Chemoselective cross-coupling reaction of sodium sulfinates with phenols under aqueous conditions†
Abstract
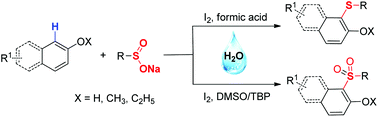
An efficient procedure for the formation of C–S bonds via direct C–H functionalization has been developed under aqueous conditions. In this process, stable and readily available sodium sulfinates were used as the sulfur source to provide aryl sulfides and sulfones in good to excellent yields. A broad range of functional groups were well tolerated in this reaction system.

 Please wait while we load your content...
Please wait while we load your content...