulation. Hydrogel capsule “strength” was dependent on the capsule wall thickness, where thicker capsule walls required greater compressive forces to rupture the capsules (Fig. 2). By coupling the ultrasound switch with the unique release kinetics of the engineered capsules, these carriers are able to release sequential payloads on demand. Though the authors presented only a proof of concept utilizing two capsule types, this general strategy may enable graded stimulation responses which could be applied to multiple types of carrier capsules.
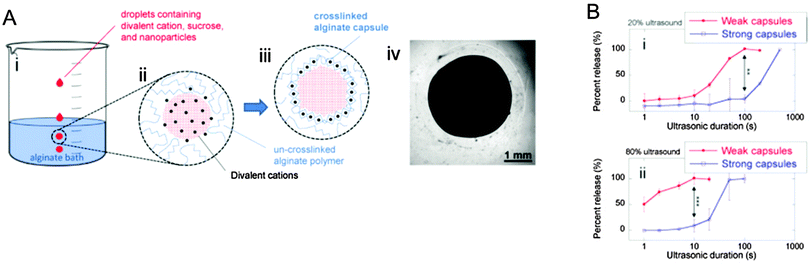 | ||
| Fig. 2 Alginate hydrogel capsule with differential release response of loaded nanoparticles. (A) Schematic representation of the production of hydrogel capsules and image of the hydrogel carrier encapsulating nanoparticles. (B) Release kinetics as a function of capsule thickness and ultrasonic stimulation, the strong and weak capsules demonstrate unique release profiles. Figure adapted with permission from Kennedy et al., Biomaterials, 2016, 75, 91 (DOI: 10.1016/j.biomaterials.2015.10.008). | ||
While technological solutions are being developed to circumvent drug delivery barriers ensuring the release of therapeutic payloads at the right time and place, drug delivery research is now beginning to address the human and societal challenges associated with drug delivery. As the previous U.S. Surgeon General C. Everett Koop suggested, “Drugs don't work if people don't take them”, and significant barriers exist that prevent patient compliance (DOI: 10.1038/519S19a). These barriers are just as important as being able to get the right drugs to the right target, and recent reports have focused on the crucial aspect of ‘convenience’ in developing these delivery systems. How might we design systems that are more convenient to use, manufacture, distribute, and administer?
To address the issue of self-administration, integrating drug delivery systems into everyday activities can ensure long term compliance. When considered as a therapeutic intervention, rates of compliance in the use of contact lenses is surprisingly high. To leverage this existing compliant act, Hsu et al. engineered contact lenses to serve as a substitute for the de facto standard of treating glaucoma patients with multiple daily eye drops containing a cocktail of drugs (DOI: 10.1016/j.ejpb.2015.06.001). By doping the contact lenses with vitamin E, they were able to create a barrier to hydrophilic glaucoma drug pairings of timolol and dorzolamide, and slow the release rate of this drug cocktail. By improving the convenience of the dosage system, these researchers aimed to improve self-administration and patient compliance with prescribed therapies.
Rather than focus on individual patient usage, other ambitious research programs aim to address issues of rising manufacturing costs in the pharmaceutical industry by repurposing infrastructure for therapeutic production, in the hope of creating a scalable and distributed manufacturing paradigm. In 2011, bioengineered plants were demonstrated as potential systems to synthesize therapeutic molecules, and have potential as a low-cost and easily distributable ‘grassroots-factory’ (DOI: 10.1038/nbt.2054). Furthermore, using plants to synthesize therapeutics allows manufacturers to exploit naturally occurring bioencapsulation processes which protect the product from degradation by digestive enzymes, a critical parameter in designing orally-delivered protein drugs. Su et al. (DOI: 10.1016/j.biomaterials.2015.08.004) demonstrated the production of bioencapsulated coagulation factor IX using lettuce cells, which was orally applied to treat hemophilia B in mice (Fig. 3). These production systems have lower startup costs, making them a potential solution to increase production and distribution. One can hence envision grassroots deployment of ‘therapeutic farms’ that have low cost of access and production (DOI: 10.1016/j.copbio.2014.12.008). As an added benefit, perhaps drugs might one day be taken in a palatable ‘salad-form’, directly supplying the therapeutics via an edible vehicle.
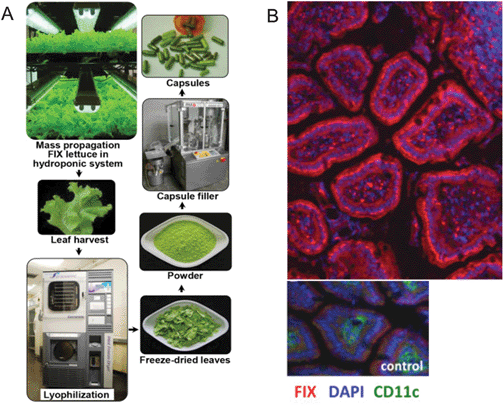 | ||
| Fig. 3 Industrial production of bioencapsulated Factor IX in lettuce and oral delivery in hemophilic mice. (A) Biomass production of bioencapsulated Factor IX in lettuce, and processing after leaf harvesting. (B) Factor IX orally delivered to the small intestine of hemophilia B mice using Factor IX in lettuce as compared to no Factor IX delivery in the control wild type lettuce. Figure adapted with permission from Su et al., Biomaterials, 2015, 70, 84 (DOI: 10.1016/j.biomaterials.2015.08.004). | ||
Taken together, these studies demonstrate innovation being applied to drug delivery studies at multiple levels. The role of the microenvironment in drug delivery is critical, and technologies that temporarily alter the microenvironment may prove fruitful in bypassing these barriers without long-term disruption of organ function. Likewise, the design of the carrier vehicle system itself may be used to target and deliver drug payloads. Finally, developing strategies to improve convenience and accessibility of these therapeutics are necessary to ensure that therapeutics will be taken. While the rest of the world is beginning to realize the importance and potential impact of disruptive innovations in delivery systems, nowhere is this better exemplified or more critically needed than in designing drug delivery systems that require integration and innovation across the boundaries of engineering, medicine and biology.
| This journal is © The Royal Society of Chemistry 2016 |
