Physical and oxidative stability of functional olive oil-in-water emulsions formulated using olive mill wastewater biophenols and whey proteins
Nicola
Caporaso
*a,
Alessandro
Genovese
a,
Róisín
Burke
b,
Catherine
Barry-Ryan
c and
Raffaele
Sacchi
a
aDepartment of Agricultural Sciences, University of Naples Federico II, 80055 Portici, NA, Italy. E-mail: nicola.caporaso3@unina.it
bSchool of Culinary Arts and Food Technology, Dublin Institute of Technology, Dublin, Ireland
cSchool of Food Science and Environmental Health, Dublin Institute of Technology, Dublin, Ireland
First published on 7th December 2015
Abstract
The present paper reports on the use of phenolic extracts from olive mill wastewater (OMW) in model olive oil-in-water (O/W) emulsions to study their effect on their physical and chemical stability. Spray-dried OMW polyphenols were added to a model 20% olive O/W emulsion stabilized with whey protein isolate (WPI) and xanthan gum, in phosphate buffer solution at pH 7. The emulsions were characterised under accelerated storage conditions (40 °C) up to 30 days. Physical stability was evaluated by analysing the creaming rate, mean particle size distribution and mean droplet size, viscosity and rheological properties, while chemical stability was assessed through the measurement of primary and secondary oxidation products. The rheological behaviour and creaming stability of the emulsions were dramatically improved by using xanthan gum, whereas the concentration of WPI and the addition of encapsulated OMW phenolics did not result in a significant improvement of physical stability. The formation of oxidation products was higher when higher concentrations of encapsulated polyphenols were used, indicating a possible binding with the WPI added in the system as a natural emulsifier. This paper might help in solving the issue of using the olive mill wastewater from olive processing in formulating functional food products with high antioxidant activity and improved health properties.
Introduction
Olive oil production technology causes major environmental problems in countries where its production is mainly localized, i.e. the Mediterranean area, as the industry produces a high output of liquid by-products represented by the olive mill wastewater (OMW). Due to the high concentration of phenolic compounds,1 this waste could be conveniently converted into a valuable source of antioxidant compounds, which can be added to a variety of foods to develop a functional product with better nutritional properties.2In the last few years, new technologies have been tested and applied for the extraction of phenolic compounds from OMW, particularly membrane processes which involve ultrafiltration in combination with nano-filtration and reverse osmosis.3 The concepts behind its production by membrane separation techniques were reported by other researchers.4,5 For their convenient storage and use, water phenolic extracts must be dried, and spray-drying has been applied to the OMW obtained from membrane filtration. The use of these phenolic extracts in emulsions is of interest at the industrial level for the production of a wide range of functional food products, such as mayonnaise, creams, sauces and other spreads.
Emulsions are kinetically unstable systems, and their instability is due to many mechanisms, including creaming, coalescence and flocculation.6,7 Therefore, stabilizers and emulsifiers are needed to provide physical stability to avoid emulsion phase separation. Food emulsions are often multiphase systems containing more than one biopolymer, e.g. mixtures of proteins and polysaccharides.8 Thickening agents are mainly polysaccharides, e.g. xanthan gum, maltodextrin, galactomannans, starches, pectin, carboxymethylcellulose, etc., used to increase the viscosity of the continuous phase.7,9
Milk proteins (caseinate and whey proteins) are hydrocolloids used in many food systems, owing to their good solubility and behaviour.10 Being surface active, whey protein isolate (WPI) is adsorbed on the oil–water interface in the form of a protective film.11–13 Proteins are usually less effective emulsifiers than synthetic surfactants, but their use in the food industry has been increasing due to the trend to use “clean label” ingredients or “natural” products.
Many factors have been studied previously for the characterisation of O/W emulsions, including the effect of pH, oil phase concentration,14 the composition of the oil phase used, the effect of stabilizers, emulsifiers, droplet size and droplet size distribution,15,16 as well as the presence of metals and phenolic compounds.17,18 The effect of environmental stresses, such as heating, chilling, freezing and drying, was previously reported, as it influences the behaviour of protein-stabilized emulsions.6
Olive oil has been studied by various researchers to formulate O/W emulsions,19,20e.g. in a mixture with lemon juice to simulate a traditional Mediterranean dressing, which was stabilized using propylene glycol alginate, xanthan or gum arabic.9 Olive oil was shown to have better physical stability in low-fat emulsions, stabilized by WPI and xanthan gum, than other oils like sesame oil.20 Previous studies also reported on the characterisation of model O/W emulsions.21–23
The complexity of real emulsions is due to the presence of mixtures of proteins and polysaccharides, and in such systems the addition of phenolic compounds implies further possible interactions, particularly with proteins, which lead to complex coacervation between macromolecules. The latter phenomenon is largely affected by the ionic strength, biopolymer ratio, biopolymer concentration and by their distribution and charge density.8 In individual proteins, e.g. β-lactoglobulin, BSA etc., the effect of polyphenol binding was reported previously.24 It is also known that in emulsions, the presence of the aqueous phase can decrease the activity of some antioxidant compounds.25 Hydroxytyrosol, the most abundant phenolic compound in olive oil and OMW, was reported to exert the highest antioxidant activity among olive phenolics, which are mainly oleuropein derivatives.17 The antioxidant activity of the more polar phenolic compounds is lower in emulsions compared to bulk oil, owing to the so-called “polar paradox”. The antioxidant activity of pure hydroxytyrosol, oleuropein and its derivatives can be also affected by the pH conditions.17
There is, however, a lack of information about the behaviour of biopolymers in O/W emulsions and their effect on emulsion stability, as the final effect of phenolic compounds depends upon many factors.21,26 Moreover, olive oil is a complex mixture of minor compounds and triacylglycerols, with stabilising effects due to the interaction with minor compounds and whey proteins.23
The food industry needs to verify these effects on real food products, and promising benefits are expected from the application of phenolic extracts from OMW, in terms of both environmental and nutritional value of foods. Indeed, the conversion of by-products into valuable ingredients for the design of functional food products has been investigated using olive oil as a source of fat, whey protein isolate and olive phenolics in a model O/W emulsion system.
Therefore, the aim of this paper was to characterise the behaviour and properties of O/W emulsions formulated with 20% olive oil and functionalised by adding polyphenolic powder extracts from OMW (P-OMW) at two concentrations, stabilized by WPI and xanthan gum at different levels.
Materials and methods
Olive oil samples, stabilizers and OMW powder
Freshly refined olive oil was donated by I.O.B.M. srl (Montesarchio, BN, Italy). Xanthan gum from Xanthomonas campestris was purchased from Sigma-Aldrich (Darmstadt, Germany). WPI was 97.5 wt% protein, and lactose content was less than 1 wt%. A phosphate buffer solution at pH 7.0 was prepared using monosodium phosphate and sodium hydroxide (Darmstadt, Germany). The buffer was used to maintain a constant pH, as this parameter can affect emulsion stability.18 All other chemicals were of analytical grade purity. A phenolic extract from OMW was kindly donated by LABS (Department of Agricultural Sciences, University of Naples Federico II, Italy). The P-OMW production process has been reported by Troise et al.27 The composition of the three main phenolic compounds analysed by HPLC-UV-Vis is as follows: OHTy 32 ± 0.2 mg g−1, Ty 1.9 ± 0.1 mg g−1, and verbascoside 2.8 ± 0.09 mg g−1.27Emulsion preparation
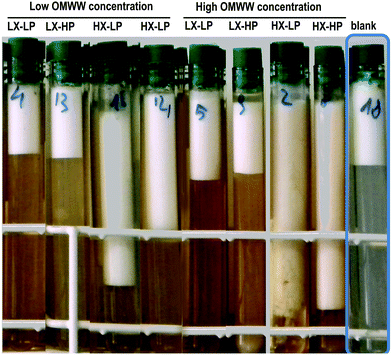
Emulsions were prepared by dispersing spray-dried P-OMW powder at two concentrations (1 or 5 mM, expressed as OHTy) and WPI (0.13 or 0.5% w/v) into a buffer solution (5 mM phosphate buffer, pH 7). The aqueous phase was gently stirred for 2 h at room temperature to ensure dissolution, using a magnetic stirring bar and a magnetic stirrer hotplate (Stuart CB162, Bibby-Scientific, Staffordshire, UK). The pH was checked and adjusted to pH 7.0 using 1 M HCl. Xanthan gum (0.06 or 0.2% w/v) was added to the emulsions and gently stirred (100 rpm) overnight at room temperature to allow complete hydration. Emulsions were produced by blending 20% (v/v) refined olive oil in the solution previously prepared using a high-speed blender at 8000 rpm for 2 min, after a pre-emulsification phase.28 The emulsions were then transferred into glass tubes for the creaming stability analysis, and stored in an incubator at 40 °C ± 1 °C for kinetic stability evaluation (Fig. 1).The concentrations of the stabilisers were chosen according to previous studies.11,22 Emulsions with low or high concentrations of stabilisers are named as follows: LX-LP, low xanthan (0.06% w/v) and low WPI (0.13% w/v); LX-HP, low xanthan and high WPI (0.5% w/v); HX-LP, high xanthan (0.2% w/v) and low WPI; HX-HP, high xanthan and high WPI. For each system, two concentrations of P-OMW were used, i.e. 1 and 5 mM as OHTy, corresponding to low and high average phenolic concentrations in commercial virgin olive oils.29 The blank system had no added P-OMW. In this case, WPI and xanthan gum concentrations were 0.5% and 0.2% (w/v), respectively.
Creaming value
Creaming value was monitored visually according to the literature.30 Duplicate samples of emulsions were stored in 75 mm × 12 mm sample tubes (York Glassware, UK) at 25 ± 0.5 °C. Measurements of the serum layer (creaming index) were carried out manually using a 60% fiberglass Measy 2000 Typ 5921 (Baty, Switzerland). Stability was evaluated as percentage decrease from the initial height using the following equation: creaming Index = 100 × (HS/HE), where HS is the serum layer formed at the bottom of glass tubes and HE is the total height of the emulsions in the tubes.31Mean particle size by image analysis (optical microscopy)
For the determination of particle size by digital image analysis, emulsions were diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 using a buffer solution to avoid droplet overlapping. A drop of emulsion was placed on a microscope slide and then covered with a cover slip. The microstructure of the emulsion was observed using an Olympus DP72 optical microscope (Japan) at 40× and 400× magnification. Digital pictures were taken by using an Olympus E-620 digital camera mounted on the microscope. The mean droplet size was calculated by analysing the microscopic images using ImageJ 1.47t 64-bit software (National Institutes of Health, USA). The function Analyse Particles was used after the colour threshold and using the following options: size: 0.01–infinity; circularity: 0.00–1.00; particle size >2 μm2; the options “exclude droplets on edge” and “include holes” were set on the software package. At least 20 pictures were taken at 100× magnification for each emulsion analysed. Image analysis was performed as described in previous studies,16 where details of the method can be found.
1000 using a buffer solution to avoid droplet overlapping. A drop of emulsion was placed on a microscope slide and then covered with a cover slip. The microstructure of the emulsion was observed using an Olympus DP72 optical microscope (Japan) at 40× and 400× magnification. Digital pictures were taken by using an Olympus E-620 digital camera mounted on the microscope. The mean droplet size was calculated by analysing the microscopic images using ImageJ 1.47t 64-bit software (National Institutes of Health, USA). The function Analyse Particles was used after the colour threshold and using the following options: size: 0.01–infinity; circularity: 0.00–1.00; particle size >2 μm2; the options “exclude droplets on edge” and “include holes” were set on the software package. At least 20 pictures were taken at 100× magnification for each emulsion analysed. Image analysis was performed as described in previous studies,16 where details of the method can be found.
Droplet size distribution of emulsions
Droplet-size distributions of the emulsions were determined by using a Mastersizer 2000 Hydro 2000S (Malvern Instruments, UK), which gives measurements based on light scattering under high dilution conditions by dispersing the samples in distilled water.15,30 To avoid multiple scattering effects, the freshly prepared emulsions were diluted to reach an obscuration rate of about 3. The refractive indices of water and refined olive oil were 1.330 and 1.418, respectively. The average droplet sizes were characterized in terms of the volume mean diameter d4,3 = ∑i·ni·di4/∑i·ni·di3, where ni is the number of droplets of diameter di. The d4,3 parameter is a useful mean diameter value, sensitive to small changes in droplet-size distribution.8 All measurements were made at room temperature and four measurements were obtained for each sample. A bimodal particle-size distribution was taken to be indicative of non-reversible flocculation.15,30Cloudiness and turbidity measurements
Cloudiness measurements, also called turbidity or opacity, were carried out according to previously published methods for O/W emulsions.32 Samples were taken after 24 h of their preparation and analysed over the storage period. In the case of separate emulsions, the supernatant was sampled, while in the absence of evident phase separation, the upper part was sampled as well. Emulsions were diluted (2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) and cloudiness was expressed from the absorbance at 660 nm.
1000) and cloudiness was expressed from the absorbance at 660 nm.
Rheological properties of emulsions
Rheological measurements were carried out in accordance with previous papers reporting on O/W emulsions stabilized by WPI and xanthan gum.13 Steady shear viscosity and small-amplitude oscillatory shear tests were conducted using a Bohlin C-VOR dynamic rheometer (Malvern Instruments Inc., Southborough, MA). Emulsion viscosity was measured at 25 °C over a shear rate range of 0.01–100 s−1 with a cone-plate geometry (CP 40/4°). All the measurements were performed within 24 h of emulsion preparation. A logarithmic progression was applied, and the sweep time was 120 s. Oscillatory tests were performed by pouring emulsion samples (typically 1–1.5 mL) directly on the holding stage and samples were covered with a thin paraffin oil layer to prevent water evaporation. In oscillatory experiments the storage (G′) and loss (G′′) moduli were recorded versus frequency (0.1–10 Hz) at a constant strain, with the increase of logarithmic scale. The linear viscoelastic region was previously determined by selecting a strain of 0.5 Pa, recording G′ and G′′ versus shear stress (0.01–100 Pa) at a constant frequency.Oxidative stability: lipid hydroperoxides
Lipid hydroperoxides were measured according to previous studies.22 Emulsions (0.3 mL) were mixed with 1.5 mL of isooctane/2-propanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), vortexing three times for 30 s and centrifuging for 2 min at 2000g (Hettich Rotanta 460R centrifuge). The supernatant (200 μL) was collected and 2.8 mL of a methanol
1, v/v), vortexing three times for 30 s and centrifuging for 2 min at 2000g (Hettich Rotanta 460R centrifuge). The supernatant (200 μL) was collected and 2.8 mL of a methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-butanol solution (3
1-butanol solution (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) were added, followed by 15 μL of 3.94 M ammonium thiocyanate and 15 μL ferrous iron solution (prepared by adding equal amounts of 0.132 M BaCl2 and 0.144 M FeSO4). After 20 min, the absorbance was measured at 510 nm using a Lambda Bio 20 spectrophotometer (PerkinElmer, Boston, MA). Hydroperoxide concentrations were determined using a calibration curve prepared with hydrogen peroxide.
1, v/v) were added, followed by 15 μL of 3.94 M ammonium thiocyanate and 15 μL ferrous iron solution (prepared by adding equal amounts of 0.132 M BaCl2 and 0.144 M FeSO4). After 20 min, the absorbance was measured at 510 nm using a Lambda Bio 20 spectrophotometer (PerkinElmer, Boston, MA). Hydroperoxide concentrations were determined using a calibration curve prepared with hydrogen peroxide.
Oxidative stability: thiobarbituric acid reactive substances
Thiobarbituric acid reactive substances (TBARs) were determined according to previously published methods.23 Emulsions (0.1–1 mL) were mixed with 2.0 mL of TBA reagent (15% w/v trichloroacetic acid and 0.375% w/v thiobarbituric acid in 0.25 M HCl) in test tubes and placed in a boiling water bath for 15 min. The tubes were cooled to room temperature for 10 minutes and then centrifuged (2000g using a Hettich Rotanta 460R centrifuge) for 15 min at 20 °C. After 10 minutes, the absorbance was measured at 532 nm. The TBAR concentration was determined by a standard curve prepared using 1,1,3,3-tetramethoxypropane.Statistical analysis
In order to better understand the influence of polyphenols, WPI and xanthan gum, as well as their interactions, a multifactor ANOVA with second-order interactions was performed. XLStat (2009.3.02), an add-in software package for Microsoft Excel (Addinsoft Corp., Paris, France), was used for data elaboration. Cluster analysis was used to construct hierarchical dendrograms, searching for the natural groupings among the samples. The sample similarities were calculated on the basis of the squared Euclidean distance, using the Unscrambler X software v. 10.3 (CAMO Software, Trondheim, Norway).Results and discussion
Creaming rate
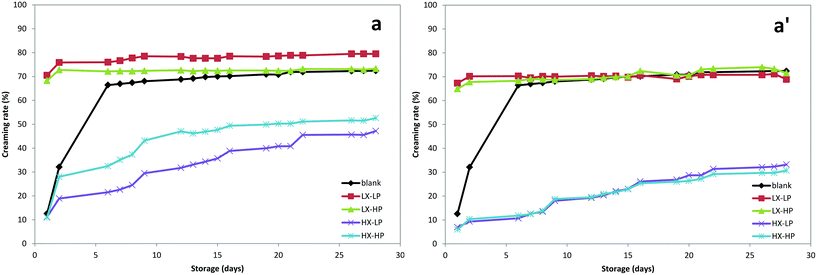
The creaming rate of olive O/W emulsions added with P-OMW and stabilized by WPI and xanthan gum is reported in Fig. 2. Different behaviour was observed mainly depending on xanthan gum levels. This hydrocolloid is a stabiliser, it was expected to delay creaming when higher concentrations are used. Indeed, xanthan gum is widely applied for its good viscoelastic and chemical properties, e.g. water solubility and pH stability.6 The control sample contained a high concentration of xanthan gum (0.2% w/v). The creaming rate observed was lower in the first storage period, i.e. up to 8 days, from which a creaming similar to the samples containing low concentration was measured.The addition of P-OMW did not cause dramatic changes in the creaming rate between low and high concentrations of xanthan gum. In the systems with low P-OMW levels (Fig. 2a), a difference in the creaming rate was observed due to the concentrations of WPI. While in the emulsions with low xanthan concentration a significant statistical difference was not reached, samples with high xanthan concentration showed higher creaming rate when higher WPI levels were used.
Two different creaming processes exist, i.e. the creaming of individual particles, with particle size around 10 μm, and the migration of flocculates, which is observed at lower particle sizes.10 Creaming occurs when the density of the droplets is lower than that of the continuous phase,33 and stabilizers act to increase emulsion viscosity and therefore they retard droplet movement.
Protein–polysaccharide conjugates are known to be formed in WPI-stabilized emulsions with polysaccharides, which leads to improved physical stability. Our results on emulsion physical stability are in agreement with previous literature data, particularly for the stabilising effect of xanthan gum on the creaming rate.28,34 However, excess xanthan gum might induce higher creaming rate by depletion flocculation, as droplet flocculation influences the creaming stability of monodisperse oil-in-water emulsions.35 The creaming rate in the case of droplet flocculation is generally higher at increased particle size and lower at increased oil phase concentration, due to hydrodynamic effects and particle–particle interactions.35 A possible effect of WPI on the creaming rate was reported by Sun and Gunasekaran (2009),13 as increasing WPI concentrations caused a slight decrease in creaming index. Indeed, it has been reported that 0.2% of WPI was not enough to cover the entire droplet surface to stabilize the emulsions containing 20–40% oil.13 The effect was explained by the unabsorbed WPI in the aqueous phase, while the presence of xanthan increased the amount of protein unabsorbed at the interface. The different behaviour observed in our emulsions with low OMW and high concentration of xanthan (HX) is likely to be due to this phenomenon. The possible protein–polyphenol interaction seems to be of little extent, as explained by the simple chemical structure of olive phenolics and their molecular size, as well as by the presence of hydrocolloids which could interfere with the binding, particularly xanthan gum, representing a barrier for the physical interaction and consequently the chemical binding. Indeed, P-OMW phenolics have a relatively simple structure and lower molecular weight than other classes of phenolics for which the protein–polyphenol effect was reported, i.e. tannins. Previous papers have shown that olive phenolics have binding affinity to caseinate and whey proteins, whereas OHTy and Ty did not interact significantly with pure BSA.26 In our previous study,36 the effect of the interaction between olive phenolics and WPI on headspace release of volatile compounds in emulsions was reported. In particular, a significant increase of some key odour compounds was observed when phenolic compounds were added to the emulsion, probably due to the polyphenol–protein interaction influencing the binding effect of volatile compounds by WPI.
A multifactorial ANOVA was carried out to evaluate the influence of polyphenols, WPI, xanthan, and their interactions over storage time (Table 1). The results showed that creaming is influenced by all the variables and by interactions between polyphenols and WPI as well between polyphenols and xanthan. Moreover, the statistical analysis has shown that storage influences all the other parameters considered. For the creaming rate, a significant effect of the interactions between P-OMW and WPI was found, as well as between P-OMW and xanthan gum.
| Creaming | Droplet size | Cloudiness | Hydroperoxides | TBARS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | F ratio | p value | F ratio | p value | F ratio | p value | F ratio | p value | F ratio | p value |
| Time | 283.631 | <0.0001 | 224.42 | <0.0001 | 18.162 | <0.0001 | 46.710 | <0.0001 | 9.459 | 0.002 |
| OMW | 105.837 | <0.0001 | 1.711 | 0.191 | 6.653 | 0.012 | 0.000 | 0.999 | 8.118 | 0.005 |
| WPI | 23.503 | <0.0001 | 0.728 | 0.394 | 6.080 | 0.016 | 0.049 | 0.825 | 0.094 | 0.760 |
| Xanthan | 20.277 | <0.0001 | 8.822 | 0.003 | 0.815 | 0.370 | 0.132 | 0.717 | 4.907 | 0.028 |
| Time × OMW | 0.140 | 0.708 | 5.154 | 0.023 | 5.462 | 0.023 | 0.208 | 0.649 | 1.851 | 0.175 |
| Time × WPI | 0.956 | 0.329 | 0.710 | 0.400 | 2.310 | 0.134 | 0.007 | 0.933 | 0.714 | 0.399 |
| Time × xanthan | 0.823 | 0.365 | 17.075 | <0.0001 | 0.218 | 0.642 | 0.002 | 0.964 | 3.286 | 0.072 |
| OMW × WPI | 6620.019 | <0.0001 | 4.437 | 0.035 | 5.741 | 0.020 | 0.255 | 0.614 | 11.817 | 0.001 |
| OMW × xanthan | 55.185 | <0.0001 | 2.695 | 0.101 | 39.212 | <0.0001 | 0.298 | 0.585 | 0.102 | 0.750 |
| WPI × xanthan | 2.570 | 0.110 | 2.278 | 0.132 | 1.069 | 0.305 | 0.989 | 0.321 | 2.813 | 0.095 |
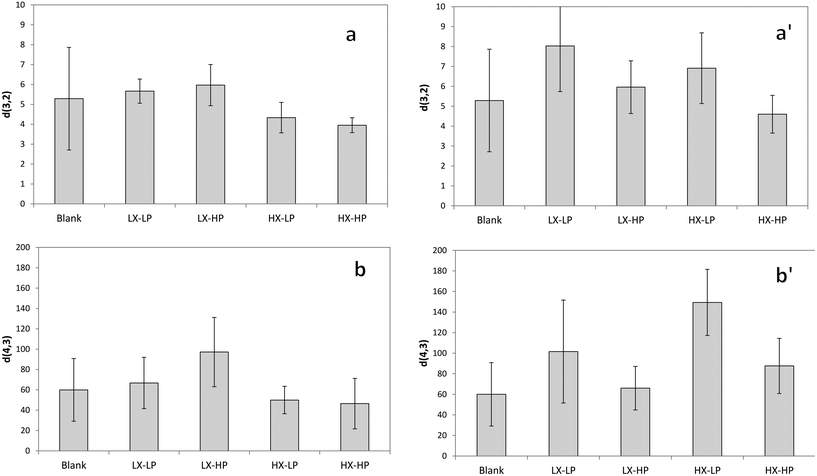
Mean droplet size assessed by image analysis and droplet size distribution
Droplet size distribution in freshly-prepared emulsions was assessed by measuring both d3,2 and d4,3 using a Mastersizer (Fig. 3). Being d4,3 more sensitive to the presence of large particles in emulsions than d3,2, it is often more sensitive to phenomena such as coalescence.6 A bimodal distribution of particle size was observed, which also implies a high standard deviation of the average particle size.Particle size in O/W emulsions is known to be influenced by the WPI level,37 as higher WPI concentrations were reported to cause lower particle size diameter.13 However, the authors applied a high pressure homogenizer to reach a considerably lower mean particle size, while in our case in the absence of dramatic differences is likely to be due to the lower exposed surface area, as the diameter of the particle size in our samples was circa ten times higher. The authors explained that the lower particle size was due to a larger area stabilized by the higher amount of added protein and to a better coverage of the droplet surface.37
In our emulsions, d3,2 was influenced by the high xanthan concentration (Fig. 3a), as at higher levels a lower average particle size was measured, probably due to the effect of this hydrocolloid on the stabilization after a few hours of emulsion production. Some emulsifier agents have been reported to have no significant effect on the mean droplet diameter, but xanthan gum was reported to be a critical factor.38 The effect of xanthan gum on droplet size of emulsions is controversial, as some papers indicated a decrease in the mean droplet diameter at increased xanthan concentration,34 while others reported an increase in droplet size attributed to the flocculation caused by the hydrocolloid.38,39 At lower concentrations, it was reported that the droplet size of O/W emulsions is unaffected by the presence of xanthan in the range 0–0.15% (w/v).40 A more obvious difference was observed between the systems containing low and high P-OMW concentrations, with generally higher d4,3 in the second case, with the only exception of LX-HP (Fig. 3b). This result suggests a possible destabilisation effect of olive oil phenolics, which caused greater droplet dimensions.
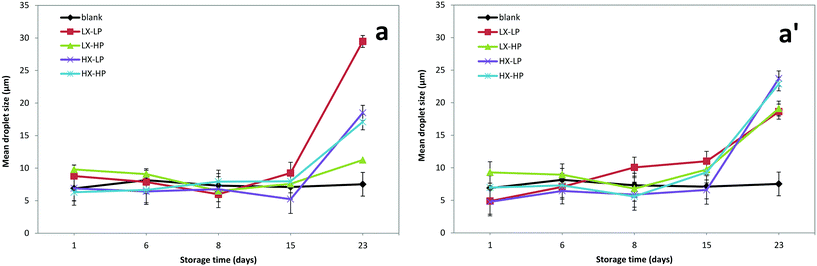
The change in mean droplet size was also assessed by digital image analysis of micrographs, over storage time (Fig. 4). A general increasing trend in the particle diameter was measured up to 23 days of storage, particularly evident in the last period of incubation. This information could appear in contrast with the creaming observation, as the main changes were observed during the first week, while a plateau was observed later. Previous studies suggest that there could be an increase in droplet size without any direct effect on the creaming rate when emulsions are stabilized by thickening agents.15 The mean droplet sizes assessed by image analysis were larger than the values obtained by the light scattering technique, i.e. Mastersizer analysis. This effect was explained by the impossibility of detecting the smallest droplets in the micrographs. From our micrographs, it was observed that the main driver affecting the structure of the O/W emulsions was xanthan gum, as the presence of some aggregates was observed at high xanthan gum concentrations (data not shown), which is in accordance with previous findings.40
No dramatic influence of P-OMW extracts on the mean droplet size was observed, except for the last sampling time. On day 23, the droplet size increased in the presence of phenolics by a factor of two as compared to the control. It was noted, moreover, that in samples with high xanthan concentration, the particle size was significantly (p < 0.05) higher than in emulsions with lower levels. The xanthan gum level had a statistically significant effect on droplet size. Interestingly, the interaction of phenolics over time, and phenolics with WPI was statistically significant (Table 1). The xanthan gum effect is explained by the way in which this compound stabilises emulsions, i.e. acting as a thickener and therefore retarding the phase separation, whereas they do not act directly on the maintenance of small particle size diameters. In fact, both the presence of an insufficient emulsifier and its excess in the aqueous solution could lead to different droplet sizes of the obtained emulsions.6
Previous literature reports described a general increase in droplet size during accelerated storage of olive O/W emulsions, also depending on the type of phenolic compound used, and their possible interference in protein rearrangements was shown.23 However, in our case, OMW contains phenolic compounds that are less reactive toward protein–protein interaction and therefore a more limited effect was expected depending on their chemical structure.
Cloudiness
Cloudiness of the emulsions under accelerated conditions showed a general decreasing trend over storage time (Fig. 5). Cloudiness is dependent upon the type and concentrations of hydrocolloids, and the variation in its value obtained in our experiment is in accordance with previous findings.32 It was explained by the changes in average droplet size induced by the aggregation of oil droplets as well as the changes in the refractive index of oil phase and aqueous phase. Turbidity loss is known to be linked to mechanisms such as flocculation, coalescence and aggregation, which are responsible for the turbidity loss over storage.6 Higher level of xanthan caused higher cloudiness values especially during the first day of storage.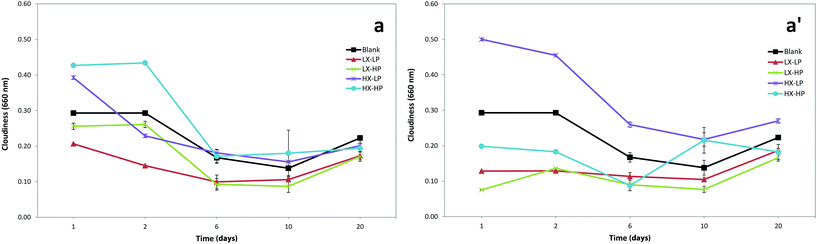 | ||
| Fig. 5 Cloudiness index in functionalised O/W emulsions with OMW at low (a) and high (a′) concentrations. | ||
P-OMW resulted in significant effects on emulsion cloudiness (Table 1), as the measured value was significantly lower when higher concentrations were added. At low P-OMW concentrations (Fig. 5a), higher levels of WPI caused greater cloudiness values, while the opposite effect was found at high P-OMW concentrations (Fig. 5a′). This phenomenon could support the idea of protein–polyphenol interactions occurring in such a system as the hydroxyl group interacts with protein, in turn leading to a lower turbid emulsion. The interaction of P-OMW with xanthan gum was statistically significant for creaming and cloudiness, while its interaction with WPI was significant for all the following parameters: creaming, droplet size, cloudiness and TBARS (Table 1). The general decrease in turbidity could be explained by emulsion phase separation. The serum and cream layers of the separate emulsions were separately analysed to verify this hypothesis, as shown in Fig. 6. The absorbance of serum was significantly higher than that of the cream layer, due to the higher presence of phenolic extract in the aqueous phase, as these phenolic compounds are highly hydro-soluble. A possible influence of the xanthan gum concentration was also observed. This was expected due to the optical properties of the phenolic extracts. Also, the oxidation status of the oil might affect this parameter, as it was suggested that linoleic acid oxidation affects the structural organisation of the micellar/emulsion system, leading to a turbidity increase.21
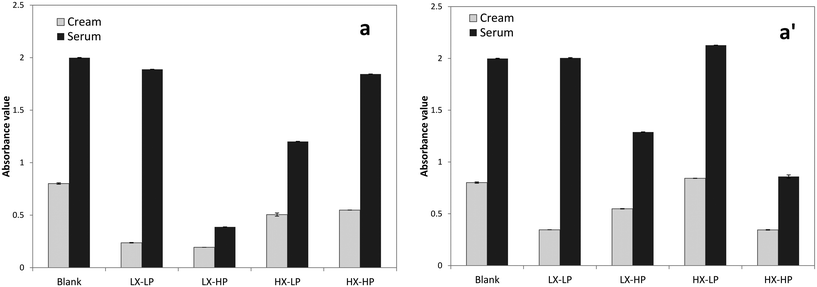 | ||
| Fig. 6 Absorbance at 600 nm of the cream and serum layer of emulsions with low (a) and high (a′) OMW levels, xanthan gum (HX/LX) and WPI (HP/LP) at day 7 of storage (40 °C). | ||
Emulsion viscosity and rheological behaviour
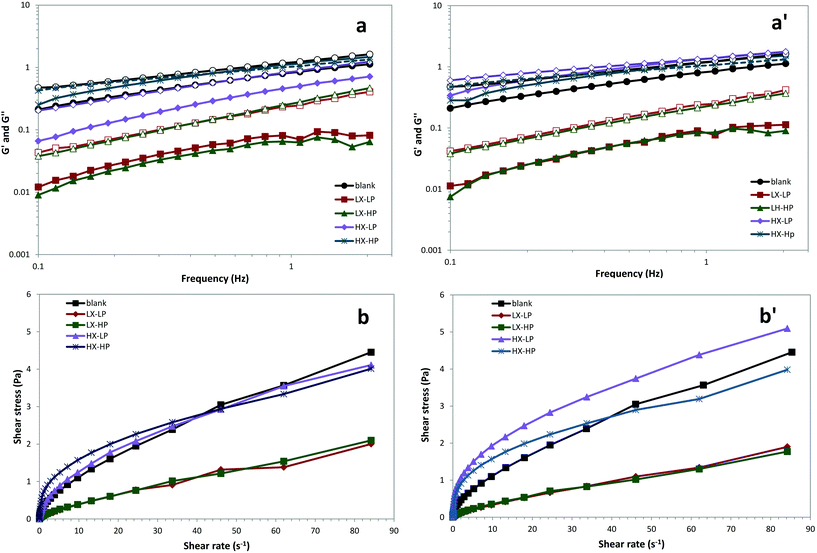
As shown in Fig. 7, the most influencing factor for emulsion viscosity and rheological properties was the xanthan gum concentration, which caused a sharp increase in the measured viscosity. A significant effect was found of xanthan gum concentration on O/W emulsion viscosity, and the shear-thinning behaviour found in the samples is in accordance with previous studies.11,40 The presence of P-OMW caused a further increase in viscosity than the control, probably due to the presence of maltodextrin as a coating agent of the phenolic powder.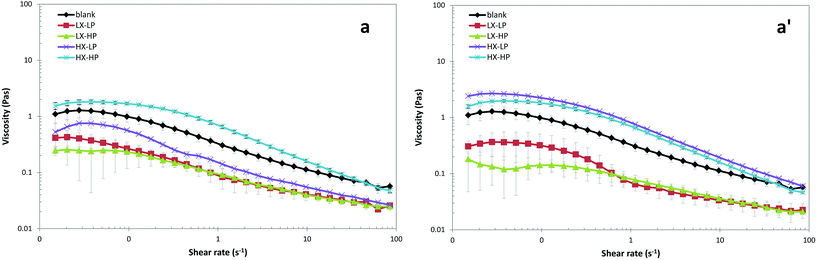 | ||
| Fig. 7 Shear-rate dependence of the apparent viscosity of O/W emulsions with low (a) and high (a′) concentrations of OMW. | ||
The viscoelastic region of the emulsions was determined by an amplitude sweep test, and both levels of xanthan gum resulted in a linear viscoelastic region (data not shown), in accordance with others.34 Small amplitude oscillatory shear measurements to define the oscillatory sweep properties of the emulsions are shown in Fig. 8. In all systems, the value of both moduli increased in the range 0.01–2 Hz (Fig. 8a). The storage and loss moduli (G′–G′′) were unaffected by the protein level, which is in accordance with previous findings.13 The only exception is represented by the emulsion with high P-OMW levels and high xanthan concentrations. In this case, the addition of higher WPI concentration caused a significant decrease in shear stress. The increase in G′ with time was attributed to the formation of strong droplet flocculation, and the gel-like rheological behaviour of protein-stabilized emulsions was attributed to the network structure formed by protein coating.33 As the viscous modulus (G′′) was higher than the elastic one (G′), it indicates pseudo-plastic behaviour. The shear stress over the shear rate of the samples showed that xanthan gum was the main driving factor, while a significant difference was observed between low and high WPI concentrations at high OMW levels (Fig. 8b). This phenomenon is likely due to the weak interactions occurring among WPI and P-OMW.
Oscillatory rheological measurements of storage modulus and loss modulus can indicate whether the emulsion system is strongly or weakly associated. Xanthan gum influenced emulsion yield stress, as low xanthan systems were obviously differentiated from those with high xanthan concentration, while both the presence of WPI and P-OMW did not significantly influence emulsion rheological behaviour. Based on the theory of droplet flocculation, the viscoelasticity of the flocculated emulsions should increase with increasing xanthan gum concentration, whereas the thickening effect of this polysaccharide causes a delay of this phenomenon.40
Lipid oxidation
The results of the lipid oxidation test are reported in Fig. 9. An increase in lipid hydroperoxide concentration was observed up to circa 1 week upon storage in both systems (low and high P-OMW), with a limited extent in those with high WPI concentration (Fig. 9a). These results indicate a possible antioxidant effect of WPI, as suggested by other workers.41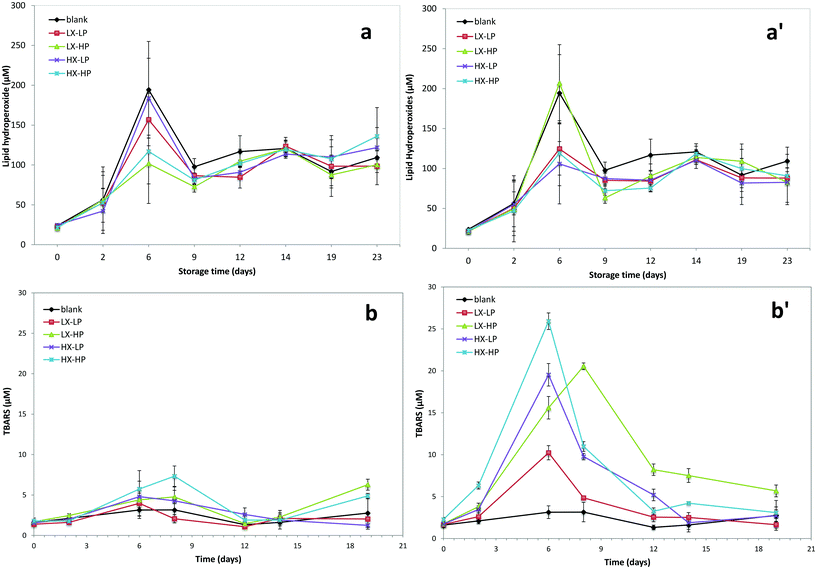 | ||
| Fig. 9 Lipid hydroperoxides (a) and secondary oxidation products TBARS (b) upon the storage of emulsions. Phenolic compound concentrations were 150 (a, b) and 750 (a′, b′) mg kg−1 expressed as OHTy. | ||
The concentration of primary oxidation products followed a stabilisation in the final storage period. In the latter period, a slight increase was observed in the system with low P-OMW, while in the high P-OMW one the oxidation products were lower in concentration than the control. The hydroperoxide concentration was dramatically lower in emulsions with phenolics, LX-HP being the only exception. However, a clear statistical significance was not reached for primary oxidation products depending on emulsion formulation, except for storage time (Table 1).
The results obtained for TBARS were quite different (Fig. 9b), as their concentration in systems with high P-OMW was circa 3 fold higher than in systems with low P-OMW. Also in this case, the highest concentration was obtained at about 1 week storage. Generally, high WPI concentration was associated with higher TBARS production, especially when olive phenolics were present at high concentration. In the latter case the TBARS value was always higher than the control. WPI and xanthan gum significantly (p < 0.05) influenced the TBARS concentration, and the interaction of WPI with P-OMW was also statistically significant (Table 1).
The higher TBARS concentrations are related to the decomposition of primary oxidation products, and therefore the formation of aldehydes and other products leading to off-flavour in the final product. In accordance with Di Mattia et al.,22,23 the presence of phenolic extracts in systems with low P-OMW had only limited effects on lipid hydroperoxide formation. On the contrary, a high P-OMW concentration was associated with a higher oxidation level in the system with high WPI (HX-HP). Accordingly, certain phenolic compounds, e.g. catechin, were reported to cause higher TBARS concentration and they are not able to delay the formation of primary oxidation products, showing pro-oxidant activity.22,23
Some phenolic compounds can exert the opposite effect on chemical stability, e.g. caffeic acid retard the production of peroxide while promoting at the same time higher concentration of secondary oxidation products.18 Zhou and Elias42 confirmed the potential of some phenolic compounds to act as antioxidants or pro-oxidants in O/W emulsions, being strongly influenced by the pH and their concentration. The authors reported that epigallocatechin-gallate had higher TBARS concentration (in the range pH 2–4) in the range 1–100 μM. Up to 500 μM, the observed effect was lower, and the authors explained this as due to the competition between antioxidant and pro-oxidant effects.42 In the present paper we used directly a complex phenolic extract, which is obviously different from pure laboratory phenolics and therefore their effect on lipid oxidation is not straightforward. Moreover, the possible presence of metallic ions as traces in our phenolic extract might have affected the antioxidant activity of the P-OMW, influencing lipid oxidation. Metallic ions affect O/W emulsion oxidation and a pro-oxidant effect was demonstrated for pure hydroxytyrosol and oleuropein in the presence of ferric ions, depending on the pH.17 This stresses the importance of further studying the lipid oxidation in O/W emulsions considering the effects of phenolics and proteins, as well as the presence of other stabilizers.
The interaction between olive oil phenolic compounds and food proteins was studied previously using other techniques.26 However, sodium caseinate, bovine serum albumin, β-lactoglobulin and gelatin were used, in comparison to gallic acid and tannic acid, which are chemically different from the compounds used in the present paper. A relatively weak binding capacity was shown, as both OHTy and Ty had little or no binding. However, previous studies have shown that free phenols in the aqueous phase can bind to proteins, both in bulk systems and in emulsions, while the phenomenon was more limited in the latter case.26 Milk proteins can be considered to be antioxidant compounds, e.g. casein hydrolysates and caseinophosphopeptides, capable of binding transitional metals and limiting lipid oxidation in O/W emulsions.43 However, the effectiveness of WPI in emulsions was assessed without considering OMW or olive phenolics in such a system.13
Whereas P-OMW seem not to have positive effects on hydroperoxides or TBARS, their effectiveness in vivo is likely, as the polysaccharide coating could be broken during digestion and therefore its phenolic content may become available to exert its antioxidant effects.
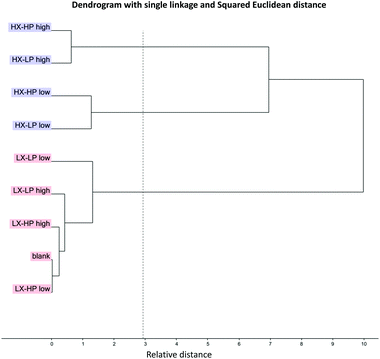
A cluster analysis was carried out to understand the similarity among the samples, based on the creaming index, mean droplet size, apparent viscosity, peroxide value and TBARS parameters after 2 weeks of storage. As shown in Fig. 10, the clustering resulted in three main groups, mainly differentiated by xanthan gum, which was in fact the main driver for emulsion behaviour, and olive phenolic extracts in the system with high level of xanthan gum. Finally, the blank sample with no added phenolic compounds was clustered at a short distance to the LX-HP sample. This result confirmed the important effect of polyphenols in an emulsion system, as discussed previously.
Conclusions
This paper reported on the stability of the model oil-in-water emulsions functionalized with polyphenols by the addition of olive mill wastewater. The presence of P-OMW can lead to higher creaming rate and physical instability; however it was shown that its influence on the rheological behaviour was limited. At high concentrations of P-OMW and WPI, both the physical and chemical stability were negatively affected, showing particularly high TBARS values. The addition of phenolic extracts is not straightforward in terms of oxidative stability, and their interaction with the hydrocolloids, mainly whey proteins, can exert a significant influence on physical stability, but can also exert a pro-oxidant effect depending on its level. These results suggest the importance of accurately choosing the concentration of stabilizers, and a practical consequence is the suggestion of using low WPI concentrations when P-OMW is added in food emulsions.The knowledge of olive O/W emulsion stability is of great importance in the formulation of emulsion-based food products having enhanced health properties owing to the widely known health benefits of olive oil and the antioxidant activity of olive phenolics. Moreover, the possibility of using by-products from the olive oil extraction process with high environmental impact is of interest for the food industry as well as for food scientists to incorporate hydrophilic phenolic compounds into fatty foods. This research has great potential in terms of applicability at the industrial level, and it is hoped that it will contribute to the “hot topic” of environmental pollution reduction and to the creation of innovative functional foods. Further work is needed to better define the optimal concentration of each hydrocolloid according to the final use of the product, as well as to understand the in vivo effect of the consumption of these functional emulsions.
Abbreviations
| OMW | Olive mill wastewater |
| P-OMW | Powder extracts from OMW |
| WPI | Whey protein isolate |
| O/W | Oil-in-water |
| Ty | Tyrosol |
| OHTy | Hydroxytyrosol |
| PV | Peroxide value |
| TBARS | Thiobarbituric acid derivative species |
Abbreviations used for the emulsion systems:
| HX | High xanthan gum (0.2%) |
| LX | Low xanthan gum (0.06%) |
| HP | High WPI (0.5%) |
| LP | Low WPI (0.13%) |
Acknowledgements
Dr Mark Traynor and Dr Lubna Ahmed are acknowledged for their assistance in emulsion production and their support. Prof. Vincenzo Fogliano is kindly acknowledged for useful suggestions and discussion. We thank Dr Alberto Fiore and Dr Antonello Paduano for spray-drying of polyphenols and HPLC analysis. Dr Maria Savarese and IOBM srl (Montesarchio, Italy) are acknowledged for supplying olive oil. Mr John Jones, Institute of Technology Tallaght (Dublin), is acknowledged for his help in droplet size distribution analysis.References
- M. Servili, M. Baldioli, R. Selvaggini, E. Miniati, A. Macchioni and G. Montedoro, J. Am. Oil Chem. Soc., 1999, 76, 873–882 CrossRef CAS.
- H. K. Obied, M. S. Allen, D. R. Bedgood, P. D. Prenzler, K. Robards and R. Stockmann, J. Agric. Food Chem., 2005, 53, 823–837 CrossRef CAS PubMed.
- P. Paraskeva and E. Diamadopoulos, J. Chem. Technol. Biotechnol., 2006, 81, 1475–1485 CrossRef CAS.
- E. O. Akdemir and A. Ozer, Desalination, 2009, 249, 660–666 CrossRef CAS.
- P. E. Gkoutsidis, K. B. Petrotos, M. I. Kokkora, A. D. Tziortziou, K. Christodouloulis and P. Goulas, Desalin. Water Treat., 2011, 30, 237–246 CrossRef CAS.
- D. J. McClements, Food emulsions: principles, practices, and techniques, CRC Press, 2004 Search PubMed.
- E. Dickinson, Food Hydrocolloids, 2009, 23, 1473–1482 CrossRef CAS.
- T. Moschakis, B. S. Murray and C. G. Biliaderis, Food Hydrocolloids, 2010, 24, 8–17 CrossRef CAS.
- A. Paraskevopoulou, D. Boskou and V. Kiosseoglou, Food Chem., 2005, 90, 627–634 CrossRef CAS.
- C. Huck-Iriart, M. S. Álvarez-Cerimedo, R. J. Candal and M. L. Herrera, Curr. Opin. Colloid Interface Sci., 2011, 16, 412–420 CrossRef CAS.
- C. Sun, S. Gunasekaran and M. P. Richards, Food Hydrocolloids, 2007, 21, 555–564 CrossRef CAS.
- D. Djordjevic, D. J. McClements and E. A. Decker, J. Food Sci., 2004, 69, C356–C362 CrossRef CAS.
- C. Sun and S. Gunasekaran, Food Hydrocolloids, 2009, 23, 165–174 CrossRef CAS.
- T. Dapčević Hadnađev, P. Dokić, V. Krstonošić and M. Hadnađev, Eur. J. Lipid Sci. Technol., 2013, 115, 313–321 CrossRef.
- L. Lethuaut, F. Métro and C. Genot, J. Am. Oil Chem. Soc., 2002, 79, 425–430 CrossRef CAS.
- K. A. Silva, M. H. Rocha-Leão and M. A. Z. Coelho, J. Food Eng., 2010, 97, 335–340 CrossRef CAS.
- F. Paiva-Martins and M. H. Gordon, J. Am. Oil Chem. Soc., 2002, 79, 571–576 CrossRef CAS.
- A.-D. M. Sørensen, A.-M. Haahr, E. M. Becker, L. H. Skibsted, B. Bergenståhl, L. Nilsson and C. Jacobsen, J. Agric. Food Chem., 2008, 56, 1740–1750 CrossRef PubMed.
- E. Bylaite, T. Nylander, R. Venskutonis and B. Jönsson, Colloids Surf., B, 2001, 20, 327–340 CrossRef CAS PubMed.
- S. Protonotariou, V. Evageliou, S. Yanniotis and I. Mandala, J. Food Eng., 2013, 117, 124–132 CrossRef CAS.
- T. G. Sotiroudis, G. Sotiroudis, N. Varkas and A. Xenakis, J. Am. Oil Chem. Soc., 2005, 82, 415–420 CrossRef CAS.
- C. Di Mattia, G. Sacchetti, D. Mastrocola and P. Pittia, Food Res. Int., 2009, 42, 1163–1170 CrossRef CAS.
- C. Di Mattia, G. Sacchetti, D. Mastrocola, D. K. Sarker and P. Pittia, Food Hydrocolloids, 2010, 24, 652–658 CrossRef CAS.
- M. P. Almajano and M. H. Gordon, J. Am. Oil Chem. Soc., 2004, 81, 275–280 CrossRef CAS.
- M. Kumamoto, T. Sonda, K. Nagayama and M. Tabata, Biosci., Biotechnol., Biochem., 2001, 65, 126–132 CrossRef CAS PubMed.
- A. H. Pripp, R. Vreeker and J. van Duynhoven, J. Sci. Food Agric., 2005, 85, 354–362 CrossRef CAS.
- A. D. Troise, A. Fiore, A. Colantuono, S. Kokkinidou, D. G. Peterson and V. Fogliano, J. Agric. Food Chem., 2014, 62, 10092–10100 CrossRef CAS PubMed.
- M. Traynor, R. Burke, J. M. Frias, E. Gaston and C. Barry-Ryan, Int. Food Res. J., 2013, 20, 9 Search PubMed.
- N. Caporaso, M. Savarese, A. Paduano, G. Guidone, E. De Marco and R. Sacchi, J. Food Compos. Anal., 2015, 40, 154–162 CrossRef CAS.
- E. Dickinson, S. J. Radford and M. Golding, Food Hydrocolloids, 2003, 17, 211–220 CrossRef CAS.
- U. Klinkesorn, P. Sophanodora, P. Chinachoti and D. J. McClements, Food Res. Int., 2004, 37, 851–859 CrossRef CAS.
- H. Mirhosseini, C. P. Tan, A. Aghlara, N. S. Hamid, S. Yusof and B. H. Chern, Carbohydr. Polym., 2008, 73, 83–91 CrossRef CAS.
- E. Dickinson and M. Golding, Food Hydrocolloids, 1997, 11, 13–18 CrossRef CAS.
- V. Krstonošić, L. Dokić, P. Dokić and T. Dapčević, Food Hydrocolloids, 2009, 23, 2212–2218 CrossRef.
- R. Chanamai and D. McClements, J. Food Sci., 2001, 66, 457–463 CrossRef CAS.
- A. Genovese, N. Caporaso, L. De Luca, A. Paduano and R. Sacchi, J. Agric. Food Chem., 2015, 63, 3838–3850 CrossRef CAS PubMed.
- F. Yuan, Y. Gao, E. A. Decker and D. J. McClements, J. Food Eng., 2013, 114, 1–7 CrossRef CAS.
- Y. Hemar, M. Tamehana, P. Munro and H. Singh, Food Hydrocolloids, 2001, 15, 513–519 CrossRef CAS.
- A. Ye, S. Anema and H. Singh, J. Dairy Sci., 2004, 87, 4013–4022 CrossRef CAS PubMed.
- T. Moschakis, B. S. Murray and E. Dickinson, J. Colloid Interface Sci., 2005, 284, 714–728 CrossRef CAS PubMed.
- M. Hu, D. J. McClements and E. A. Decker, J. Agric. Food Chem., 2003, 51, 1696–1700 CrossRef CAS PubMed.
- L. Zhou and R. J. Elias, Food Chem., 2013, 138, 1503–1509 CrossRef CAS PubMed.
- M. Díaz, C. M. Dunn, D. J. McClements and E. A. Decker, J. Agric. Food Chem., 2003, 51, 2365–2370 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2016 |