Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Rapid production of benzazole derivatives by a high-pressure and high-temperature water microflow chemical process†
I.
Nagao
*,
T.
Ishizaka
and
H.
Kawanami
*
Research Institute for Chemical Process Technology, Department of Materials and Chemistry, 4-2-1, Nigatake, Miyaginoku, Sendai 983-8551, Japan. E-mail: i.nagao@aist.go.jp; h-kawanami@aist.go.jp
First published on 25th May 2016
Abstract
A high-pressure and high-temperature (HPHT) water microflow chemical process was utilized for the synthesis of benzazole derivatives. The current approach enables the extremely rapid production of various 2-arylbenzazoles including benzimidazoles, benzoxazoles, and benzthiazole in excellent yields.
Introduction
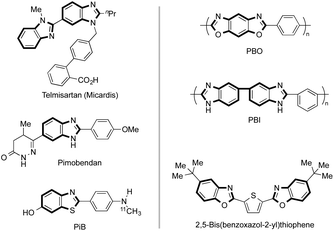
Due to the increasing concern for the development of sustainable chemical technologies, there has been huge demand for processes that combine a high production efficiency, low environmental burden, high hygiene level, and economic viability. In this regard, the utilization of water as a reaction medium in a chemical process would be one of the most attractive solutions.1–3 Water is highly abundant, chemically stable and non-toxic in nature, thus possessing significant relevant aspects in availability, cost, and safety, compared with most volatile organic solvents commonly used in traditional chemical processes. Meanwhile, water under subcritical or supercritical conditions, namely, high-pressure and high-temperature (HPHT) water, exhibits a variety of physical properties such as lowered polarity, increased ionic constants, generation of radical species, and high diffusion coefficients, etc., which are not observed under non-HPHT conditions. HPHT water with these characteristics would possess great potential to act as a solvent with high solubilizing power and/or as a catalyst/reagent itself, inducing an extremely accelerated reaction rate. In addition, because of the significant difference in properties between non-HPHT and HPHT water, there might be opportunities to reduce the number of process steps related to workup and/or purification procedures.It should be of significant interest to develop production methods of 2-aryl-substituted benzazole derivatives,4 since these structures are often found as a key unit in various natural compounds, biologically active agents, pharmaceuticals, and functional chemicals, etc.5 For example, Telmisartan and Pimobendan are two well known medicines, for high blood pressure (hypertension) and congestive heart failure, respectively (Fig. 1). Recently, Pittsburgh compound B (PiB), which is a positron emission tomography (PET) imaging agent for Alzheimers disease, has entered the stages of clinical trials. Functional chemicals such as polybenzoxazole (PBO) and polybenzimidazole (PBI) have long been developed for use as super engineering plastics. Another example is fluorescent 2,5-bis(benzoxazol-2-yl)thiophene, which is known to be utilized as an optical brightener for textiles. The compounds shown here are a very few selected examples of already commercialized materials. Furthermore, in the literature, incomparably great numbers of 2-arylbenzazole-based materials have been reported, potentially waiting for industrialization.5
In this contribution, it will be shown that a HPHT water process in association with a microflow reaction system allows to produce various 2-arylbenzazoles including benzimidazoles, benzoxazoles, and benzthioazoles within 10 seconds in excellent yields. To the best of our knowledge, the results shown here would be one of the best demonstrations of the acceleration of benzazole synthesis via dehydration.
A set of our investigations was initiated with the aim of developing a process for 2-arylbenzazoles as a future “green” chemical technology. For this purpose, we specifically paid attention to the condensation reaction between ortho-substituted aniline and benzoic acid derivatives (Scheme 1).6 The route principally consists two fundamental organic reactions; (i) an intramolecular N-acylation and (ii) a dehydration cyclization.4 It has been already reported by our research group that N-acylation of amine and aniline derivatives with acid anhydrides was performed efficiently in ambient-to-subcritical water using microreaction systems.3 Therefore, it is quite plausible that the intramolecular N-acylation proceeds effectively in HPHT water if utilizing benzoic anhydrides as benzoic acid derivatives. In this context, our assumptions leading to this achievement were that; (i) overall reaction completion of the condensation should be extremely fast due to the high energetic state of HPHT water; (ii) the dehydration cyclization step would be facilitated without any addition of acid or base catalysts, since HPHT water itself acts as a catalyst; (iii) the exceptional characteristics of HPHT water would ensure production of the desired benzazoles by preventing undesirable retroreactions such as hydrolysis.
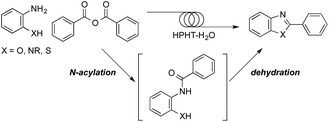 | ||
| Scheme 1 Production of benzazoles by a high-pressure high-temperature (HPHT) water microflow process. | ||
Results and discussion
Standard microflow chemistry techniques8 were adopted for the reactions. Details of the process are available in the ESI.† As a microreactor, SUS316 tubes with an inner diameter of 0.5 mm were used. To attain the highest yields of products, different parameters were varied; pressure, solution temperature, and length of the microreactor tube which therefore corresponds to the reactor volume, while keeping the total flow rate of solutions constant at 5.0 mL min−1.Table 1 summarizes selected results of the effects by HPHT water in intramolecular dehydration of N-phenylbenzamides 1.9 At first, N-[2-(phenylamino)phenyl]benzamide 1a was reacted at 400 °C and 30 MPa in a reactor of 0.88 cm3 volume, affording the desired product 1,2-diphenyl-1H-benzo[d]imidazole 2a in 59% yield (Table 1, entry 1). Then, maintaining a constant reaction temperature of 400 °C, pressure was gradually increased from 35 MPa up to 45 MPa. As a result, the yield of 2a was increased from 76% (35 MPa) to 94% (45 MPa). Subsequently, fixing the pressure at 45 MPa, the temperature was increased up to 445 °C, and finally the yield of 2a achieved was quantitative (entry 5). Under these conditions using the reactor of 0.88 cm3 volume, the residence time was estimated to be in the order of ca. 4–5 s. So as to prolong the residence time, a reactor of 4.99 cm3 volume was utilized instead, allowing the product to be given quantitatively under relatively ambient conditions of 400 °C and 30 MPa (entry 6). It should be noted that by-products were not observed in any of the cases above (entries 1–6); 2a was generated as the sole product, otherwise, starting substrate 1a was recovered.10
| Entry | X | R. Vol.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (cm3) a (cm3) |
Temp. (°C) | Pres. (MPa) | Yieldb (%) | Timec (s) |
|---|---|---|---|---|---|---|
| a Reactor volume. b GC yield. c Estimated residence time in microreactor. d 50 mM of substrate solution was processed. e 1.0 M of substrate solution was processed. | ||||||
| 1d | NPh (1a) | 0.88 | 400 | 30 | 59 (2a) | 3.79 |
| 2d | NPh (1a) | 0.88 | 400 | 35 | 76 (2a) | 5.04 |
| 3d | NPh (1a) | 0.88 | 400 | 40 | 87 (2a) | 5.55 |
| 4d | NPh (1a) | 0.88 | 400 | 45 | 94 (2a) | 5.88 |
| 5d | NPh (1a) | 0.88 | 445 | 45 | >99 (2a) | 3.87 |
| 6d | NPh (1a) | 4.9 | 400 | 30 | >99 (2a) | 21.1 |
| 7e | O (1b) | 0.88 | 400 | 40 | 41 (2b) | 5.55 |
| 8e | O (1b) | 0.88 | 445 | 45 | 57 (2b) | 3.87 |
Fig. 2 shows a graphical representation of product yields in a more extensive range of temperature and pressure. The presented data shows a tendency to reach higher yields at higher pressure and temperature. This suggests that the increased thermal energy at a higher pressure/temperature and prolonged residence time by higher pressure work in favor of affording the product. It should be noted that a local maximum of product yield was observed around at 375 °C under each pressure. This correlates very well to the fact that the ionic constants of HPHT water are maximized up to ca. 370 °C, then lower gradually at higher temperature. This might imply that catalytic function of HPHT water as an acid/base also plays a significant role in achieving a high yield of the product.11
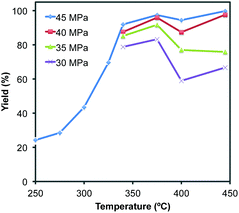 | ||
| Fig. 2 Effects of temperature and pressure on the yield of benzimidazole 2a using a microreactor tube of 0.88 cm3 volume. | ||
For production of 2-phenylbenzoxazole 2b, N-(2-hydroxyphenyl)benzamide 1b was subjected to HPHT conditions. For example, the reaction at 400 °C and 40 MPa using a 0.88 cm3 reactor proceeded in 41% yield (entry 7). Higher temperature/pressure (entry 8, 445 °C, 45 MPa) improved the yield up to 57%. The residence time in these reactions was estimated to be 5.55 and 3.87 s, respectively. Contrary to the case of imidazole 2a, a small amount of by-products such as 2-aminophenol and benzoic acid was observed, because of hydrolysis of 1a.10
To clarify characteristics of the current process using HPHT water, we ran the dehydration reactions under non-HPHT conditions (Table 2). For example, reactions of 1a or 1b were performed in aqueous solution under reflux (120 °C, 0.1 MPa) for 24 h (entries 1 and 2 for 1a; entries 4 and 5 for 1b). However, the desired products were hardly detected in each case. Then, acetic acid was added to the reaction mixtures; 1a was converted into 2a in quantitative yield (entry 3), on the other hand, 1b was never transformed into 2b in satisfactory yield (entry 6). These results most clearly point to the distinguishing features of the HPHT process, which completes the reactions without any additional catalyst within only a few seconds.12
| Entry | X | Solvent | Recovery | Yieldb (%) | Product | Yieldb (%) |
|---|---|---|---|---|---|---|
a 5 mM of 1 in solvent.
b GC yield.
c H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NMP = 9 NMP = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
d H2O 1.
d H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AcOH = 9 AcOH = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1.
|
||||||
| 1 | NPh | H2O | 69 | (1a) | 9 | (2a) |
| 2 | NPh | H2O/NMPc | 87 | (1a) | 12 | (2a) |
| 3 | NPh | H2O/AcOHd | 0 | (1a) | >99 | (2a) |
| 4 | O | H2O | >99 | (1b) | trace | (2b) |
| 5 | O | H2O/NMPc | >99 | (1b) | trace | (2b) |
| 6 | O | H2O/AcOHd | 54 | (1b) | trace | (2b) |
After confirming that the intramolecular dehydration step proceeded very well under HPHT water conditions as shown above, we extended the current approach to an N-acylation/dehydration sequential process. An example is presented in Scheme 2; N-phenyl-o-phenylenediamine and benzoic anhydride (1.25 eq.) were applied to the HPHT water process, producing 2a efficiently as well.
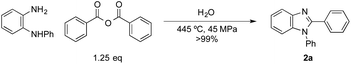 | ||
| Scheme 2 N-Acylation/dehydration condensation sequence leading to 1,2-diphenyl-1H-benzo[d]imidazole (2a). | ||
Finally, scope and limitations are shown in Table 3. All the benzazoles were produced by an N-acylation/dehydration sequential process. Reactions of 1a with MeO- and CF3 substituted benzoic anhydrides proceeded smoothly at 445 °C and 45 MPa, giving the corresponding 1,2-diphenyl-1H-benzo[d]imidazoles 2c and 2d, respectively, in high yields (entries 1 and 2). As a benzazole, 2-phenylbenzimidazole 2e was attainable by condensation between o-phenylenediamine and benzoic anhydride at 445 °C and 35 MPa (entry 3). In the case of 2e, conditions of 445 °C and 45 MPa achieved the most accelerated reaction completion time, 0.17 s (entry 4). Various 2-phenylbenzimidazoles with Br, F, PhCO, and NO2 substituents at the 5 position and a Me substituent at the 1 position, were obtained in excellent yields under the corresponding HPHT conditions (entries 5–9, 2f–2j). The process was also applicable to the efficient production of benzoxazoles 2b, 2k, 2l, benzthiazole 2m, and bis(benzimidazole) 2n (entries 10–14).
| Entry | Product/functional groups (2) | Conditionsb (°C/MPa/s) | Yieldc (%) | |
|---|---|---|---|---|
| a A 50 mM NMP solution of starting substrates including o-substituted aniline (1.0 eq.) and benzoic anhydride derivative (1.25 eq.) was applied to the HTHP water process. Reactor volume = 0.88 cm3. b Process conditions: temperature (°C); pressure (MPa); residence time (s). c GC yield. d Reactor volume = 0.039 cm3. e 1.0 M NMP solution of 2-aminophenol and benzoic anhydride (1.25 eq.) was processed. | ||||
| 1 |
|
R1 = OMe (2c) | 445/45/3.87 | 90 |
| 2 | R1 = CF3 (2d) | 445/45/3.87 | >99 | |
| 3 |
|
R2 = H (2e) | 445/35/2.26 | 98 |
| 4d | 445/45/0.17 | 81 | ||
| 5 | R2 = Br (2f) | 400/25/1.77 | >99 | |
| 6 | R2 = F (2g) | 400/40/5.55 | >99 | |
| 7 | R2 = COPh (2h) | 445/45/3.87 | >99 | |
| 8 |
|
R2 = NO2 (2i) | 340/45/7.45 | >99 |
| 9 | X = NMe, R3 = H (2j) | 400/25/1.77 | >99 | |
| 10e | X = O, R3 = H (2b) | 445/45/3.87 | 81 | |
| 11 | X = O, R3 = CF3 (2k) | 445/45/3.87 | 69 | |
| 12 | X = O, R3 = OMe (2l) | 445/45/3.87 | 84 | |
| 13 | X = S, R3 = H (2m) | 400/30/3.79 | >99 | |
| 14 |
|
400/40/5.55 | 92 | |
Conclusions
In conclusion, it was demonstrated that the condensation reaction between ortho-substituted aniline and benzoic anhydride derivatives for the synthesis of 2-arylbenzazoles can be successfully achieved using a HPHT water microflow chemical process. The reactions proceeded extremely rapidly, in very high yields, and as well are tolerant to a wide range of functional groups. Additional catalysts such as acid/base were not required in the dehydration step. The authors trust that “updated classic” organic synthesis using HPHT water would be definitely significant as a sustainable chemical process for 2-arylbenzazoles. Also taking advantages of microflow chemistry such as rapid screening, ease of scale up, and the capability for point of demand synthesis, etc., the current approach would accelerate the exploration of novel functional materials based on 2-arylbenzazoles, directing towards their industrial manufacture in the future.Acknowledgements
This research was partly supported by a Grant-in-Aid for Research Activity start-up (JSPS KAKENHI Grant Number 15H06892). We thank Dr Maya Chatterjee for her helpful discussions and graceful support.Notes and references
- B. Subramanlam and M. A. McHugh, Ind. Eng. Chem. Process Des. Dev., 1986, 25, 1–12 Search PubMed; D. Bröll, C. Kaul, A. Krämer, P. Krammer, T. Richter, M. Jung, H. Vogel and P. Zehner, Angew. Chem., Int. Ed., 1999, 38, 2998–3014 CrossRef CAS; P. E. Savage, Chem. Rev., 1999, 99, 603–621 CrossRef PubMed; M. Siskin and A. R. Katritzky, Chem. Rev., 2001, 101, 825–836 CrossRef PubMed; A. R. Katritzky, D. A. Nichols, M. Siskin, R. Murugan and M. Balasubramanian, Chem. Rev., 2001, 101, 837–892 CrossRef PubMed; R. Scott Oakes, A. A. Clifford and C. M. Rayner, J. Chem. Soc., Perkin Trans. 1, 2001, 917–941 RSC; N. Akiya and P. E. Savage, Chem. Rev., 2002, 102, 2725–2750 CrossRef PubMed; M. Watanabe, T. Sato, H. Inomata, R. L. Smith Jr., K. Arai, A. Kruse and E. Dinjus, Chem. Rev., 2004, 104, 5803–5821 CrossRef PubMed; S. E. Hunter and P. E. Savage, Chem. Eng. Sci., 2004, 59, 4903–4909 CrossRef; D. Prajapati and M. Gohain, Tetrahedron, 2004, 60, 815–833 CrossRef; H. Weingärtner and E. U. Franck, Angew. Chem., Int. Ed., 2005, 44, 2672–2692 CrossRef PubMed; C.-J. Li, Chem. Rev., 2005, 105, 3095–3165 CrossRef PubMed; M. Sato, Y. Ikushima, K. Hatakeda and R. Zhang, Anal. Sci., 2006, 22, 1409–1416 CrossRef PubMed; J. F. Dubreuil and M. Poliakoff, Pure Appl. Chem., 2006, 78, 1971–1982 Search PubMed; A. Kruse and E. Dinjus, J. Supercrit. Fluids, 2007, 39, 362–380 CrossRef; A. Kruse and E. Dinjus, J. Supercrit. Fluids, 2007, 41, 361–379 CrossRef; H. C. Hailes, Org. Process Res. Dev., 2007, 11, 114–120 CrossRef; V. Hessel, B. Cortese and M. H. J. M. de Croon, Chem. Eng. Sci., 2011, 66, 1426–1448 CrossRef; N. S. Kus, Tetrahedron, 2012, 68, 949–958 CrossRef; M. Akizuki, T. Fujii, R. Hayashi and Y. Oshima, J. Biosci. Bioeng., 2014, 117, 10–18 CrossRef PubMed.
- H. Kawanami, K. Matsushima, M. Sato and Y. Ikushima, Angew. Chem., Int. Ed., 2007, 46, 5129–5132 CrossRef CAS PubMed; M. Sato, K. Matsushima, H. Kawanami and Y. Ikushima, Angew. Chem., Int. Ed., 2007, 46, 6284–6288 CrossRef PubMed; M. Sato, N. Otabe, T. Tuji, K. Matsushima, H. Kawanami, M. Chatterjee, T. Yokoyama, Y. Ikushima and T. M. Suzuki, Green Chem., 2009, 11, 763–766 RSC; A. Suzuki, H. Kawanami, S. Kawasaki and K. Hatakeda, Synthesiology, 2010, 3, 151–161 CrossRef; R. Javaid, H. Kawanami, M. Chatterjee, T. Ishizaka, A. Suzuki and T. M. Suzuki, Chem. Eng. J., 2011, 167, 431–435 CrossRef; H. Kawanami, M. Sato, M. Chatterjee, N. Otabe, T. Tuji, Y. Ikushima, T. Ishizaka, T. Yokoyama and T. M. Suzuki, Chem. Eng. J., 2011, 167, 572–577 CrossRef.
- M. Sato, K. Matsushima, H. Kawanami, M. Chatterjee, T. Yokoyama, Y. Ikuhsima and T. M. Suzuki, Lab Chip, 2009, 9, 2877–2880 RSC.
- As early examples of condensation between ortho-substituted aniline and benzoic acid derivatives for benzazoles synthesis: M. A. Phillips, J. Chem. Soc., 1928, 2393–2399 RSC; E. L. Holljes Jr. and E. C. Wagner, J. Org. Chem., 1944, 9, 31–49 CrossRef CAS; D. W. Hein, R. J. Alheimand and J. J. Leavit, J. Am. Chem. Soc., 1957, 79, 427–429 CrossRef; J. B. Hendrickson and Md. S. Hussoin, J. Org. Chem., 1987, 52, 4137–4139 CrossRef.
- J. B. Wright, Chem. Rev., 1951, 48, 397–541 CrossRef CAS PubMed; P. N. Preston, Chem. Rev., 1974, 74, 279–314 CrossRef; I. J. Turchi and M. J. S. Dewar, Chem. Rev., 1975, 75, 389–437 CrossRef; I. J. Turchi, Ind. Eng. Chem. Prod. Res. Dev., 1981, 20, 32–78 CrossRef; V. V. Korshak, G. V. Kazakova and A. L. Rusanov, Polym. Sci. U.S.S.R., 1989, 1, 4–21 CrossRef; D. A. Horton, G. T. Bourne and M. L. Smythe, Chem. Rev., 2003, 103, 893–930 CrossRef PubMed; R. V. Kumar, Asian J. Chem., 2004, 16, 1241–1260 Search PubMed; N. R. Candeias, L. C. Branco, P. M. P. Gois, C. A. M. Afonso and A. F. Trindade, Chem. Rev., 2009, 109, 2703–2802 CrossRef PubMed; A. Gupta and S. Rawat, J. Curr. Pharm. Res., 2010, 3, 13–23 Search PubMed; L. C. R. Carvalho, E. Fernandes and M. M. B. Marques, Chem. – Eur. J., 2011, 17, 12544–12555 CrossRef PubMed.
- As a pioneering work, there has been a report of benzimidozole synthesis in high-temperature water using a novel batch-type autoclave. L. M. Dudd, E. Venardou, E. Garcia-Verdugo, P. Licence, A. J. Blake, C. Wilson and M. Poliakoff, Green Chem., 2003, 5, 187–192 RSC.
- H. Z. Boeini and K. H. Najafabadi, Eur. J. Org. Chem., 2009, 4926–4929 CrossRef CAS; R. P. Karuvalam, K. R. Haridas and S. N. Shetty, J. Chil. Chem. Soc., 2011, 57, 1122–1125 CrossRef; D. Rambabu, P. R. K. Murthi, B. Dulla, M. V. B. Rao and M. Pal, Synth. Commun., 2013, 43, 3083–3092 CrossRef; S. S. Panda, M. A. Ibrahim, A. A. Oliferenko, A. M. Asiri and A. R. Katritzky, Green Chem., 2013, 15, 2709–2712 RSC; Y. D. Reddy, C. V. R. Reddy and P. K. Dubey, RSC Adv., 2014, 4, 2974–2979 RSC; H. Hikawa, M. Imani, H. Suzuki, Y. Yokoyama and I. Azumaya, RSC Adv., 2014, 4, 3768–3773 RSC; P. P. Kattimani, R. R. Kamble and G. Y. Meti, RSC Adv., 2015, 5, 29447–29455 RSC; B. Maleki, M. Baghayeri, S. M. Vahdat, A. Mohammadzadeh and S. Akhoondi, RSC Adv., 2015, 5, 46545–46551 RSC.
- Selected reviews on microflow reactor technology for applications in organic synthesis; references therein lead to other reviews before 2009. T. Razzaq and C. O. Kappe, Chem. – Asian J., 2010, 5, 1274–1289 Search PubMed; C. G. Frost and L. Mutton, Green Chem., 2010, 12, 1687–1703 RSC; R. L. Hartman, J. P. McMullen and K. F. Jensen, Angew. Chem., Int. Ed., 2011, 50, 7502–7519 CrossRef CAS PubMed; J. Wegner, S. Ceylan and A. Kirschning, Chem. Commun., 2011, 47, 4583–4592 RSC; C. Wiles and P. Watts, Green Chem., 2012, 14, 38–54 RSC; L. M. Sanz and F. Susanne, J. Med. Chem., 2012, 55, 4062–4098 CrossRef PubMed; S. Marre, Y. Roig and C. Aymonier, J. Supercrit. Fluids, 2012, 66, 251–264 CrossRef; V. Hessel, D. Kralisch, N. Kockmann, T. Noël and Q. Wang, ChemSusChem, 2013, 6, 746–789 CrossRef PubMed; S. G. Newman and K. F. Jensen, Green Chem., 2013, 15, 1456–1472 RSC; K. S. Elvira, X. C. i Solvas, R. C. R. Wootton and A. J. deMello, Nat. Chem., 2013, 5, 905–915 CrossRef PubMed; L. Vaccaro, D. Lanari, A. Marrocchi and G. Strappaveccia, Green Chem., 2014, 16, 3680–3704 RSC; S. V. Ley, D. E. Fitzpatrick, R. J. Ingham and R. M. Myers, Angew. Chem., Int. Ed., 2015, 54, 3449–3464 CrossRef PubMed; B. Gutmann, D. Cantillo and C. O. Kappe, Angew. Chem., Int. Ed., 2015, 54, 6688–6728 CrossRef PubMed; S. V. Ley, D. E. Fitzpatrick, R. M. Myers, C. Battilocchio and R. J. Ingham, Angew. Chem., Int. Ed., 2015, 54, 10122–10136 CrossRef PubMed.
- Details are found in the ESI.†.
- GC charts after the reactions are found in the ESI.†.
- A graphical representation of residence time and ionic constants of water at various temperatures and pressures are found in the ESI.†.
- Reported syntheses of benazaole derivatives by dehydration often require catalysts/reagensts such as strong acids and a reaction time in the order from minutes to hours. For examples, please see ref. 4, 6 and 7.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6gc01195k |
| This journal is © The Royal Society of Chemistry 2016 |