Open Access Article
Open Access ArticleAn efficient Passerini tetrazole reaction (PT-3CR)†
Ajay L.
Chandgude
and
Alexander
Dömling
*
Department of Drug Design, University of Groningen, Antonius Deusinglaan 1, 9713 AV Groningen, The Netherlands. E-mail: a.s.s.domling@rug.nl; Web: http://www.drugdesign.nl/
First published on 19th May 2016
Abstract
A sonication accelerated, catalyst free, simple, high yielding and efficient method for the Passerini-type three-component reaction (PT-3CR) has been developed. It comprises the reaction of an aldehyde/ketone, an isocyanide and a TMS-azide in methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (1
water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the solvent system. The use of sonication not only accelerated the rate of the reaction but also provided good to excellent quantitative yields. This reaction is applicable to a broad scope of aldehydes/ketones and isocyanides.
1) as the solvent system. The use of sonication not only accelerated the rate of the reaction but also provided good to excellent quantitative yields. This reaction is applicable to a broad scope of aldehydes/ketones and isocyanides.
Introduction
Tetrazole scaffolds are extensively used in medicinal chemistry and in industries like agriculture, explosives and photography.1 1,5-Disubstituted tetrazoles are important ring systems, having applications as bio-active agents or in drugs like cilostazol, pentylenetetrazole, latamoxef, BMS-317180 and cis-amide bond isosteres in peptides (Fig. 1). This propels the need for efficient synthetic methods for tetrazoles.2 Different reactions have been developed for the direct access to diverse 1,5-disubstituted tetrazoles, but three- and four-component reactions (MCR) are mostly preferred due to their convergent, atom-efficient and flexible nature.3 Multi-component reactions are considered ideal syntheses, and that's why their use in synthetic chemistry is increasing tremendously.4In 1921, a three-component reaction between carboxylic acids, oxo components and isocyanides for the synthesis of α-acyloxy amide was discovered by Passerini (P-3CR).5,7c In 1961, Ugi reported the synthesis of tetrazoles via a Passerini-type 3CR (PT-3CR) for the first time using HN3 and Al(N3)3.6 Even though the use of HN3 or NaN3 in Passerini reactions for the synthesis of tetrazoles was reported, the highly toxic and explosive nature of HN3 and NaN3 limit its application.7 The use of TMSN3 as a safe substitute for HN3 was then introduced by Hulme.8 However the use of TMSN3 as an azide source in the PT-3CR resulted in a very low yield, and the TMS-ether was found as a major product instead. Similarly protected amino aldehydes in DCM also resulted in generally low yields9 and the described reaction times were up to 96 hours.9a Reported PT-3CRs are not very suitable for aromatic aldehydes.7 The use of different Lewis acids as catalysts, like AlCl3, to activate aldehydes forms inseparable mixtures of the desired product with α-hydroxy-amide, with a maximum yield of 30%.10 Zhu and co-workers used TMSN3 as a test reaction component in the asymmetric PT-3CR; nevertheless, they could not avoid the formation of α-hydroxy-amide.7b
To the best of our knowledge, no efficient, diverse and high yielding PT-3CR reaction has yet been reported. We report herein a sonication-promoted catalyst free, TMSN3-modified PT-3CR using methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (1
water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as solvent with diverse scope and affording good to excellent yields.
1) as solvent with diverse scope and affording good to excellent yields.
Results and discussion
We started our investigation by using tert-butyl isocyanide, phenylacetaldehyde and TMSN3 as starting materials (Table 1). We hypothesized that the use of fluoride ion sources like TBAF, CsF and KF could trigger TMSN3 activation.11 However, when the reaction was carried out with TBAF with different solvents like DCM or water, or in neat, the product was formed only in trace amounts (Table 1, entries 1–3). Surprisingly, using methanol as a solvent increased the isolated yield to 25%. Carrying out the reaction with alternative F-sources, such as KF in DCM or CsF in DCM, methanol and water, resulted only in small amounts of product formation.| Entry | Catalyst | Solvent | Time (h) | Product yieldb (%) |
|---|---|---|---|---|
| a The reaction was carried out with phenylacetaldehyde (1 mmol), tert-butyl isocyanide (1 mmol), and TMSN3 (1 mmol) at room temperature. b Yield of isolated product. c 1 equivalent TBAF·3H2O. d 1 equivalent TBAF in 1 M THF. e 1 equivalent KF. f 1 equivalent CsF. g Reaction carried out at 70 °C. nd = not determined. | ||||
| 1 | TBAFc | — | 12 | Trace |
| 2 | TBAFd | DCM | 12 | Trace |
| 3 | TBAFc | H2O | 12 | Trace |
| 4 | TBAFc | MeOH | 12 | 25 |
| 5 | KFe | DCM | 12 | nd |
| 6 | CsFf | DCM | 12 | nd |
| 7 | CsFf | MeOH | 12 | nd |
| 8 | CsFf | H2O | 12 | nd |
| 9 | I2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) f f |
DCM | 12 | nd |
| 10 | I2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) f f |
H2O | 12 | nd |
| 11 | H2O | 12 | 17 | |
| 12 | TBAFc | MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
12 | 63 |
| 13 | MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
12 | 64 | |
| 14 | Sonication |
MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) H
2
O (1 H
2
O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) 1)
|
2 | 97 |
| 15 | Sonicationg | — | 3 | 31 |
| 16 | Sonication | DCM | 2 | 34 |
| 17 | Sonication | H2O | 2 | 71 |
The use of iodine, to trap TMS as TMSI, also failed to improve the reaction yield. 17% product formed when the reaction was carried out in water without any additive. TBAF in methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (1
water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) enhanced the yield up to 63%; however comparable yields were obtained when the reaction was carried out without TBAF in the same solvent system. Thus we concluded that the use of TBAF is not fruitful, whereas the solvent system has a major impact.
1) enhanced the yield up to 63%; however comparable yields were obtained when the reaction was carried out without TBAF in the same solvent system. Thus we concluded that the use of TBAF is not fruitful, whereas the solvent system has a major impact.
We foresaw that the accelerating effect of sonication could potentially speed up the reaction and increase yields. Ultrasound in general12 and also in the context of MCR12d is often used in organic synthesis due to its advantages such as increasing the reaction efficacy while decreasing waste byproducts, short reaction times, cleaner reactions, easier experimental procedures and having low energy requirements. Recently, the popularity of sonication-assisted synthesis as a green synthetic approach has significantly increased and has resulted in a plethora of ‘better’ reactions.13 Ultrasound in chemical reactions works via a physical phenomenon called acoustic cavitation, which forms, expands and collapses gaseous and vaporous cavities in an ultrasound irradiated liquid. The mechanical effect of cavitation destroys the attractive forces of molecules in the liquid phase and so accelerates reaction rates by facilitating mass transfer in the microenvironment.13 To our delight, the use of sonication not only accelerated the reaction from 12 to two hours, but provided excellent quantitative yields using methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (1
water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the solvent system, noteworthily without the necessity of any previously used additive (Table 1, entry 14). We used a simple ultrasonic cleaning bath which is the most widely available and cheapest source of ultrasonic irradiation. A recent study has shown that both ultrasonic cleaning baths and ultrasonic probe systems are efficient in Passerini reactions.14 The ultrasonic cleaning bath offers further advantages; for example, the reaction vessel can be put directly into the bath without any adaptation. This is in contrast to the ultrasonic probe system, which is more expensive and also requires special vessels, making it inconvenient to use.
1) as the solvent system, noteworthily without the necessity of any previously used additive (Table 1, entry 14). We used a simple ultrasonic cleaning bath which is the most widely available and cheapest source of ultrasonic irradiation. A recent study has shown that both ultrasonic cleaning baths and ultrasonic probe systems are efficient in Passerini reactions.14 The ultrasonic cleaning bath offers further advantages; for example, the reaction vessel can be put directly into the bath without any adaptation. This is in contrast to the ultrasonic probe system, which is more expensive and also requires special vessels, making it inconvenient to use.
Lastly, reactions under sonication in DCM or in neat conditions provided smaller yields, of 34% and 31% respectively, and the formation of TMS-ether as a side product was observed. The use of pure water as the solvent under sonication conditions provided the product in 71% yield. The use of 1 equivalent of TMSN3 avoids the danger of forming hydrazide from excess azide. This catalyst free reaction doesn't require any work-up.
With these optimized conditions in hand, we next examined the generality of this PT-3CR by reacting different aldehydes with different isocyanides (Table 2). Good to excellent yields were obtained with linear and branched aliphatic aldehydes. Aromatic aldehydes are also compatible substrates for this process (Table 2, entries 15–22). Electron donating (methoxy) and withdrawing groups (Cl, Br, NO2) at different positions like ortho, meta and para are valid, providing moderate to good yields. Paraformaldehyde also reacts when pure water was used as the solvent. Reaction with one or six equivalents of paraformaldehyde in a methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water system only forms mono-substituted tetrazole. The reaction of benzyl isocyanide with aliphatic aldehydes gave excellent yields.
water system only forms mono-substituted tetrazole. The reaction of benzyl isocyanide with aliphatic aldehydes gave excellent yields.
| Entry | 1 | R3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
Yieldc (%) |
|---|---|---|---|
| a The reaction was carried out with 1 mmol 1, 1 mmol 2, 1 mmol TMSN3. b cy = cyclohexyl, octyl = 2-isocyano-2,4,4-trimethylpentane. c Yield of isolated product. d 6 equivalents of paraformaldehyde in water as solvent and at 60 °C. iPr = isopropyl. | |||
| Aldehydes | |||
| 1 | C6H5–CH2–CHO | C6H5–CH2 | 96 (3a) |
| 2 | iPr-CHO | (CH3)3–C | 98 (3b) |
| 3 | CH3–(CH2)2–CHO | C6H5–CH2 | 80 (3c) |
| 4 | C6H5–CH2–CHO | t Octyl | 77 (3d) |
| 5 | iPr-CHO | CN–CH2–CH2 | 72 (3e) |
| 6 | C6H5–(CH2)2–CHO |
|
53 (3f) |
| 7 | C6H5–(CH2)2–CHO | Cy | 76 (3g) |
| 8 | C6H5–CH2–CHO | 2-BrC6H4–CH2 | 77 (3h) |
| 9 | H–CHOd | 2-BrC6H4–CH2 | 42 (3i) |
| 10 | iPr-CHO | 2-BrC6H4–CH2 | 80 (3j) |
| 11 | C6H5–(CH2)2–CHO | (CH3)3–C | 88 (3k) |
| 12 | CH3–CH2–CHO | C6H5–CH2 | 91 (3l) |
| 13 | (CH3)2–CH–CH2–CHO | C6H5–CH2 | 92 (3m) |
| 14 | C6H5–CH2–CHO | (CH3)3–C | 97 (3n) |
| 15 | C6H5–CHO | (CH3)3–C | 41 (3o) |
| 16 | 2,6-(Cl)2C6H3–CHO | C6H5–CH2 | 71 (3p) |
| 20 | 2,3-(Cl)2C6H3–CHO | Cy | 73 (3q) |
| 17 | 2-MeO-5-BrC6H3–CHO | C6H5–CH2–CH2 | 46 (3r) |
| 18 | 2-BrC6H4–CHO | Cy | 60 (3s) |
| 19 | 2-Cl-3,4-(OCH3)2C6H2–CHO | Cy | 42 (3t) |
| 21 |

|
Cy | 39 (3u) |
| 22 | 2,5-(OCH3)2C6H3–CHO | Cy | 48 (3v) |
| Ketones | |||
| 23 | Cyclohexanone | C6H5–CH2 | 84 (3w) |
| 24 | 1-Benzylpiperidin-4-one | C6H5–CH2 | 46 (3x) |
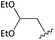
Isocyanides, easy to deprotect in acidic and basic conditions, are compatible with the developed methodology (Table 2, entries 2, 4 and 5). The functional group tolerance of the isocyanide (Table 2, entries 5–6 and 8–10), in this protocol provides multiple opportunities for various further chemical manipulations. For example, the compatibility of 1,1-diethoxy-2-isocyanoethane as the isocyanide component could be used in further reactions as aldehyde or halogen functional groups for coupling reactions.
We also explored the scope of ketones in the developed method (Table 2, entries 23 and 24). Cyclohexanone gives a good yield of 84%. The important building block piperidone is also compatible with the reaction.
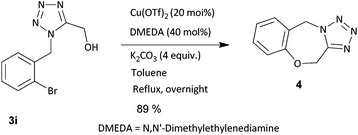
Fused tetrazoles are important scaffolds as they possess a wide spectrum of activity and vast industrial applications. As functional groups bearing isocyanides are compatible in our developed method, we foresaw a quick and easy access to fused tetrazoles. According to our synthetic plan, the use of functionalized PT-3CR product for post modification would allow an anticipated cyclization process. (1-(2-Bromobenzyl)-1H-tetrazol-5-yl)methanol (3i), when refluxed with copper(II) triflate in the presence of base, formed 5,11-dihydrobenzo[f]tetrazolo[5,1-c][1,4]oxazepine in 89% yield (Scheme 1).
Conclusions
In conclusion, we have developed a novel, efficient, safe and general sonication assisted Passerini tetrazole reaction (PT-3CR) to access 5-(1-hydroxyalkyl)tetrazoles in good to excellent yields. The herein described Passerini tetrazole procedure provides multiple advantages over previously described procedures. The reaction does not use highly toxic and explosive staring materials like HN3, Al(N3)3 or NaN3. This catalyst free reaction avoids the use of any dangerous or adverse catalysts such as the Al–salen chiral complexes or AlCl3. Sonifiaction was found to provide superior reaction conditions, resulting in high conversion and giving high yields of Passerini products and no TMS-ether side products, as often observed previously. Sonification is also well known to be compatible with upscaling procedures. The scope of the reaction could be dramatically extended, including aliphatic, aromatic aldehydes and also ketones. Due to the extended functional group compatibility of the reaction, many new scaffolds amenable by post-condensation reactions can be foreseen, as we have illustrated by the synthesis of a Cu-mediated fused tetrazole. Altogether, we believe that our procedure is superior to all previously reported Passerini tetrazole reactions and will be the method of choice for the future.Acknowledgements
We thank the University of Groningen. The Erasmus Mundus Scholarship “Svaagata” is acknowledged for a fellowship to A. Chandgude. The work was financially supported by the NIH (1R01GM097082-01) and by Innovative Medicines Initiative (grant agreement no. 115489).Notes and references
- For a review on the importance of tetrazole derivatives, see: (a) C. X. Wei, M. Bian and G. H. Gong, Molecules, 2015, 20, 5528–5553 CrossRef CAS PubMed; (b) J. Roh, K. Vavrova and A. Hrabalek, Eur. J. Org. Chem., 2012, 6101–6118 CrossRef CAS; (c) P. B. Mohite and V. H. Bhaskar, Int. J. PharmTech. Res., 2011, 3, 1557–1566 CAS; (d) L. M. Frija, A. Ismael and M. L. S. Cristiano, Molecules, 2010, 15, 3757–3774 CrossRef CAS PubMed; (e) L. V. Myznikov, A. Hrabalek and G. I. Koldobskii, Chem. Heterocycl. Compd., 2007, 43, 1–9 CrossRef CAS.
- For a review on the synthesis of tetrazole derivatives, see: (a) M. Malik, M. Wani, S. Al-Thabaiti and R. Shiekh, J. Inclusion Phenom. Macrocyclic Chem., 2014, 78, 15–37 CrossRef CAS; (b) G. I. Koldobskii, Russ. J. Org. Chem., 2006, 42, 469–486 CrossRef CAS; (c) R. J. Herr, Bioorg. Med. Chem., 2002, 10, 3379–3393 CrossRef CAS PubMed; (d) V. Y. Zubarev and V. A. Ostrovskii, Chem. Heterocycl. Compd., 2000, 36, 759–774 CrossRef CAS; (e) S. J. Wittenberger, Org. Prep. Proced. Int., 1994, 26, 499–531 CrossRef CAS.
- (a) A. Sarvary and A. Maleki, Mol. Diversity, 2015, 19, 189–212 CrossRef CAS PubMed; (b) A. L. Chandgude and A. Dömling, Eur. J. Org. Chem., 2016, 2383–2387 CrossRef CAS.
- For a general review on the importance of MCR reactions, see: (a) A. Dömling, W. Wang and K. Wang, Chem. Rev., 2012, 112, 3083–3135 CrossRef PubMed; (b) T. Zarganes-Tzitzikas, A. L. Chandgude and A. Dömling, Chem. Rec., 2015, 15, 981–996 CrossRef CAS PubMed.
- (a) M. Passerini and L. Simone, Gazz. Chim. Ital., 1921, 51, 126–129 CAS; (b) M. Passerini and G. Ragni, Gazz. Chim. Ital., 1931, 61, 964–969 CAS.
- I. Ugi and R. Meyer, Chem. Ber., 1961, 94, 2229–2233 CrossRef CAS.
- (a) T. Sela and A. Vigalok, Adv. Synth. Catal., 2012, 354, 2407–2411 CrossRef CAS; (b) T. Yue, M. X. Wang, D.-X. Wang and J. Zhu, Angew. Chem., Int. Ed., 2008, 47, 9454–9457 CrossRef CAS PubMed; (c) L. Banfi and R. Riva, Org. React., 2005, 65, 1–140 CAS.
- T. Nixey and C. Hulme, Tetrahedron Lett., 2002, 43, 6833–6835 CrossRef CAS.
- (a) I. Monfardini, J.-W. Huang, B. Beck, J. F. Cellitti, M. Pellecchia and A. Domling, J. Med. Chem., 2011, 54, 890–900 CrossRef CAS PubMed; (b) E. S. Schremmer and K. T. Wanner, Heterocycles, 2007, 74, 661–671 CrossRef CAS.
- M. Giustiniano, T. Pirali, A. Massarotti, B. Biletta, E. Novellino, P. Campiglia, G. Sorba and G. C. Tron, Synthesis, 2010, 4107–4118 CAS.
- D. Amantini, R. Beleggia, F. Fringuelli, F. Pizzo and L. Vaccaro, J. Org. Chem., 2004, 69, 2896–2898 CrossRef CAS PubMed.
- (a) R. B. Nasir Baig and R. S. Varma, Chem. Soc. Rev., 2012, 41, 1559–1584 RSC; (b) G. Cravotto and P. Cintas, Chem. Soc. Rev., 2006, 35, 180–196 RSC; (c) P. Cintas and J. Luche, Green Chem., 1999, 1, 115–125 RSC; (d) C. Cui, C. Zhu, X.-J. Du, Z.-P. Wang, Z.-M. Li and W.-G. Zhao, Green Chem., 2012, 14, 3157–3163 RSC.
- (a) T. J. Mason, Chem. Soc. Rev., 1997, 26, 443–451 RSC; (b) R. F. Abdulla, Adrichimica Acta, 1988, 21, 31–42 CAS; (c) A. Loupy and J. L. Luche, in Synthetic Organic Sonochemistry, ed. J. L. Luche, Plenum Press, New York, 1998, pp. 107–166 Search PubMed; (d) L. H. Thompson and L. K. Doraiswamy, Ind. Eng. Chem. Res., 1999, 38, 1215–1249 CrossRef CAS.
- C. Cui, C. Zhu, X.-J. Du, Z.-P. Wang, Z.-M. Li and W.-G. Zhao, Green Chem., 2012, 14, 3157–3163 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6gc00910g |
| This journal is © The Royal Society of Chemistry 2016 |