Open Access Article
Open Access ArticleBioactive and functional properties of sour cherry juice (Prunus cerasus)
Guillermo
Cásedas
a,
Francisco
Les
a,
Maria Pilar
Gómez-Serranillos
b,
Carine
Smith
c and
Víctor
López
 *a
*a
aDepartment of Pharmacy, Faculty of Health Sciences, Universidad San Jorge, 50.830 Villanueva de Gállego, Zaragoza, Spain. E-mail: ilopez@usj.es
bDepartment of Pharmacology, Faculty of Pharmacy, University Complutense of Madrid, Plaza Ramón y Cajal s/n, 28040 Madrid, Spain
cDepartment of Physiological Sciences, Science Faculty, Stellenbosch University, Stellenbosch, South Africa
First published on 6th October 2016
Abstract
Sour cherry juice (Prunus cerasus) is consumed as a nutritional supplement claiming health effects. The aim of the study was to evaluate the different properties of sour cherry juice in terms of antioxidant activity and inhibition of target enzymes in the central nervous system and diabetes. The content of polyphenols and anthocyanins was quantified. Different experiments were carried out to determine the radical scavenging properties of the juice. The activity of sour cherry juice was also tested in physiological relevant enzymes of the central nervous system (acetylcholinesterase, monoamine oxidase A, tyrosinase) and others involved in type 2 diabetes (α-glucosidase, dipeptidyl peptidase-4). Sour cherry juice showed significant antioxidant effects but the activity of the lyophilized juice was not superior to compounds such as ascorbic, gallic or chlorogenic acid. Furthermore, sour cherry juice and one of its main polyphenols known as chlorogenic acid were also able to inhibit monoamine oxidase A and tyrosinase as well as enzymes involved in diabetes. This is the first time that sour cherry juice is reported to inhibit monoamine oxidase A, α-glucosidase and dipeptidyl peptidase-4 in a dose dependent manner, which may be of interest for human health and the prevention of certain diseases.
1. Introduction
Cherry belongs to the Rosaceae family, and specifically to the genus Prunus. The most common types of Prunus are Prunus cerasus and Prunus avium, the first one is known as sour cherry and the other is called sweet cherry. Both are considered nutrient dense food with a relatively low caloric content and a significant amount of important nutrients and bioactive food components.1 Several studies have confirmed that eating a diet rich in fruit is related to a reduced risk of oxidative stress, cardiovascular disease, cancer, neurodegenerative disorders and diabetes.2–6 This may be due to dietary polyphenols, which are formed by at least one aromatic ring with one or more hydroxyl groups attached.7Some of the most common dietary polyphenols present in fruits and berries are anthocyanidins, which generate several anthocyanins. These anthocyanins are responsible for the red colour of fruits and the potential antioxidant activity. Although cherry is botanically classified as a stone fruit (drupe) due to the pit in the centre, it has the appearance of a berry. Several studies in animal models and in human subjects have demonstrated that dietary polyphenols are bioavailable and exert a protective role against oxidative stress and free radical damage.7 Antioxidants have the ability to scavenge or to neutralize free radicals, or are necessary to enable other molecules to perform such a function.8
There is strong evidence demonstrating that several ROS-mediated pathways may be involved in the neurodegenerative diseases, like Alzheimer's disease (AD) and Parkinson's disease (PD). It has been described that the accumulation of iron ions in the brain leads to higher ROS generation, involvement of mitochondrial pathways and to a decrease of endogenous antioxidants levels. Thus, natural antioxidants may prevent neurodegenerative disorders.9
Although mechanisms remain unclear, a body of evidence links type-2 diabetes with dementia and neurodegenerative diseases.10 One therapeutic approach to treat diabetes is to retard the absorption of glucose via inhibition of enzymes, such as α-glucosidase, in the digestive organs. It has been confirmed that α-glucosidase activity in vitro can be inhibited by berry extracts, i.e. blueberry, blackcurrant, strawberry, and raspberry rich in polyphenols.11 In recent years, there has also been an increasing interest in the ability of dietary factors to treat diabetes via modulating GLP-1 levels. GLP-1 is secreted from enteroendocrine L cells, which are present in the lower small intestine and large intestine, and stimulates insulin secretion in a blood glucose concentration dependent manner. GLP-1 is inactivated by dipeptidyl peptidase-4 (DPP-4), a circulating catabolic enzyme, resulting in a rather short half-life of about two minutes in the blood. There are reports that non-nutrient dietary factors such as polyphenols can affect GLP-1 levels.12
The aim of this study is to evaluate the bioactive properties of sour cherry juice in terms of antioxidant potential as well as activity in pharmacological targets of neurological diseases and diabetes. Antioxidant and protective effects of the juice have been studied in cellular and cell free systems. Potential inhibition of enzymes with relevant biological properties such as acetylcholinesterase, monoamine oxidase-A, tyrosinase, α-glucosidase and dipeptidyl peptidase 4 has also been carried out.
2. Materials and methods
2.1. Reagents and chemicals
Chemical reagents were acquired through Sigma-Aldrich, Cayman Chemical, Cymit química and Panreac (Spain). Sour cherry juice (Prunus cerasus) from Rabenhorst® was kindly supplied by Natur Import. According to the manufacturer, the juice is 100% organic, additives free and was obtained by pressing and pasteurization. The juice is bottled into amber bottles (expiration date: 20/01/2017).2.2. Sour cherry juice lyophilization
330 mL of Rabenhorst® sour cherry juice were lyophilized using a Genesis VirTis 25 EL lyophilizer (Wizard 2.0 control system) over 7 days. The liquid sample was frozen at −80 °C for 2 h while the lyophilizer was freezing at −80 °C. Afterwards, the temperature was modified to −30 °C for a couple of hours and for 96 h to −60 °C. Next transition was to −40 °C again (4 h) and 24 h to −60 °C. Finally, temperature reached until −15 °C (7 h) and dried 22 h at 20 °C. The last 2 h temperature was fixed at 40 °C. A sticky red residue was obtained and kept at −20 °C in a freezer until experiments were done.2.3. Phytochemical analyses of lyophilized sour cherry juice
2.4. Cytotoxicity screening in HeLa cells
HeLa cells were used to perform a cell viability test (MTT assay).15 HeLa cells were grown in DMEM supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin–glutamine. Cultures were incubated in the presence of 5% CO2 at 37 °C under a 100% relative humidified atmosphere. Cells were seeded in 96-well microplates at a density of 7 × 103 cells per well and grown for 24 h at 37 °C. Cells were then treated with various concentrations of lyophilized sour cherry juice (0.001–1 mg mL−1) for 72 h and a MTT solution was added and incubated for 3 h at 37 °C. Cell survival was measured as the reduction of MTT into formazan at 550 nm in a microplate reader. Experiments were performed twice.2.5. Antioxidant activity assays
As the viability of Artemia salina nauplii was not affected by different concentrations of sour cherry juice, the experiment was performed adding hydrogen peroxide to the wells at a concentration of 0.4 g L−1. Two different control wells without sour cherry juice were also set, one with hydrogen peroxide and another with seawater. The viability of the nauplii study was measured every 24 h for 72 h.12
2.6. Bioassays regarding CNS enzymes
2.7. Bioassays regarding enzymes involved in type 2 diabetes
2.8. Statistical analysis
Results were expressed as the mean ± standard error of experiments performed in triplicate. GraphPad Prism v.5 was required to perform data analyses, nonlinear regressions and statistics.3. Results
3.1. Phytochemical analysis of lyophilized sour cherry juice
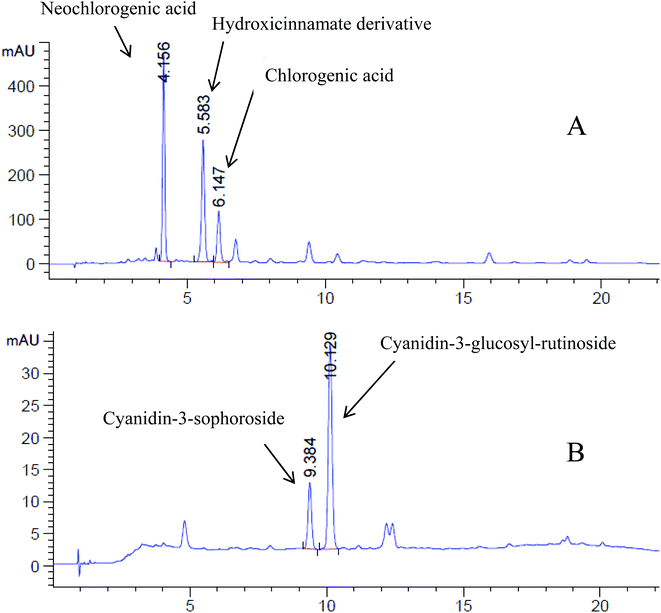
The polyphenol content was measured by the Folin–Ciocalteu method and expressed as gallic acid equivalents (GAE). Sour cherry juice contained 9.835 ± 1.092 μg GAE mg−1 of lyophilized juice. Five compounds (ascorbic acid, gallic acid, chlorogenic acid, neochlorogenic acid and cyanidin 3-glucosil-rutinoside) out of different monitored polyphenolic compounds were detected, quantified and confirmed comparing retention times and UV spectra with standards (Table 1 and Fig. 1). Total anthocyanins were quantified by HPLC at 520 nm using cyanidin-3-o-rutinoside (keracyanin chloride) as the external standard following the literature. Anthocyanins were found to be 0.194 ± 0.004 μg cyanidin-3-o-rutinoside equivalents per mg. However, more anthocyanins different from cyanidin 3-glucosil-rutinoside might be responsible for the red colour as other minor peaks can be seen at 520 nm (Fig. 1B).| Compound | μg mg−1 of lyophilized juice (S.E.) |
|---|---|
| —: Not detected; S.E. = standard error. | |
| Ascorbic acid | 0.011 (0.003) |
| Gallic acid | 0.047 (0.001) |
| Ellagic acid | — |
| Catechin | — |
| Chlorogenic acid | 0.593 (0.001) |
| Neochlorogenic acid | 1.580 (0.012) |
| Cyanidin 3-glucosil-rutinoside | 0.081 (0.000) |
| Total polyphenols (Folin method) | 9.835 ± 1.092 |
| Total anthocyanins (HPLC method) | 0.194 (0.003) |
3.2. Cytotoxicity screening in HeLa cells
The lyophilized juice showed very mild antiproliferative effects in HeLa cells. Significant differences were detected at concentrations over 0.125 mg mL−1, which indicates that this cell line seems to be partially sensitive to cherry components. Cell viability was approximately 60% at the highest tested concentration (1 mg mL−1), which means that the juice is not considered cytotoxic in this type of cervical cancer cells (Fig. 2).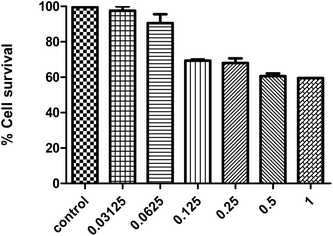 | ||
| Fig. 2 Viability of HeLa cells exposed to different concentration (mg mL−1) of sour cherry juice for 72 hours in the MTT assay. IC50 was not calculated as percentages of viability were more than 50%. | ||
3.3. Antioxidant activity assays
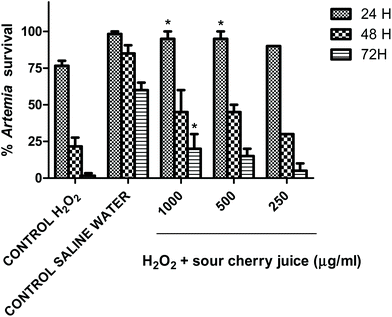
Fig. 3 indicates that sour cherry juice increased the survival of Artemia salina nauplii compared to 0.4 g L−1 hydrogen peroxide at 24, 48 and 72 h. Different concentrations of lyophilized juice enhanced survival of nauplii exposed to hydrogen peroxide, reaching more than 90% at 24 h. At 48 h, survival of nauplii was around 30–50% and finally, at 72 h between 10–20% of the nauplii survived compared to 0% survival of nauplii exposed to hydrogen peroxide. Significant differences were only obtained at doses of 1000 and 500 μg mL−1 at 24 h and 72 h.Fig. 4 shows the antioxidant effect of the juice compared to a reference standard such as gallic acid on superoxide radicals generated by the xanthine/xanthine oxidase system. IC50 values in this case were 54 μg mL−1 for sour cherry juice and 0.044 μg mL−1 for gallic acid.
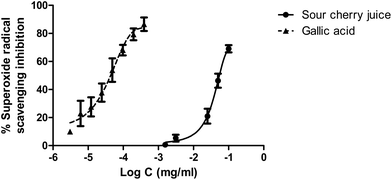 | ||
| Fig. 4 Antioxidant activity of sour cherry juice and gallic acid against superoxide radicals generated by the xanthine/xanthine oxidase system. | ||
The DPPH radical scavenging effects of sour cherry juice are shown in Fig. 5. The antiradical activity of the juice was compared to gallic, ascorbic and chlorogenic acid. IC50 values were also calculated by nonlinear regression. IC50 values were 236 μg mL−1 for cherry juice, 10 μg mL−1 for chlorogenic acid, 3 μg mL−1 for ascorbic acid and 1 μg mL−1 for gallic acid.
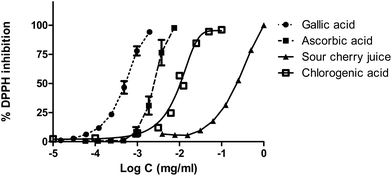 | ||
| Fig. 5 Antiradical activity of sour cherry juice, ascorbic acid, gallic acid and chlorogenic acid against DPPH. | ||
All these data indicate that the antioxidant and potential protective effects of sour cherry juice may be due to radical scavenging properties as it has been demonstrated through the DPPH and superoxide radical assays. In addition, the presence of polyphenols such as chlorogenic acid and anthocyanins in the juice, which was confirmed in the phytochemical analyses, seems to be crucial for the antioxidant and antiradical activities.
3.4. Bioassays regarding CNS enzymes
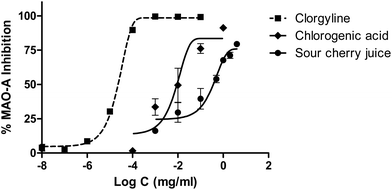
Sour cherry juice did not show activity in the AChE assay; however, it showed a clear dose dependent MAO-A inhibitory activity. These inhibitions are shown in Fig. 6. IC50 values were calculated by nonlinear regression (0.02 μg mL−1 for the selective inhibitor clorgyline, 246.19 μg mL−1 for sour cherry juice). TYR inhibitory activity was not as clear as for MAO-A. Sour cherry juice produced a very mild inhibition (28% at 1 mg mL−1) like Fig. 7 shows. In the MAO-A and TYR bioassays, chlorogenic acid showed a higher inhibition than the lyophilized juice.3.5. Bioassays regarding type 2 diabetes enzymes
Sour cherry juice exhibited in vitro an inhibition of α-GLU, but this activity was moderate compared to chlorogenic acid and acarbose, which is a reference inhibitor of this enzyme. As shown in Fig. 8, IC50 values were calculated by nonlinear regression (2783 μg mL−1 for sour cherry juice, 996 μg mL−1 for chlorogenic acid and 380 μg mL−1 for acarbose).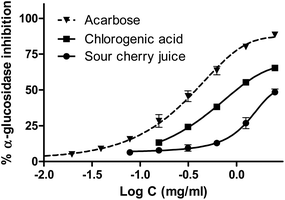 | ||
| Fig. 8 α-Glucosidase inhibition performed by sour cherry juice, chlorogenic acid and acarbose as the standard. | ||
Sitagliptin, an antidiabetic drug, showed a clear dose dependent DPP-4 inhibition. In addition, the effects of the juice and chlorogenic acid in this enzyme are shown in Fig. 9. IC50 values were calculated by nonlinear regression (0.1 μg mL−1 for sitagliptin and 1003.41 μg mL−1 for sour cherry juice) (Fig. 9).
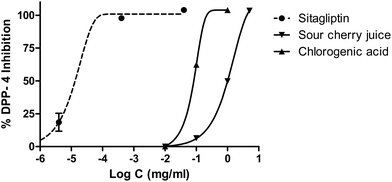 | ||
| Fig. 9 Dipeptidyl peptidase-4 inhibition performed by sour cherry juice, chlorogenic acid and sitagliptin as the standard. | ||
4. Discussion
Sour cherry juice is a good source of phytochemicals, specifically polyphenols and anthocyanins, which are the polyphenols responsible for the red skin and flesh colour.22The concentration of total phenolics (TP) was 9.835 ± 1.092 μg GAE per mg of lyophilized sour cherry juice (approx. 100 mg per 100 g), which is a lower concentration compared to other juices such as pomegranate13 but can still be of significant importance to produce health benefits. HPLC-DAD analysis showed a peak of chlorogenic acid and previous studies reveal that hydroxycinnamates such as caffeoylquinic acids are the main polyphenols in sweet and sour cherries.22 Anthocyanins were also quantified by HPLC-DAD, obtaining approximately 20 mg per 100 g. Other authors such as Wojdyło et al. compared 33 types of sour cherry in terms of the polyphenol content and antioxidant activity;23 our results are within the range of anthocyanins calculated for different cherry cultivars (7.56–94.20 mg per 100 g) although the authors studied the fruit content instead of juices as in our case.
Sour cherry juice is used in sports medicine to prevent muscle damage as some studies have shown that this product is able to prevent these symptoms through anti-inflammatory and antioxidant properties.24–29 Our results confirm the antioxidant potential of sour cherry juice. The protective effects against toxicity induced by hydrogen peroxide were measured using living organisms (Artemia salina). This experiment was performed by the authors using lyophilized sour cherry juice as a co-treatment with hydrogen peroxide and the study demonstrated significant differences of 1000 and 500 μg mL−1 at 24 h and 1000 μg mL−1 at 72 h. The juice was also able to scavenge both DPPH and superoxide radicals. It can be deduced that the antioxidant activity is mainly provided by polyphenols such as chlorogenic acid and anthocyanins. Other studies quantified Prunus cerasus antioxidant capacity using trolox as the standard, which makes it difficult to compare with our results.30
In addition, the study of the activity of sour cherry juice on enzymes was divided into two main groups, one related to the central nervous system and the other to glucose metabolism. We found for the first time that sour cherry juice was able to inhibit MAO-A and TYR. MAO-A is involved in the deamination of catecholamines and serotonin and certain polyphenols such as anthocyanins are involved in this inhibition, which can drive an antidepressant and anxiolytic effect.31 Tyrosinase is a copper-containing enzyme essential for tyrosine-melanin pigmentation and the role of toxic quinones in dopamine-induced neuronal damage catalyzed by TYR has been cleared in a number of studies.32 According to our data, this juice may have potential as a neuroprotective agent via MAO-A or TYR inhibition; in fact, a recent interventional human study showed that the consumption of anthocyanin-rich cherry juice for 12 weeks improves memory and cognition in older adults with mild-to-moderate dementia.33
Finally, the inhibition of enzymes involved in glucose metabolism and type 2 diabetes was studied; this is the first time that sour cherry juice is reported to inhibit α-glucosidase and DPP-4 in a dose dependent manner. The anthocyanin content in fruits is also related with α-glucosidase inhibition.34 According to our results, chlorogenic acid is also responsible for the activity. Polyphenols have also shown to facilitate the insulin response and attenuate secretion of glucose-dependent insulinotropic polypeptide and GLP-1. The DPP-4 enzyme also regulates glycaemia and its inhibitors such as sitagliptin represent some of the new treatments for type 2 diabetes. Taking in consideration that type 2 diabetes is linked to neurodegenerative diseases due to the production of superoxide radicals, sour cherry (Prunus cerasus) juice might be an interesting antioxidant nutritional tool to prevent these disorders.
Conflicts of interest
The authors declare that they do not have any conflicts of interest.Acknowledgements
Universidad San Jorge is acknowledged for financial support and providing Guillermo Casedas and Francisco Les PhD grants. Dr Olga Abian (IACS, BIFI-Universidad de Zaragoza) is thanked for providing HeLa cells. Natur Import is acknowledged for supplying the juice and CITA Aragón for lyophilization.References
- L. M. McCune, C. Kubota, N. R. Stendell-Hollis and C. A. Thomson, Cherries and Health: A Review, Crit. Rev. Food Sci. Nutr., 2010, 51(1), 1–12 CrossRef PubMed.
- C. Del Bo’, D. Martini, M. Porrini, D. Klimis-Zacas and P. Riso, Berries and oxidative stress markers: an overview of human intervention studies, Food Funct., 2015, 6(9), 2890–2917 Search PubMed.
- L. Wu, D. Sun and Y. He, Fruit and vegetables consumption and incident hypertension: dose–response meta-analysis of prospective cohort studies, J. Hum. Hypertens., 2016, 30(10), 573–580 CAS.
- S. Afrin, F. Giampieri, M. Gasparrini, T. Y. Forbes-Hernandez, A. Varela-López, J. L. Quiles, B. Mezzetti and M. Battino, Chemopreventive and Therapeutic Effects of Edible Berries: A Focus on Colon Cancer Prevention and Treatment, Molecules, 2016, 21(2), 169 CrossRef PubMed.
- D. J. Lamport, C. Saunders, L. T. Butler and J. P. Spencer, Fruits, vegetables, 100% juices, and cognitive function, Nutr. Rev., 2014, 72(12), 774–789 CrossRef PubMed.
- H. Guo and W. Ling, The update of anthocyanins on obesity and type 2 diabetes: experimental evidence and clinical perspectives, Rev. Endocr. Metab. Disord., 2015, 16(1), 1–13 CrossRef CAS PubMed.
- D. Del Rio, A. Rodriguez-Mateos, J. P. E. Spencer, M. Tognolini, G. Borges and A. Crozier, Dietary (Poly)phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases, Antioxid. Redox Signaling, 2013, 18(14), 1818–1892 CrossRef CAS PubMed.
- R. L. Prior, Fruits and vegetables in the prevention of cellular oxidative damage, Am. J. Clin. Nutr., 2003, 78(3 Suppl.), 570–578 Search PubMed.
- A. Ebrahimi and H. Schluesener, Natural polyphenols against neurodegenerative disorders: Potentials and pitfalls, Ageing Res. Rev., 2012, 11(2), 329–345 CrossRef CAS PubMed.
- G. Verdile, S. J. Fuller and R. N. Martins, The role of type 2 diabetes in neurodegeneration, Neurobiol. Dis., 2015, 84, 22–38 CrossRef CAS PubMed.
- G. J. McDougall, F. Shpiro, P. Dobson, P. Smith, A. Blake and D. Stewart, Different polyphenolic components of soft fruits inhibit α-amylase and α-glycosidase, J. Agric. Food Chem., 2005, 53(7), 2760–2766 CrossRef CAS PubMed.
- T. Tsuda, Possible abilities of dietary factors to prevent and treat diabetes via the stimulation of glucagon-like peptide-1 secretion, Mol. Nutr. Food Res., 2015, 59(7), 1264–1273 CAS.
- F. Les, J. M. Prieto, J. M. Arbonés-Mainar, M. S. Valero and V. López, Bioactive properties of commercialised pomegranate (Punica granatum) juice: antioxidant, antiproliferative and enzyme inhibiting activities, Food Funct., 2015, 6(6), 2049–2057 CAS.
- M. C. Díaz-García, J. M. Obón, M. R. Castellar, J. Collado and M. Alacid, Quantification by UHPLC of total individual polyphenols in fruit juices, Food Chem., 2013, 138(2–3), 938–949 CrossRef PubMed.
- T. Mosmann, Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays, J. Immunol. Methods, 1983, 65, 55–63 CrossRef CAS PubMed.
- J. L. Rodríguez-Chávez, E. Coballase-Urrutia, A. Nieto-Camacho and G. Delgado-Lamas, Antioxidant Capacity of “Mexican Arnica” Heterotheca inuloides Cass Natural Products and Some Derivatives: Their Anti-Inflammatory Evaluation and Effect on C. elegans Life Span, Oxid. Med. Cell. Longevity, 2015, 2015, 843237 Search PubMed.
- V. Lopez, S. Akerreta, E. Casanova, J. M. Garcia-Mina, R. Y. Cavero and M. I. Calvo, In vitro antioxidant and anti-rhizopus activities of Lamiaceae herbal extracts, Plant Foods Hum. Nutr., 2007, 62, 151–155 CrossRef PubMed.
- I. K. Rhee, M. van de Meent, K. Ingkaninan and R. Verpoorte, Screening for acetylcholinesterase inhibitors from Amaryllidaceae using silica gel thin-layer chromatography in combination with bioactivity staining, J. Chromatogr., A, 2001, 915(1–2), 217–223 CrossRef CAS PubMed.
- H. T. Olsen, G. I. Stafford, J. van Staden, S. B. Christensen and A. K. Jäger, Isolation of the MAO-inhibitor naringenin from Mentha aquatica L, J. Ethnopharmacol., 2008, 117(3), 500–502 CrossRef CAS PubMed.
- F. Sezer Senol, I. E. Orhan, U. Ozgen, G. Renda, G. Bulut, L. Guven, E. S. Karaoglan, H. G. Sevindik, K. Skalicka-Wozniak, U. Koca Caliskan and N. Sekeroglu, Memory-vitalizing effect of twenty-five medicinal and edible plants and their isolated compounds, S. Afr. J. Bot., 2016, 102, 102–109 CrossRef CAS.
- M. I. Kazeem, J. O. Adamson and I. A. Ogunwande, Modes of inhibition of α-amylase and α-glucosidase by aqueous extract of Morinda lucida benth leaf, BioMed Res. Int., 2013, 2013, 527570 CAS.
- G. Ferretti, T. Bacchetti, A. Belleggia and D. Neri, Cherry antioxidants: from farm to table, Molecules, 2010, 15(10), 6993–7005 CrossRef CAS PubMed.
- A. Wojdyło, P. Nowicka, P. Laskowski and J. Oszmiański, Evaluation of sour cherry (Prunus cerasus L.) fruits for their polyphenol content, antioxidant properties, and nutritional components, J. Agric. Food Chem., 2014, 62(51), 12332–12345 CrossRef PubMed.
- D. A. Connolly, M. P. McHugh, O. I. Padilla-Zakour, L. Carlson and S. P. Sayers, Efficacy of a tart cherry juice blend in preventing the symptoms of muscle damage, Br. J. Sports Med., 2006, 40(8), 679–683 CrossRef CAS PubMed.
- G. Howatson, M. P. McHugh, J. A. Hill, J. Brouner, A. P. Jewell, K. A. van Someren, R. E. Shave and S. A. Howatson, Influence of tart cherry juice on indices of recovery following marathon running, Scand. J. Med. Sci. Sports, 2010, 20(6), 843–852 CrossRef CAS PubMed.
- K. S. Kuehl, E. T. Perrier, D. L. Elliot and J. C. Chesnutt, Efficacy of tart cherry juice in reducing muscle pain during running: a randomized controlled trial, J. Int. Soc. Sports Nutr., 2010, 7, 17 CrossRef PubMed.
- J. L. Bowtell, D. P. Sumners, A. Dyer, P. Fox and K. N. Mileva, Montmorency cherry juice reduces muscle damage caused by intensive strength exercise, Med. Sci. Sports Exercise, 2011, 43(8), 1544–1551 CrossRef CAS PubMed.
- K. S. Kuehl, Cherry juice targets antioxidant potential and pain relief, Med. Sport Sci., 2012, 59, 86–93 CAS.
- L. Dimitriou, J. A. Hill, A. Jehnali, J. Dunbar, J. Brouner, M. P. McHugh and G. Howatson, Influence of a Montmorency cherry juice blend on indices of exercise-induced stress and upper respiratory tract symptoms following marathon running–a pilot investigation, J. Int. Soc. Sports Nutr., 2015, 12, 22 CrossRef PubMed.
- J. Cao, Q. Jiang, J. Lin, X. Li, C. Sun and K. Chen, Physicochemical characterisation of four cherry species (Prunus spp.) grown in China, Food Chem., 2015, 173, 855–863 CrossRef CAS PubMed.
- S. M. Nabavi, M. Daglia, N. Braidy and S. F. Nabavi, Natural products, micronutrients, and nutraceuticals for the treatment of depression: A short review, Nutr. Neurosci., 2015 DOI:10.1080/1028415X.2015.1103461.
- T. Masuda, D. Yamashita, Y. Takeda and S. Yonemori, Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent inhibitors from Garcinia subelliptica, Biosci., Biotechnol., Biochem., 2005, 69(1), 197–201 CrossRef CAS PubMed.
- K. Kent, K. Charlton, S. Roodenrys, M. Batterham, J. Potter, V. Traynor, H. Gilbert, O. Morgan and R. Richards, Consumption of anthocyanin-rich cherry juice for 12 weeks improves memory and cognition in older adults with mild-to-moderate dementia, Eur. J. Nutr., 2015 DOI:10.1007/s00394-015-1083-y.
- G. J. McDougall, F. Shpiro, P. Dobson, P. Smith, A. Blake and D. Stewart, Different polyphenolic components of soft fruits inhibit α-amylase and α-glycosidase, J. Agric. Food Chem., 2005, 53(7), 2760–2766 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |