Effect of chronic consumption of blackberry extract on high-fat induced obesity in rats and its correlation with metabolic and brain outcomes
Manuela
Meireles
 a,
Luís M.
Rodríguez-Alcalá
b,
Cláudia
Marques
a,
Sónia
Norberto
a,
Joana
Freitas
a,
Iva
Fernandes
c,
Nuno
Mateus
c,
Ana
Gomes
b,
Ana
Faria
acd and
Conceição
Calhau
*ae
a,
Luís M.
Rodríguez-Alcalá
b,
Cláudia
Marques
a,
Sónia
Norberto
a,
Joana
Freitas
a,
Iva
Fernandes
c,
Nuno
Mateus
c,
Ana
Gomes
b,
Ana
Faria
acd and
Conceição
Calhau
*ae
aDepartamento de Bioquímica, Faculdade de Medicina, Universidade do Porto, Al. Prof. Hernâni Monteiro, 4200-319 Porto, Portugal. E-mail: ccalhau@med.up.pt; Fax: +351 22 551 3624; Tel: +351 22 551 3624
bCentro de Biotecnologia e Química Fina (CBQF), Escola Superior de Biotecnologia, Universidade Católica Portuguesa, Rua Dr. António Bernardino de Almeida, 4200-072 Porto, Portugal
cREQUIMTE, Laboratório Associado de Química Verde, Faculdade de Ciências, Universidade do Porto, 4169-009 Porto, Portugal
dFaculdade de Ciências da Nutrição e Alimentação, Universidade do Porto, Rua Dr. Roberto Frias, 4200-465 Porto, Portugal
eCentro de Investigação em Tecnologias e Sistemas de Informação em Saúde (CINTESIS), Universidade do Porto, Al. Prof. Hernâni Monteiro, 4200-319 Porto, Portugal
First published on 25th September 2015
Abstract
Flavonoids have been presented as potential protectors against metabolic and cognitive dysfunction. However, mechanisms underlying these ‘claims’ have not been sufficiently explored. To analyse the effect of long-term supplementation with blackberry extract (BE) in the context of a high-fat or a standard diet, Wistar rats were divided into 4 groups (n = 6) fed with a standard or a high-fat diet, with or without BE supplementation at 25 mg per kg body weight per day. A high-fat diet significantly impaired glucose tolerance and increased body weight, caloric ingestion, very-low-density lipoprotein, triglycerides and cholesterol. Furthermore, it was observed that a high-fat diet increased dopamine content in the prefrontal cortex and decreased brain derived neurotrophic factor (BDNF) levels both in the prefrontal cortex and in plasma. BE supplementation only affected some of these aspects. BE slightly improved glucose metabolism and significantly decreased levels of lactate, independent of diet. BE decreased levels of BDNF and also interacted with the dopaminergic system, increasing dopamine turnover in the striatum, and reverting dopamine content induced by a high-fat diet in the prefrontal cortex. This study shows that, despite some particular benefits of anthocyanin supplementation, some long-term effects may not be desirable and further studies are needed to optimize ingestion conditions.
1. Introduction
Obesity is a health problem that has reached epidemic proportions. In some European countries, more than one quarter of the population are already obese, presenting a body mass index (BMI) of greater than 30 kg m−2.1 This phenomenon is of equal or larger proportions in several other industrialized countries, i.e. in USA the incidence of obesity reached 35% in 2011–2012.2 Overall, more than 60% of the US and European adult population are obese or overweight, with BMI > 25 kg m−2.3 This epidemic represents an economic and social burden since several co-morbidities are associated with obesity.4 People with obesity have, among other features, an increased risk of suffering from diabetes, hypertension and cardiovascular diseases, cancer, and neurodegenerative diseases.5 In the past decade, much attention has been paid to the potential negative effects of obesity on brain function and general risk of dementia.3,6 Like obesity, dementia is also becoming a worldwide problem and an international priority, with millions of new cases every year.7 As obesity and its co-morbidities are associated with brain dysfunction, the development of therapeutic strategies to treat one condition can have worthy improvements in the other.Diet is an undeniable modifiable factor which may influence both the risk of obesity and impaired brain function. The exacerbation of both conditions by westernized diets, typically rich in fat and sugar, is well documented. In vivo studies have also shown that animals fed high-fat diets have several brain disorders, among them dysfunction of the dopaminergic system and impairment of neurogenesis.8–10
Fruits and vegetables, in contrast to high-fat and high-sugar diets, have been proven to reduce the risk of obesity and associated co-morbidities.11,12 In addition, intake of fruits and vegetables has been associated with a decreased risk of cognitive decline or Parkinson’s disease, in part due to their high content of natural polyphenols, including flavonoids.13,14 In recent years, berries have attracted interest as a nutritional approach to improve health, metabolic and cognitive outcomes.15–17 Blackberries, in particular, are naturally rich in flavonoids, specifically in anthocyanins, and although blackberry extracts have already shown positive effects in improving cognitive function and antioxidant status in animal studies,18–21 there remains a need to further explore and clarify the full potential of anthocyanins.
This study aimed to explore the beneficial potential of long-term supplementation with blackberry anthocyanin extract in the context of a standard or a high-fat diet. For this purpose male Wistar rats fed with a standard or a high-fat diet for 17 weeks were supplemented with blackberry anthocyanin extract (25 mg per kg b.w.) and metabolic evolution as well as biomarkers of brain function were analysed.
2. Methods
2.1. Animal care and study design
Twenty-four male Wistar rats (200–250 g body weight; 7 weeks) were acquired from Harlan Laboratories (Santiga, Spain) and after two weeks of acclimatization were divided into four groups (n = 6 rats per group): (C) standard diet; (BE) standard diet + blackberry extract; (HF) high-fat diet; (HFBE) high-fat diet + blackberry extract. Animals were fed ad libitum with commercial diets: standard chow (4% fat) from Harlan (2014S, Teklad Diets, Harlan Laboratories, Santiga, Spain) and high-fat chow (45% fat) from Research Diet D12451 (New Brunswick, NJ, USA) for 17 weeks. Blackberry extract dose was weekly weight adjusted (25 mg per kg body weight), dissolved daily in sterile water and embedded in food pellets that animals had daily access to. Animals were maintained at 23–25 °C with a 12/12 h light–dark cycle, and housed two per cage. Food ingestion was measured twice a week. Animal handling and housing protocols followed European Union guidelines (86/609/EEC) and the Portuguese Act (129/92) for the use of experimental animals. The study obtained ethical approval from the Ethical Committee of the Faculty of Medicine, University of Porto and the São João Hospital Center.2.2. Preparation of blackberry extract
Preparation of blackberry anthocyanin extract was achieved using previously described methods.22,23 Briefly, blackberries (Rubus fruticosus) were extracted with 50% aqueous ethanol (pH 1.5, acidified with HCl) for 24 hours at 22 °C. The solution obtained was filtered (50 μm nylon membrane) and concentrated using a rotary evaporator under reduced pressure at 30 °C. The concentrated extract was added to a polyamide gel column (mesh 100–120) to remove sugars. The sugar-free anthocyanin extract was freeze-dried and stored at −20 °C. The anthocyanin extract was analyzed by HPLC at 520 nm, as previously described.24 Following preparative HPLC, the purified extract was characterised for anthocyanin content by analytical HPLC coupled to UV-Vis, DAD-ESI/MS and NMR techniques.2.3. Insulin tolerance test and glucose tolerance test
Insulin and glucose tolerance tests were performed at weeks 3, 8 and 15 of the treatment period with one day intervals between tests. For the insulin tolerance test, after 5 hours of food deprivation, a solution of insulin (0.5 U per kg weight) was administered by intraperitoneal injection.25 Glucose levels were measured from tail blood with a Precision Xceed® glucometer before (0) and 30, 60, 90 and 120 minutes after insulin injection. The glucose tolerance test was performed after 5 hours of food deprivation; a solution of glucose (2 g per kg weight) was administered by oral gavage as previously described.26 Glucose levels were measured before (0) and 15, 30, 60, 90 and 120 minutes after glucose administration.2.4. Blood pressure measurements
Systolic blood pressure was measured with a tail cuff method.27 To minimize stress-induced variations in blood pressure all measurements were taken in a peaceful environment and after two trials had been performed in the previous week to allow animals to become accustomed to the procedure. Values were registered when consistent between three consecutive measurements, and when the pulse was stable. This procedure took place at weeks 9 and 14 of the treatment period.2.5. Tissue collection and body composition measurements
At the end of 17 weeks animals were anesthetized with ketamine + xylazine (50 mg kg−1 + 1 mg kg−1). Nasoanal lengths and abdominal circumference were obtained using a measuring tape, and the body composition of each rat was determined by bioelectrical impedance (quantum/S bioelectrical impedance analyser, RJL Systems, Akern SRL, Florence, Italy), according to the procedure described in the literature.28 Animals were maintained with isoflurane during blood collection from the left ventricle. Blood was collected with heparinised needles and PMSF (phenylmethylsulfonyl fluoride), a protease inhibitor, was added at a final concentration of 100 μM. After centrifugation at 2000g for 15 min, plasma was collected and stored at −80 °C. Rats were decapitated for brain removal. Prefrontal cortex, striatum, the remaining brain, liver, subcutaneous adipose tissue (scAT) and mesenteric adipose tissue (mAT) were collected, immediately frozen with liquid nitrogen and stored at −80 °C until use. A small portion of scAT and mAT from each animal was also fixed in 10% buffered formaldehyde.2.6. Biochemical analysis
For urine collection, rats were placed in metabolic cages, after being acclimated during the previous week. Routine plasma and urine biochemical analyses were performed in a certified Clinical Analysis Laboratory. In addition, adiponectin, leptin and brain derived neurotrophic factor (BDNF) were quantified using the following enzyme linked immunoabsorbent assay (ELISA) commercial kits: adiponectin (Life Technologies Ltd, Paisley, UK), leptin (Merck Millipore, Madrid, Spain) and chemiKine brain derived neurotrophic factor (Merck Millipore, Madrid, Spain). For BDNF determination, a small portion of the whole brain was homogenized using 100 mM Tris/HCl, pH 7, containing 2% bovine serum albumin (BSA), 1 M NaCl, 4 mM EDTA·Na2, 2% Triton X-100, 0.1% sodium azide and a protease inhibitor (cOmplete mini, Roche Diagnostics, USA). Homogenates were prepared using 4 μL of the described buffer per mg of tissue weight. All following procedures in plasma and brain homogenate were performed according to the manufacturers’ instructions.2.7. Morphometric analysis of adipose tissue
Following at least 48 h (4 °C) of formaldehyde fixation, the adipose tissues were dehydrated and finally embedded in paraffin. All samples were coded for a blind analysis and 3 μm-thick sections were obtained with a Leica® Microtome (RM2125RT, Lisbon, Portugal), and stained with haematoxylin and eosin to assess morphology. Digital images were acquired with a fluorescence microscope (Nikon Eclipse 50i®, Melville, USA), at a magnification of ×200 from randomly-selected different optical fields. Adipocyte area measurement was performed in 100 random adipocytes, using ImageJ Software® (National Institute of Health, Bethesda, USA).2.9. Fatty acid analysis
For analysis of the total fatty acid (FA) composition, 200 mg of liver and 30 mg of adipose tissue were accurately weighed and prepared as previously described.29 For quantification purposes, samples were mixed with 100 μL of tritridecanoin (1.34 mg ml−1), used as an internal standard prior to derivatization. Fatty acid methyl esters (FAME) were analyzed in a gas chromatograph HP6890A (Hewlett-Packard, Avondale, PA, USA), equipped with a flame-ionization detector (GLC-FID) and a BPX70 capillary column (50 m × 0.32 mm × 0.25 μm; SGE Europe Ltd, Courtaboeuf, France). Analysis conditions were as follows: injector (split 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; injection volume 1 μl) and detector temperatures were 250 °C and 270 °C respectively, carrier gas was hydrogen (11 psi) and the oven temperature program was started at 60 °C (held for 2 min) raised by 10 °C min−1 to 135 °C (held for 2 min), then raised by 10 °C min−1 to 165 °C (held for 2 min) and finally raised by 10 °C min−1 to 230 °C (held for 7 min). Supelco 37 and CRM-164 were used for the identification of fatty acids. GLC-Nestlé36 was assayed for calculation of response factors and detection and quantification limits (LOD: 0.079 μg FA ml−1; LOQ: 0.264 μg FA ml−1).
1; injection volume 1 μl) and detector temperatures were 250 °C and 270 °C respectively, carrier gas was hydrogen (11 psi) and the oven temperature program was started at 60 °C (held for 2 min) raised by 10 °C min−1 to 135 °C (held for 2 min), then raised by 10 °C min−1 to 165 °C (held for 2 min) and finally raised by 10 °C min−1 to 230 °C (held for 7 min). Supelco 37 and CRM-164 were used for the identification of fatty acids. GLC-Nestlé36 was assayed for calculation of response factors and detection and quantification limits (LOD: 0.079 μg FA ml−1; LOQ: 0.264 μg FA ml−1).
2.10. Catecholamine determination
The quantification of dopamine (DA) and its metabolite 3,4-dihydroxyphenylacetic acid (DOPAC) in striatum was performed by high pressure liquid chromatography with electrochemical detection (HPLC-ED), as previously described.30 In brief, aliquots of 1.5 ml of 0.2 M perchloric acid in which tissues were kept were placed in 5 ml conical-based glass vials with 50 mg alumina, and the pH of the samples was immediately adjusted to 8.6 with Tris buffer. The adsorbed catecholamines were then eluted from the alumina with 200 μL of 0.2 M perchloric acid and centrifuged at 1200g in Spin-X tubes with 0.22 μm pore membrane (Costar®, Tewksbury MA, EUA); 50 μL of the eluant was injected into HPLC (Gilson Medical Electronics, Villiers, le Bel, France) and 3,4-dihydroxybenzylamine was used as an internal standard. The results were adjusted for tissue weight.2.11. Statistical analysis
All the groups were tested for the effects of diet (D), treatment (BE) and their interaction (D × BE) by two-way ANOVA using GraphPad Prism® 6.0 Software. When interaction between diet and treatment were significantly different, means were compared using Fisher's LSD multiple-comparison post-test. Correlations were tested using the Pearson correlation test. Statistical significance was considered when p < 0.05.3. Results
3.1. Body weight, weight gain and caloric ingestion
All animals started the treatment at similar weights (246.5 ± 1.9 g), and at end of the treatment animals maintained similar weights between groups C and BE (429 ± 28 g and 433 ± 29 g, respectively). The body weights of animals from HF and HFBE were 551 ± 68 g and 581 ± 63 g, respectively. The increase in body weight over time was more pronounced in groups fed the HF diet (Fig. 1A). Weight gain was significantly increased by diet (Fig. 1B). Effect of supplementation with BE on body weight gain was not significant.Food ingestion was converted from g to kCal, knowing that standard and high-fat chow have 2.9 kCal and 4.73 kCal per gram, respectively. As show in Fig. 1C, caloric ingestion was affected by both diet and by BE supplementation (Fig. 1C). Curiously, there was a significant interaction between the effects of type of diet and BE supplementation on caloric ingestion. Caloric ingestion of animals supplemented with BE on the HF diet was slightly higher (82 kCal per animal per day) than those not supplemented (75 kCal per animal per day), however it was not reflected in weight gain.
3.2. Metabolic parameters
Glucose and insulin tolerance tests were performed at weeks 3, 8 and 15 of the treatment. HF diet impaired the overall glucose sensibility of the animals, seen as an increase of total area under the curve (AUC) (Fig. 2A). This effect was seen as early as after three weeks of study, and it was maintained until the end of treatment (Fig. 2B–D). Animals from the BE group presented a tendency to a lower AUC, significant at the end of the test on week 8.After 15 weeks of treatment, both animals’ basal blood glucose and glucose tolerance response were significantly affected by diet but not by BE supplementation (Fig. 2D).
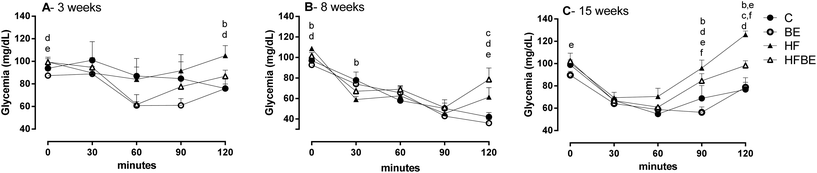
Glycaemic levels in insulin tolerance test was affected by diet after only three weeks of treatment (Fig. 3A). BE supplementation significantly increase insulin response at 60 min. Curiously, at week 8 (Fig. 3B) animals fed with the HF diet had a higher response to insulin, presenting a lower peak at 30 min; however the animals fed with the standard diet were the ones that sustained the decrease in glycaemia as seen at 120 min. As can be seen in Fig. 3C, after 15 weeks, animals from the HFBE group had a better response to insulin than those from the HF group.
Systolic blood pressure seemed to be affected by diet but not by BE supplementation after 8 weeks of treatment. No effect was seen after 14 weeks of treatment (Table 1).
| C | BE | HF | HFBE | D | BE | D × BE | |
|---|---|---|---|---|---|---|---|
| SBP – systolic blood pressure. Standard diet (C); standard diet + blackberry anthocyanin extract (BE); high-fat diet (HF) or high-fat diet + blackberry anthocyanin extract (HFBE). Values are expressed as mean (SD). The significance of diet (D), blackberry extract (BE) or interaction between both factors (D × BE) were tested by two-way ANOVA and expressed as p values. | |||||||
| SBP – 9 weeks | 137.5 (11.2) | 129.6 (5.7) | 145.2 (9.8) | 139.7 (14.5) | 0.058 | 0.15 | 0.79 |
| SBP – 15 weeks | 129.9 (14.3) | 133.3 (9.0) | 137.3 (13.4) | 135.9 (20.5) | 0.42 | 0.86 | 0.69 |
3.3. Biochemical parameters
At the end of treatment plasmatic biochemical analyses were performed. As described in Table 2, the HF diet affected lipid metabolism, increasing very low density lipoprotein (VLDL), triglycerides (TG) and cholesterol levels of the animals. Regarding hepatic enzymes, only alkaline phosphatase (ALP) activity was strongly increased by the HF diet. Also, levels of urea were increased by the HF diet. BE supplementation decreased aspartate aminotransferase activity (ASAT) and decreased creatinine kinase activity (CK). Plasmatic lactate levels were strongly affected by BE supplementation, but not with the high-fat diet. Interaction between diet and BE supplementation affected albumin and creatinine levels. While in C animals albumin seemed to decrease and creatinine increase, the opposite effect was seen in animals fed with the HF diet.| C | BE | HF | HFBE | D | BE | D × BE | |
|---|---|---|---|---|---|---|---|
| Standard diet (C); standard diet + blackberry anthocyanin extract (BE); high-fat diet (HF) or high-fat diet + blackberry anthocyanin extract (HFBE); HDL – high density lipoprotein; VLDL – very low density lipoprotein; TG – triglycerides; ASAT – aspartate aminotransferase; ALAT – alanine aminotransferase; ALP – alkaline phosphatase; CK – creatinine kinase. Values are expressed as mean (SD). The significance of diet (D), blackberry extract (BE) or interaction between both factors (D × BE) were tested by two-way ANOVA and expressed as p values. Post-hoc Fishers's LSD test was performed when interaction between factors was present. Mean values with different superscript letters are significantly different (p < 0.05). | |||||||
| Plasma analyses | |||||||
| Cholesterol (mg dL−1) | 103.8 (16.1) | 100.0 (19.7) | 105.6 (17.2) | 133.2 (13.6) | <0.05 | 0.14 | 0.05 |
| HDL (mg dL−1) | 80.8 (14.9) | 88.3 (16.7) | 75.0 (12.8) | 89.2 (13.8) | 0.72 | 0.14 | 0.64 |
| VLDL (mg dL−1) | 30.0 (9.5) | 26.3 (2.5) | 37.2 (5.9) | 36.3 (5.3) | <0.01 | 0.45 | 0.64 |
| TG (mg dL−1) | 150.2 (47.6) | 131.5 (11.8) | 185.0 (29.8) | 181.3 (27.2) | <0.01 | 0.45 | 0.62 |
| ASAT (U L−1 at 37 °C) | 163.6 (67.1) | 101.0 (31.0) | 168.6 (41.6) | 117.3 (25.0) | 0.61 | <0.05 | 0.78 |
| ALAT (U L−1 at 37 °C) | 47.2 (9.2) | 51.5 (11.9) | 41.4 (8.2) | 41.2 (4.5) | 0.07 | 0.60 | 0.63 |
| ALP (U L−1 at 37 °C) | 79.7 (11.6) | 92.8 (5.8) | 132.0 (29.8) | 135.2 (17.0) | <0.001 | 0.33 | 0.55 |
| Proteins (g dL−1) | 6.08 (0.35) | 6.00 (0.22) | 6.16 (0.19) | 6.47 (0.33) | 0.05 | 0.41 | 0.15 |
| Albumin (g dL−1) | 3.67 (0.2)a,b | 3.37 (0.2)a | 3.52 (0.2)a,b | 3.77 (0.3)b | 0.26 | 0.81 | <0.05 |
| Creatinine (mg dL−1) | 0.40 (0.10)a | 0.53 (0.02)b | 0.52 (0.05)b | 0.44 (0.11)a,b | 0.62 | 0.54 | <0.01 |
| CK (U L−1 at 37 °C) | 1087 (358) | 443 (189) | 783 (236) | 485 (155) | 0.27 | <0.001 | 0.15 |
| Urea (mg dL−1) | 30.2 (1.7) | 32.2 (2.6) | 36.6 (4.5) | 40.2 (5.8) | <0.001 | 0.14 | 0.67 |
| Uric acid (mg dL−1) | 0.83 (0.17) | 0.74 (0.14) | 0.92 (0.23) | 0.93 (0.31) | 0.20 | 0.71 | 0.62 |
| Iron (μg dL−1) | 291.0 (35.3) | 271.8 (37.6) | 259.4 (32.2) | 268.2 (14.8) | 0.29 | 0.75 | 0.40 |
| Sodium (mmol L−1) | 140.8 (4.6) | 142.5 (3.3) | 136.0 (1.6) | 141.5 (4.7) | 0.14 | 0.06 | 0.32 |
| Potassium (mmol L−1) | 5.50 (0.75) | 5.55 (0.53) | 5.78 (0.61) | 6.00 (0.82) | 0.29 | 0.69 | 0.80 |
| Chlorides (mmol L−1) | 103.2 (3.7)a,b | 100.8 (1.6)a | 100.2 (3.3)a | 107.8 (8.0)b | 0.40 | 0.27 | <0.05 |
| Calcium (mg dL−1) | 9.9 (0.97) | 10.7 (0.30) | 10.7 (0.45) | 11.1 (0.59) | 0.07 | 0.07 | 0.60 |
| Phosphorus (mg dL−1) | 10.3 (0.71) | 10.2 (1.25) | 10.3 (0.37) | 10.8 (0.66) | 0.40 | 0.67 | 0.46 |
| Magnesium (mg dL−1) | 2.66 (0.16) | 2.66 (0.16) | 2.32 (0.23) | 2.20 (0.17) | <0.001 | 0.40 | 0.36 |
| Lactate (mg dL−1) | 7.85 (1.94) | 5.58 (1.00) | 7.90 (1.85) | 3.80 (1.82) | 0.25 | <0.001 | 0.23 |
| Urine analyses | |||||||
| Total urine (24 h) | 17.3 (6.0)a,b | 12.7 (5.5)a | 12.5 (6.5)a | 22.5 (7.1)b | 0.38 | 0.35 | <0.05 |
| Glucose (mg dL−1) | 14.8 (1.3) | 19.0 (3.7) | 10.2 (1.5) | 11.2 (0.7) | <0.001 | 0.08 | 0.09 |
| Urea 24 h (g per day) | 0.17 (0.03) | 0.19 (0.05) | 0.24 (0.03) | 0.35 (0.11) | <0.01 | <0.05 | 0.14 |
| Sodium (mmol per day) | 0.53 (0.26) | 0.62 (0.31) | 0.70 (0.14) | 0.93 (0.25) | <0.05 | 0.17 | 0.51 |
| Potassium (mmol per day) | 1.78 (0.36) | 2.02 (0.71) | 1.95 (0.35) | 2.58 (0.62) | 0.14 | 0.08 | 0.41 |
| Microalbuminuria (mg per day) | 0.04 (0.04) | 0.05 (0.02) | 0.16 (0.13) | 0.12 (0.07) | <0.05 | 0.50 | 0.82 |
Renal function was affected by the HF diet: there was an increase in urine excretion of urea, sodium and albumin and a decrease in glycosuria. BE also contributed to an increase in renal excretion of urea.
Plasmatic levels of brain derived neurotropic factor (BDNF) were significantly decreased in the HF fed animals. The BE group showed decreased levels, but the supplementation had no effect regarding HF fed groups (Table 3). BDNF levels in the brain frontal cortex were measured to correlate with plasmatic ones. Cortical BDNF levels were decreased both by diet and by BE supplementation. There was not a correlation between plasmatic and central BDNF (r = 0.361 p = 1.129) considering all tested animals. However, when considering each group isolated, control groups (C) had a strong association between both localizations (r = −0.967 p = 0.033) but both HF diet and BE supplementation disrupted this correlation.
| C | BE | HF | HFBE | D | BE | D × BE | |
|---|---|---|---|---|---|---|---|
| Standard diet (C); standard diet + blackberry anthocyanins extract (BE); high-fat diet (HF) or high-fat diet + blackberry anthocyanin extract (HFBE); values are expressed as mean (SD). The significance of diet (D), blackberry extract (BE) or interaction between both factors (D × BE) were tested by two-way ANOVA, expressed as p values, and followed by Fishers's LSD test. Mean values with different superscript letters are significantly different (p < 0.05). | |||||||
| BDNF plasmatic (pg mL−1) | 18.84 (4.0)a | 12.16 (3.7)b | 8.07 (2.1)b | 8.29 (3.7)b | <0.001 | 0.06 | <0.05 |
| BDNF brain (pg per mg tissue) | 0.88 (0.15)a | 0.57 (0.10)b | 0.68 (0.10)b | 0.57 (0.04)b | <0.05 | <0.001 | <0.05 |
3.4. Adipose tissue distribution, adipokine levels, and fatty acid composition
HF diet was the main contributing factor to variance in nasoanal length, waist circumference, free fatty mass, fatty mass weight and percentage, leading to an increase in all these parameters (Table 4). The plasmatic levels of adipokines adiponectin and leptin (Fig. 4A and B) were both increased by HF diet. BE supplementation also contributed to an increase in animals’ leptin levels but not in adiponectin levels. The ratio between the pro-inflammatory leptin and the anti-inflammatory adiponectin showed that there is still an increase induced by the HF diet but no effect of BE (Fig. 4C).| C | BE | HF | HFBE | D | BE | D × BE | |
|---|---|---|---|---|---|---|---|
| Standard diet (C); standard diet + blackberry anthocyanins extract (BE); high-fat diet (HF) or high-fat diet + blackberry anthocyanins extract (HFBE). Values are expressed as mean (SD). The significance of diet (D), blackberry extract (BE) or interaction between both factors (D × BE) were tested by two-way ANOVA and expressed as p values. | |||||||
| Nasoanal length (cm) | 25.2 (0.18) | 25.0 (0.58) | 26.7 (1.14) | 27.1 (0.82) | <0.0001 | 0.752 | 0.348 |
| Waist circumference (cm) | 18.3 (0.87) | 18.6 (0.83) | 21.1 (1.10) | 21.7 (1.25) | <0.0001 | 0.296 | 0.724 |
| Free fatty mass (g) | 246.6 (11.5) | 248.3 (11.0) | 295.1 (27.5) | 307.3 (26.1) | <0.0001 | 0.417 | 0.539 |
| Fatty mass (g) | 181.2 (16.6) | 183.9 (16.9) | 259.2 (41.2) | 278.3 (40.0) | <0.0001 | 0.400 | 0.525 |
The morphology of adipose tissue was only affected by type of diet (Fig. 5A). Surprisingly, the HF diet increased adipocyte area only in subcutaneous depots (Fig. 5A). Fatty acid composition was analysed in the liver and on two distinct deposits of adipose tissue, known to have different metabolic implications: the subcutaneous and mesenteric adipose tissue. The concentrations of each fatty acid are described in Tables 5–9. Compared with C animals, HF diet animals showed a greater accumulation of total monounsaturated fatty acids (MUFA) in mesenteric adipose tissue, fewer polyunsaturated fatty acids (PUFA) (Table 5) and no differences in total saturated fatty acids (SFA) (Table 6). This effect was mainly due to greater accumulation of fatty acids as the monounsaturated oleic acid (C18:1 c9) and less as polyunsaturated linoleic acid (C18:2 c9c12). In subcutaneous adipose tissue (Tables 7 and 8), the HF diet increased SFA and MUFA with no effect on PUFA. BE supplementation did not have an effect on the global SFA, MUFA or PUFA. Nevertheless, some differences were observed in particular fatty acids within these classes, specifically in C17i and C15:1 c10, both increased in the mAT of the BE group; also C17i was increased in the scAT of these animals. There were no significant effects of BE on fatty acid composition in the liver (Table 9).
| C | BE | HF | HFBE | |
|---|---|---|---|---|
| ai: branched chain fatty acid, anteiso; i: branched chain fatty acid, iso. Standard diet (C); standard diet + blackberry anthocyanin extract (BE); high-fat diet (HF) or high-fat diet + blackberry anthocyanin extract (HFBE). Values are expressed as mean (SD). a,b,c,d Superscript letters in a row for significant differences among groups (p < 0.05) after one-way ANOVA followed by Bonferroni adjustment. | ||||
| SFA | 205.56 (33.31) | 208.37 (29.45) | 212.44 (35.87) | 233.19 (27.53) |
| C12 | 0.32 (0.08) | 0.29 (0.06) | 0.29 (0.03) | 0.35 (0.06) |
| C14 | 6.39a (1.34) | 5.97a (1.23) | 4.63b (0.36) | 5.61a,b (0.53) |
| C15 ai | 0.07a (0.03) | 0.07a (0.02) | 0.03b (0.00) | 0.04b (0.00) |
| C15 | 2.02a (0.37) | 1.99a (0.21) | 0.89b (0.11) | 0.99b (0.06) |
| C16 | 170.20a (27.52) | 170.35a (26.99) | 142.96b (21.97) | 161.19a,b (15.16) |
| C17i | 0.52a (0.14) | 0.75b (0.13) | 0.35b (0.07) | 0.40b (0.05) |
| C17 ai | 0.54a (0.13) | 0.55a (0.14) | 0.42b (0.07) | 0.48b (0.06) |
| C17 | 1.70a (0.37) | 1.79a (0.22) | 2.52b (0.47) | 2.62b (0.41) |
| C18 i | 1.13a (0.29) | 1.47a (0.23) | 0.72b (0.20) | 0.74b (0.19) |
| C18 | 20.44a (4.53) | 22.59a (3.16) | 57.96b (13.41) | 59.09b (12.53) |
| C20 | 0.70 (0.24) | 0.80 (0.17) | 0.81 (0.23) | 0.75 (0.27) |
| C21 | 0.07a (0.02) | 0.09a (0.02) | 0.05b (0.02) | 0.05b (0.01) |
| C22 | 0.23a (0.09) | 0.27a (0.07) | 0.18b (0.06) | 0.17b (0.06) |
| C23 | 0.05a,b (0.01) | 0.06b (0.01) | 0.04a (0.01) | 0.04a (0.00) |
| C24 | 1.18a (0.30) | 1.33a (0.15) | 0.57b (0.13) | 0.68b (0.12) |
| C | BE | HF | HFBE | |
|---|---|---|---|---|
| c: cis double bond; t: trans double bond; AA: arachidonic acid; n3: omega 3 fatty acid; n6: omega 6 fatty acid; DHA: docosahexanoic fatty acid. Standard diet (C); standard diet + blackberry anthocyanin extract (BE); high-fat diet (HF) or high-fat diet + blackberry anthocyanin extract (HFBE). Values are expressed as mean (SD). a,b,c,d Superscript letters in a row for significant differences among groups (p < 0.05) after one-way ANOVA followed by Bonferroni adjustment. | ||||
| MUFA | 235.25a (35.89) | 242.47a (30.67) | 331.81b (50.51) | 353.60b (36.72) |
| C14:1 | 0.39a (0.15) | 0.37a (0.12) | 0.10b (0.01) | 0.13b (0.04) |
| C15:1 c10 | 0.62a (0.15) | 0.76a (0.13) | 0.34b (0.10) | 0.38b (0.07) |
| C16:1 t9 | 0.13a (0.04) | 0.13a (0.02) | 0.22b (0.03) | 0.25b (0.02) |
| C16:1 c7 | 2.78a (0.38) | 2.65a (0.38) | 3.56b (0.52) | 3.79b (0.33) |
| C16:1 c9 | 24.14a (7.77) | 22.47a (8.71) | 7.29b (1.00) | 10.00b (2.47) |
| C16:1 c11 | 0.33a (0.09) | 0.28a (0.08) | 0.12b (0.02) | 0.16b (0.02) |
| C17:1 c9 | 0.20a (0.03) | 0.19a (0.03) | 0.15b (0.02) | 0.17a,b (0.02) |
| C17:1 c10 | 1.08a (0.24) | 1.04a (0.21) | 1.26b (0.15) | 1.48b (0.12) |
| C18:1 t | 0.87a (0.33) | 0.95a (0.24) | 2.48b (0.40) | 2.47b (0.30) |
| C18:1 c9 | 167.98a (25.95) | 174.29a (18.91) | 290.91b (45.08) | 307.25b (34.37) |
| C18:1 c11 | 30.29a (4.53) | 32.11a (4.34) | 20.01b (3.08) | 21.89b (2.19) |
| C18:1 c12 | 0.13a (0.05) | 0.14a (0.05) | 0.38b (0.03) | 0.41b (0.03) |
| C18:1 c13 | 0.57a (0.11) | 0.56a (0.09) | 0.44b (0.06) | 0.54a,b (0.06) |
| C18:1 t16 | 0.48 (0.13) | 0.55 (0.13) | 0.51 (0.09) | 0.53 (0.09) |
| C20:1 c9 | 2.02a (0.44) | 2.32a (0.34) | 3.19b (0.78) | 3.21b (0.63) |
| C20:1 c11 | 3.20a (0.76) | 3.57a (0.59) | 0.88b (0.24) | 0.99b (0.21) |
| C22:1 c9 | 0.11a (0.05) | 0.15a,b (0.03) | 0.08b (0.03) | 0.08b (0.02) |
| C24:1 | 0.10a (0.05) | 0.11a (0.03) | 0.06b (0.01) | 0.05b (0.02) |
| PUFA | 245.70a (30.41) | 249.96a (30.35) | 181.19b (15.12) | 194.72b (12.22) |
| C18:2 t9t12 | 0.17 (0.04) | 0.17 (0.04) | 0.15 (0.02) | 0.18 (0.02) |
| C18:2 c9t12 | 0.06a (0.02) | 0.05a (0.01) | 0.09b (0.02) | 0.12b (0.02) |
| C18:2 t9c12 | 0.57a (0.11) | 0.53a (0.11) | 0.27b (0.04) | 0.30b (0.06) |
| C18:2 c9c12 | 228.73a (28.13) | 233.44a (28.34) | 168.69b (14.20) | 179.88b (11.73) |
| C18:3 t9t12c15 | 0.34 (0.06) | 0.37 (0.04) | 0.36 (0.06) | 0.37 (0.05) |
| C18:3 c6c9c12 | 0.83a (0.16) | 0.75a,b (0.11) | 0.64b (0.08) | 0.76b (0.05) |
| C18:3 c9t12t15 | 0.37 (0.09) | 0.45 (0.07) | 0.43 (0.07) | 0.45 (0.08) |
| C18:3 c9c12c15 | 7.64a (1.77) | 7.37a (1.64) | 4.80b (0.53) | 5.83b (0.99) |
| C18:2 c9t11 | 0.22a (0.09) | 0.24a (0.08) | 0.63b (0.06) | 0.70b (0.06) |
| C20:2 c11c14 | 1.16a (0.28) | 1.27a (0.16) | 2.08b (0.45) | 2.37b (0.36) |
| C20:3 c8c11c14 | 0.65 (0.21) | 0.64 (0.09 | 0.52 (0.08) | 0.66 (0.09) |
| C20:4 AA | 3.19a (1.08) | 2.98a (0.37) | 1.65b (0.23) | 2.05b (0.35) |
| C20:3 c11c14c17 | 0.11a (0.04) | 0.10a (0.01) | 0.19b (0.03) | 0.23b (0.05) |
| C20:5 n3 | 0.18a (0.04) | 0.16a (0.03) | 0.08b (0.01) | 0.09b (0.02) |
| C22:2 c13c16 | 0.14a (0.04) | 0.13a (0.02) | 0.09b (0.02) | 0.08b (0.02) |
| C22:5 n6 | 0.39a (0.16) | 0.41a (0.08) | 0.16b (0.03) | 0.18b (0.04) |
| C22:5 n3 | 0.42a (0.16) | 0.44a (0.13) | 0.20b (0.04) | 0.23b (0.06) |
| C22:6 DHA | 0.54a (0.29) | 0.46a (0.08) | 0.18b (0.04) | 0.25b (0.07) |
| μg mg−1 | 686.51 (92.98) | 700.80 (81.61) | 725.44 (98.09) | 781.51 (70.09) |
| C | BE | HF | HFBE | |
|---|---|---|---|---|
| ai: branched chain fatty acid, anteiso; i: branched chain fatty acid, iso. Standard diet (C); standard diet + blackberry anthocyanin extract (BE); high-fat diet (HF) or high-fat diet + blackberry anthocyanin extract (HFBE). Values are expressed as mean (SD). a,b,c,d Superscript letters in a row for significant differences among groups (p < 0.05) after one-way ANOVA followed by Bonferroni adjustment. | ||||
| SFA | 212.75a,b (29.98) | 249.04a,b (22.50) | 263.69b (41.84) | 285.47b (46.28) |
| C12 | 0.49 (0.14) | 0.45 (0.11) | 0.55 (0.12) | 0.51 (0.10) |
| C14 | 7.44 (0.91) | 7.75 (1.82) | 7.69 (1.33) | 7.86 (1.14) |
| C15 ai | 0.08a (0.03) | 0.08a (0.02) | 0.06b (0.01) | 0.05b (0.01) |
| C15 | 2.25a (0.45) | 2.57a (0.34) | 1.32b (0.21) | 1.36b (0.18) |
| C16 | 176.36 (24.19) | 204.10 (18.35) | 185.34 (29.67) | 201.54 (30.12) |
| C17i | 0.47a (0.14) | 0.82b (0.14) | 0.47a (0.05) | 0.52a (0.09) |
| C17 ai | 0.54 (0.10) | 0.61 (0.10) | 0.60 (0.09) | 0.65 (0.09) |
| C17 | 1.73a (0.45) | 2.20a (0.42) | 2.92b (0.47) | 3.20b (0.45) |
| C18i | 1.03a (0.15) | 1.48a (0.20) | 0.80b (0.07) | 0.91b (0.23) |
| C18 | 20.10a (5.20) | 26.15a (5.26) | 61.63b (10.49) | 66.43b (15.68) |
| C20 | 0.49a (0.13) | 0.64a,b (0.15) | 0.63a,b (0.13) | 0.74b (0.20) |
| C21 | 0.07 (0.02) | 0.10 (0.01) | 0.05 (0.02) | 0.12 (0.21) |
| C22 | 0.16 (0.04) | 0.22 (0.04) | 0.14 (0.02) | 0.16 (0.03) |
| C23 | 0.06 (0.01) | 0.08 (0.03) | 0.07 (0.01) | 0.07 (0.02) |
| C24 | 1.48a,b (0.30) | 1.80a (0.21) | 1.41a,b (0.31) | 1.37b (0.19) |
| C | BE | HF | HFBE | |
|---|---|---|---|---|
| c: cis double bond; t: trans double bond; AA: arachidonic acid; n3: omega 3 fatty acid; n6: omega 6 fatty acid; DHA: docosahexanoic fatty acid. Standard diet (C); standard diet + blackberry anthocyanin extract (BE); high-fat diet (HF) or high-fat diet + blackberry anthocyanin extract (HFBE). Values are expressed as mean (SD). a,b,c,d Superscript letters in a row for significant differences among groups (p < 0.05) after one-way ANOVA followed by Bonferroni adjustment. | ||||
| MUFA | 249.77a (32.29) | 281.16a (29.12) | 407.67b (60.69) | 449.86b (61.37) |
| C14:1 | 0.44a (0.06) | 0.45a (0.12) | 0.21b (0.04) | 0.22b (0.05) |
| C15:1 c10 | 0.67a (0.13) | 0.98b (0.16) | 0.50c (0.05) | 0.52c (0.11) |
| C16:1 t9 | 0.17a (0.03) | 0.16a (0.03) | 0.33b (0.06) | 0.35b (0.04) |
| C16:1 c7 | 3.20a (0.47) | 3.35a (0.41) | 4.65b (0.82) | 4.80b (0.59) |
| C16:1 c9 | 28.26a (7.49) | 27.48a (6.84) | 15.68b (2.53) | 18.55b (5.64) |
| C16:1 c11 | 0.39a (0.07) | 0.38a (0.08) | 0.21b (0.05) | 0.21b (0.03) |
| C17:1 c9 | 0.21 (0.03) | 0.24 (0.04) | 0.21 (0.04) | 0.21 (0.03) |
| C17:1 c10 | 1.26a (0.23) | 1.34a (0.17) | 1.88b (0.27) | 2.10b (0.29) |
| C18:1 t | 1.10a (0.27) | 1.19a (0.34) | 3.30b (0.51) | 3.33b (0.46) |
| C18:1 c9 | 176.73a (22.18) | 201.44a (22.99) | 348.64b (52.56) | 383.85b (53.61) |
| C18:1 c11 | 31.49a,b (4.43) | 37.04a (4.08) | 25.13c (3.82) | 28.02c (3.76) |
| C18:1 c12 | 0.15a (0.05) | 0.15a (0.03) | 0.51b (0.07) | 0.54b (0.07) |
| C18:1 c13 | 0.60 (0.11) | 0.68 (0.07) | 0.68 (0.09) | 0.75 (0.11) |
| C18:1 t16 | 0.42a (0.08) | 0.48a (0.08) | 0.61b (0.07) | 0.66b (0.09) |
| C20:1 c9 | 1.92a (0.37) | 2.35a (0.44) | 3.94b (0.60) | 4.37b (0.80) |
| C20:1 c11 | 2.79a (0.79) | 3.43a (0.63) | 1.21b (0.20) | 1.41b (0.30) |
| C22:1 c9 | 0.10 (0.03) | 0.14 (0.02) | 0.10 (0.02) | 0.11 (0.02) |
| C24:1 | 0.08 (0.01) | 0.10 (0.03) | 0.07 (0.01) | 0.09 (0.05) |
| PUFA | 275.73a,b (49.14) | 324.67b (54.96) | 255.31b (38.89) | 261.10b (34.10) |
| C16:2 c9t12 | 0.02 (0.01) | 0.03 (0.01) | 0.02 (0.01) | 0.02 (0.01) |
| C16:2 c9c12 | 0.07a (0.01) | 0.08a (0.03) | 0.05b (0.01) | 0.04b (0.01) |
| C18:2 t9t12 | 0.21 (0.04) | 0.24 (0.04) | 0.22 (0.03) | 0.25 (0.03) |
| C18:2 c9t12 | 0.07a (0.02) | 0.08a (0.03) | 0.13b (0.03) | 0.15b (0.02) |
| C18:2 t9c12 | 0.68a (0.11) | 0.72a (0.12) | 0.41b (0.08) | 0.43b (0.06) |
| C18:2 c9c12 | 253.66a,b (44.95) | 299.16b (51.39) | 230.87a (35.64) | 236.42a (31.56) |
| C18:2 c9c15 | n.d.a | n.d.a | 0.26b (0.07) | 0.27b (0.04) |
| C18:3 t9t12c15 | 0.36 (0.06) | 0.40 (0.06) | 0.44 (0.06) | 0.49 (0.07) |
| C18:3 c6c9c12 | 0.96 (0.15) | 1.02 (0.19) | 0.93 (0.14) | 1.04 (0.13) |
| C18:3 c9t12t15 | 0.31a (0.06) | 0.43a (0.08) | 0.51b (0.07) | 0.55b (0.09) |
| C18:3 c9c12c15 | 9.55a,b (2.16) | 11.22b (2.20) | 8.61a (1.55) | 8.53a (1.32) |
| C18:2 c9t11 | 0.29a (0.13) | 0.28a (0.10) | 0.89b (0.15) | 0.96b (0.14) |
| C20:2 c11c14 | 1.58a (0.40) | 1.81a (0.18) | 4.38b (0.58) | 4.42b (0.68) |
| C20:3 c8c11c14 | 0.82a (0.22) | 0.93a,b (0.14) | 1.09b,c (0.16) | 1.20c (0.20) |
| C20:4 AA | 4.79 (0.98) | 5.46 (1.06) | 4.24 (0.80) | 4.16 (0.55) |
| C20:3 c11c14c17 | 0.16a (0.04) | 0.19a (0.06) | 0.46b (0.07) | 0.44b (0.06 |
| C20:5 n3 | 0.21a,b (0.06) | 0.24b (0.08) | 0.17a (0.04) | 0.16a (0.03) |
| C22:2 c13c16 | 0.12a,b (0.02) | 0.14b (0.05) | 0.10a (0.02) | 0.11a (0.04) |
| C22:5 n6 | 0.53a (0.12) | 0.64a (0.16) | 0.33b (0.06) | 0.36b (0.07) |
| C22:5 n3 | 0.61 (0.21) | 0.76 (0.27) | 0.62 (0.16) | 0.56 (0.12) |
| C22:6 DHA | 0.73a (0.21) | 0.82a (0.19) | 0.58b (0.13) | 0.56b (0.12) |
| μg mg−1 | 738.25a (101.11) | 854.86a (102.26) | 926.67b (137.22) | 996.42b (133.87) |
| C | BE | HF | HFBE | |
|---|---|---|---|---|
| c: cis double bond; t: trans double bond; AA: arachidonic acid; n3: omega 3 fatty acid; n6: omega 6 fatty acid; DHA: docosahexanoic fatty acid. Standard diet (C); standard diet + blackberry anthocyanin extract (BE); high-fat diet (HF) or high-fat diet + blackberry anthocyanin extract (HFBE). Values are expressed as mean (SD). a,b,c,d Superscript letters in a row for significant differences among groups (p < 0.05) after one-way ANOVA followed by Bonferroni adjustment. | ||||
| SFA | 11.05 (1.54) | 10.47 (1.14) | 12.87 (2.54) | 12.30 (2.34) |
| C14 | 0.09a (0.02) | 0.09a (0.02) | 0.13b (0.06) | 0.14b (0.04) |
| C15 | 0.05 (0.00) | 0.05 (0.01) | 0.05 (0.01) | 0.04 (0.01) |
| C16 | 5.70 (0.82) | 5.45 (0.71) | 6.46 (1.54) | 6.10 (1.49) |
| C17 | 0.11 (0.01) | 0.11 (0.02) | 0.11 (0.02) | 0.10 (0.02) |
| C18 | 4.91a,b (0.75) | 4.58b (0.59) | 5.90a (1.25) | 5.70a (0.95) |
| C24 | 0.14 (0.03) | 0.14 (0.03) | 0.19 (0.05) | 0.18 (0.06) |
| MUFA | 3.47 (0.64) | 3.36 (0.76) | 6.79 (2.61) | 6.60 (2.01) |
| C81:1 t | 0.05 (0.01) | 0.04 (0.01) | 0.10 (0.03) | 0.10 (0.03) |
| C16:1 c7 | 0.05a (0.01) | 0.05a (0.01) | 0.12b (0.06) | 0.12b (0.06) |
| C16:1 c9 | 0.44a (0.04) | 0.49a (0.10) | 0.29b (0.08) | 0.36a,b (0.14) |
| C18:1 c9 | 1.75a (0.50) | 1.53a (0.49) | 5.42b (2.23) | 5.17b (1.62) |
| C18:1 c11 | 1.08a (0.13) | 1.16a (0.17) | 0.75b (0.21) | 0.74b (0.19) |
| C18:1 t16 | 0.05a (0.01) | 0.05a (0.01) | 0.03b (0.01) | 0.03b (0.00) |
| C20:1 c9 | 0.03a (0.02) | 0.04a (0.01) | 0.09b (0.03) | 0.08b (0.03) |
| C20:1 c11 | 0.07a (0.01) | 0.06a (0.01) | 0.03b (0.01) | 0.03b (0.01) |
| PUFA | 11.63 (1.51) | 11.26 (1.54) | 12.97 (2.67) | 12.24 (1.91) |
| C18:2 c9c12 | 4.42 (0.44) | 4.15 (0.51) | 5.70 (1.48) | 5.06 (1.14) |
| C18:3 c6c9c12 | 0.08a,b (0.02) | 0.06b (0.01) | 0.11a (0.03) | 0.11a (0.03) |
| C18:3 c9c12c15 | 0.07a (0.02) | 0.05a (0.02) | 0.13b (0.05) | 0.12b (0.03) |
| C20:2 c11c14 | 0.09a (0.01) | 0.10a (0.02) | 0.14b (0.03) | 0.14b (0.03) |
| C20:3 c8c11c14 | 0.20 (0.03) | 0.22 (0.04) | 0.19 (0.04) | 0.21 (0.04) |
| C20:4 AA | 5.64 (1.19) | 5.54 (1.14) | 5.35 (1.50) | 5.28 (1.11) |
| C20:3 c11c14c17 | 0.05 (0.01) | 0.05 (0.01) | 0.05 (0.01) | 0.05 (0.01) |
| C20:5 n3 | 0.05 (0.01) | 0.05 (0.01) | 0.04 (0.01) | 0.04 (0.01) |
| C22:5 n6 | 0.11 (0.02) | 0.11 (0.04) | 0.10 (0.02) | 0.12 (0.02) |
| C22:5 n3 | 0.17 (0.03) | 0.19 (0.04) | 0.17 (0.03) | 0.16 (0.04) |
| C22:6 DHA | 0.75a (0.12) | 0.74a (0.17) | 1.00b (0.25) | 0.97b (0.18) |
| μg mg−1 | 26.15a,b (2.97) | 25.09b (2.57) | 32.63a (6.91) | 31.14a (5.78) |
3.5. Dopamine content
Dopamine (DA) levels were quantified in the prefrontal cortex and the striatum. DA levels were affected by type of diet in the prefrontal cortex and there was an interaction of BE extract with this effect. As can be seen in Fig. 6A, although not having an effect on C animals, supplementation with BE reverted the increase in DA content seen in HF animals. In the striatum, type of diet did not affect DA levels while BE supplementation significantly decreased DA content (Fig. 6B). Levels of the dopamine metabolite 3,4-dihydroxyphenylacetic acid (DOPAC) were not affected by any factor (Fig. 6C). This reflected an increase in DOPAC/DA ratio, indicating a higher DA turnover with BE supplementation (Fig. 6D).4. Discussion
This study aimed to analyse the impact of long-term BE anthocyanin consumption with a global metabolic approach in rats with or without diet-induced obesity. Several metabolic parameters were analysed: resistance to insulin, glucose tolerance, blood pressure, biochemical parameters and adipose tissue morphology, function and fatty acid storage.Wistar rats were supplemented with an anthocyanin BE in a normal and in an obesity context for 17 weeks. The HF diet insult resulted, as expected, in overweight animals and some metabolic dysfunctions such as impairment in glucose tolerance and insulinemic response, increase in percentage of fat body weight, increase in scAT adipocyte size and increase in adipokine production. Although after 9 weeks of treatment, systolic blood pressure seemed altered, hypertension was not observed at the end.
As diet seems to be a key factor in controlling and counteracting the induction of obesity and associated co-morbidities, an extract from blackberry, rich in anthocyanins, was simultaneously given to a group of animals. This supplementation seemed to induce a better glycaemic response in both BE and HFBE animals. Some studies have shown a decrease in glycaemic response after anthocyanin consumption. The mechanisms behind this effect are not completely clear, but it is possible that it involves an effect on glucose transport.31 Cyanidin-3-glucoside and delphinidin-3-glucoside have been associated with anti-diabetic properties in different in vitro and in vivo models.32 Obesity has an associated low-grade chronic inflammation component, and it is still unclear whether it is a cause or a consequence of associated co-morbidities. An increase in adipocyte size, for instance, prompts adipose tissue inflammation, which is also dependent on the type of fatty acids stored.33 Adipocyte size has been shown to be an indirect measurement of inflammation as it correlates with several risk factors of metabolic dysfunction.33 The propensity to lower adipocyte size in mesenteric tissue may be predictive of a decreased risk of inflammation by BE supplementation. Localization of adipose tissue can predict the risk of metabolic consequences, more associated with visceral fat, while subcutaneous fat can have a protective effect.34 Our results show that type of diet significantly increases cholesterol levels, but curiously this effect was only visible on the HFBE group, and not on the HF group, as would be expected from previous literature.35,36 This effect may be, in part, due to the decrease in HDL seen in the HF groups and prevented in the HFBE group.
A very unusual but relevant result was the reduction of lactate release to the bloodstream after BE consumption. Hyperlactatemia has been found in obese humans37,38 and previous studies have shown that, besides muscle, lactate can also be produced in other tissues including adipose tissue.39 These lower lactate levels in groups with anthocyanin BE extract consumption may be seen as positive, since lactate levels are co-related with cardiovascular diseases and overall mortality.40
BE intake decreased BDNF content in both the plasma and the brain of standard-fed animals, which is in agreement with the results of Klein et al., who have shown that blood BDNF concentrations correlate positively with BDNF levels in the hippocampus of rats and pigs.41 It is well documented that BDNF is involved in synaptic plasticity, neuronal differentiation and survival of neurons, and thus its increase is usually associated with beneficial outcomes. Nevertheless, Miyazaki et al.42 observed significant increases in serum BDNF levels in patients with neurodevelopmental disorders, such as autism or mental retardation, compared to normal controls. Also, Si-Hoon demonstrated that plasma BDNF levels have a significant positive correlation with the severity of inattention symptoms in children.43 While El-Gharbawy et al.44 showed in 2006 that plasmatic BDNF concentrations were decreased in obese individuals, a recent study has also shown that obesity does not affect BDNF levels.45 Low levels of BDNF have been associated with changes in dopamine receptors,46 whereas increasing levels of BDNF were found to increase dopamine turnover.47,48 BDNF gene expression in the frontal cortex of DAT knockout mice was shown to be reduced.49 Both the HF diet and BE supplementation decreased BDNF levels, but the mechanisms behind this same effect may be different.
In previous animal studies supplementation with blueberries, also rich in anthocyanins, or with the flavonoids alone, for six weeks resulted in increased hippocampal levels of BDNF.50,51 Supplementation with BE, although decreasing BDNF levels, showed increased dopamine turnover in the striatum. Previous studies have suggested an association between glucose and dopamine levels,52 with low levels of glucose in the brain inhibiting dopamine release.52,53 Interference of flavonoids with glucose transporters, which has already been shown in previous reports31,54 and includes modulation of glucose access to the brain, could play a role in the dopamine changes observed in this study.
The leptin/adiponectin ratio has been correlated with metabolic parameters and predictor of cardiovascular risk.55 Diet increased this ratio with no effects of BE supplementation. These animals have also increased production of adiponectin and leptin, produced by adipose tissue which is known to be involved in metabolic regulation.
Anthocyanins have been suggested as potentially increasing plasma long chain fatty acid levels, including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), the main very long-chain (n-3) PUFA,56 although this issue remains controversial.57 In the present study, animals fed with the anthocyanin-rich extract did not show any difference in long-chain fatty acid composition. Although the biological significance was not clear, BE significantly increased methylhexadecanoic acid (C17i) in standard fed animals in both adipose tissue deposits. Branched-chain FAs are ubiquitous in nature and present in particularly large quantities in bacteria, but are rarely found in other organisms.58 This fact, coupled with a lack of detection of this fatty acid on the standard diet (data not shown), suggested that somehow BE could be changing either the animals’ metabolism or the production of fatty acids by intestinal bacteria.59
The effect of flavonoids, similar to that of many other xenobiotics, varies with the supplemented dose, many times without a dose-dependency, especially regarding in vivo effects.60,61 The dose given to the animals – 25 mg/kg body weight – would correspond to approximately 243 mg of blackberry extract in a human adult weighing 60 kg, using the formula to human equivalent dose (HED) based on body surface area as described by Reagan-Shaw.62 This dose could be easily achieved by diet, by eating as much as 100 g of blackberries a day,63 or could also be introduced as a food supplement if its risk–benefit so justifies. Future studies should test different doses to maximize the potential of blackberry supplementation whilst minimizing undesirable effects.
This study confirmed that high-fat high-carbohydrate diet-induced obesity can prompt several features of metabolic dysfunction in Wistar rats, some of them being partially reverted with low doses of blackberry extract supplementation for a long period. A decrease of plasma lactate levels appeared to be the strongest effect of blackberry supplementation, independent of the fat content of the diet. Blackberry supplementation was also able to modulate levels of dopamine and its clearance while reducing BDNF levels, the biological relevance of which is still a matter of debate. Further interventional studies should clarify these outcomes. The interest in effective intervention strategies to prevent/treat obesity and related pathologies has been increasing, along with a recent interest regarding the close associations between obesity and brain dysfunction. These results have advanced the knowledge of the therapeutic potential of berries, and may empower the achievement of specific recommendations for berry intake or purified blackberry extract in the future.
Authors’ contributions
MM contributed to the experimental design, data acquisition and analysis and drafted the manuscript. CM, SN, JF and LA contributed to data acquisition. IF and NM were responsible for the preparation of the blackberry extracts. AF and CC were responsible for study conception, conduction of experiments, data interpretation, preparation and critical revision of the manuscript.Conflict of interest
The authors declare no competing financial interests.Acknowledgements
The authors would like to thank to Drª Luisa Guardão, responsible for animal facilities, and Liliana Leite, for the technical assistance with animal procedures, and Eng. Paula Serrão from the Institute of Pharmacology and Therapeutics, Faculty of Medicine, University of Porto, Porto, Portugal for the technical help on catecholamine measurements. Financial support from Fundação para Ciência e Tecnologia and POPH/FSE (1 grant project PTDC/AGR-TEC/2227/2012 and 4 fellow grants SFRH/BPD/75294/2010, SFRH/BD/78367/2011, SFRH/BPD/86173/2012 and SFRH/BD/93073/2013) are gratefully acknowledged.References
- A. Berghofer, T. Pischon, T. Reinhold, C. M. Apovian, A. M. Sharma and S. N. Willich, BMC Public Health, 2008, 8, 200 CrossRef PubMed.
- C. L. Ogden, M. D. Carroll, B. K. Kit and K. M. Flegal, JAMA, J. Am. Med. Assoc., 2014, 311, 806–814 CrossRef CAS PubMed.
- A. K. Dahl and L. B. Hassing, Epidemiol. Rev., 2013, 35, 22–32 CrossRef PubMed.
- F. Muller-Riemenschneider, T. Reinhold, A. Berghofer and S. N. Willich, Eur. J. Epidemiol., 2008, 23, 499–509 CrossRef PubMed.
- G. A. Bray, J. Clin. Endocrinol. Metab., 2004, 89, 2583–2589 CrossRef CAS PubMed.
- M. A. Beydoun, H. A. Beydoun and Y. Wang, Obes. Rev., 2008, 9, 204–218 CrossRef CAS PubMed.
- M. Prince, R. Bryce, E. Albanese, A. Wimo, W. Ribeiro and C. P. Ferri, Alzheimer's Dementia, 2013, 9, 63–75 CrossRef PubMed.
- S. E. Kanoski and T. L. Davidson, Physiol. Behav., 2011, 103, 59–68 CrossRef CAS PubMed.
- H. R. Park, M. Park, J. Choi, K. Y. Park, H. Y. Chung and J. Lee, Neurosci. Lett., 2010, 482, 235–239 CrossRef CAS PubMed.
- J. K. Morris, G. L. Bomhoff, B. K. Gorres, V. A. Davis, J. Kim, P. P. Lee, W. M. Brooks, G. A. Gerhardt, P. C. Geiger and J. A. Stanford, Exp. Neurol., 2011, 231, 171–180 CrossRef CAS PubMed.
- B. Buijsse, E. J. Feskens, M. B. Schulze, N. G. Forouhi, N. J. Wareham, S. Sharp, D. Palli, G. Tognon, J. Halkjaer, A. Tjonneland, M. U. Jakobsen, K. Overvad, A. D. van der, H. Du, T. I. Sorensen and H. Boeing, Am. J. Clin. Nutr., 2009, 90, 202–209 CrossRef CAS PubMed.
- M. González-Castejón and A. Rodriguez-Casado, Pharmacol. Res., 2011, 64, 438–455 CrossRef PubMed.
- L. Letenneur, C. Proust-Lima, A. Le Gouge, J. F. Dartigues and P. Barberger-Gateau, Am. J. Epidemiol., 2007, 165, 1364–1371 CrossRef CAS PubMed.
- X. Gao, A. Cassidy, M. Schwarzschild, E. Rimm and A. Ascherio, Neurology, 2012, 78, 1138–1145 CrossRef CAS PubMed.
- S. Norberto, S. Silva, M. Meireles, A. Faria, M. Pintado and C. Calhau, J. Funct. Foods, 2013, 5, 1518–1528 CrossRef CAS.
- A. Basu, M. Rhone and T. J. Lyons, Nutr. Rev., 2010, 68, 168–177 CrossRef PubMed.
- C. Rendeiro, J. D. Guerreiro, C. M. Williams and J. P. Spencer, Proc. Nutr. Soc., 2012, 71, 246–262 CrossRef CAS PubMed.
- B. O. Cho, H. W. Ryu, C. H. Jin, D. S. Choi, S. Y. Kang, D. S. Kim, M. W. Byun and I. Y. Jeong, J. Agric. Food Chem., 2011, 59, 11442–11448 CrossRef CAS PubMed.
- B. Shukitt-Hale, V. Cheng and J. A. Joseph, Nutr. Neurosci., 2009, 12, 135–140 CrossRef CAS PubMed.
- N. M. Hassimotto and F. M. Lajolo, J. Sci. Food Agric., 2011, 91, 523–531 CrossRef CAS PubMed.
- I. Serraino, L. Dugo, P. Dugo, L. Mondello, E. Mazzon, G. Dugo, A. P. Caputi and S. Cuzzocrea, Life Sci., 2003, 73, 1097–1114 CrossRef CAS PubMed.
- J. Azevedo, I. Fernandes, A. Faria, J. Oliveira, A. Fernandes, V. de Freitas and N. Mateus, Food Chem., 2010, 119, 518–523 CrossRef CAS.
- J. Oliveira, V. Fernandes, C. Miranda, C. Santos-Buelga, A. Silva, V. de Freitas and N. Mateus, J. Agric. Food Chem., 2006, 54, 6894–6903 CrossRef CAS PubMed.
- I. Fernandes, J. Azevedo, A. Faria, C. Calhau, V. de Freitas and N. Mateus, J. Agric. Food Chem., 2009, 57, 735–745 CrossRef CAS PubMed.
- C. Barrientos, R. Racotta and L. Quevedo, Nutr. Res., 2010, 30, 791–800 CrossRef CAS PubMed.
- C. van den Brom, C. Bulte, B. Kloeze, S. Loer, C. Boer and R. Bouwman, Cardiovasc. Diabetol., 2012, 11, 74 CrossRef CAS PubMed.
- J. Ibrahim, B. C. Berk and A. D. Hughes, Clini. Exp. Hypertens., 2006, 28, 57–72 CrossRef PubMed.
- K. Rutter, L. Hennoste, L. C. Ward, B. H. Cornish and B. J. Thomas, Lab. Anim., 1998, 32, 65–71 CrossRef CAS PubMed.
- P. Castro-Gómez, J. Fontecha and L. M. Rodríguez-Alcalá, Talanta, 2014, 128, 518–523 CrossRef PubMed.
- P. Soares-Da-Silva and M. H. Fernandes, Acta Physiol. Scand., 1991, 143, 287–293 CrossRef CAS PubMed.
- A. Faria, D. Pestana, J. Azevedo, F. Martel, V. de Freitas, I. Azevedo, N. Mateus and C. Calhau, Mol. Nutr. Food Res., 2009, 53, 1430–1437 CAS.
- P. V. Babu, D. Liu and E. R. Gilbert, J. Nutr. Biochem., 2013, 24, 1777–1789 CrossRef CAS PubMed.
- M. Itoh, T. Suganami, R. Hachiya and Y. Ogawa, Int. J. Inflammation, 2011, 2011, 720926 Search PubMed.
- I. J. Neeland, C. R. Ayers, A. K. Rohatgi, A. T. Turer, J. D. Berry, S. R. Das, G. L. Vega, A. Khera, D. K. McGuire, S. M. Grundy and J. A. de Lemos, Obesity, 2013, 21, 19 Search PubMed.
- S. K. Panchal, L. Ward and L. Brown, Eur. J. Nutr., 2013, 52, 559–568 CrossRef CAS PubMed.
- X.-Y. Li, Z.-X. Zhao, M. Huang, R. Feng, C.-Y. He, C. Ma, S.-H. Luo, J. Fu, B.-Y. Wen, L. Ren, J.-W. Shou, F. Guo, Y. Chen, X. Gao, Y. Wang and J.-D. Jiang, J. Transl. Med., 2015, 13, 278 CrossRef PubMed.
- J. Lovejoy, F. D. Newby, S. S. Gebhart and M. DiGirolamo, Metabolism, 1992, 41, 22–27 CrossRef CAS PubMed.
- M. DiGirolamo, F. D. Newby and J. Lovejoy, FASEB J., 1992, 6, 2405–2412 CAS.
- G. van Hall, Acta Physiol., 2010, 199, 499–508 CrossRef CAS PubMed.
- K. Matsushita, E. K. Williams, M. L. Mongraw-Chaffin, J. Coresh, M. I. Schmidt, F. L. Brancati, R. C. Hoogeveen, C. M. Ballantyne and J. H. Young, Am. J. Epidemiol., 2013, 178, 401–409 CrossRef PubMed.
- A. B. Klein, R. Williamson, M. A. Santini, C. Clemmensen, A. Ettrup, M. Rios, G. M. Knudsen and S. Aznar, Blood BDNF concentrations reflect brain-tissue BDNF levels across species, 2011 Search PubMed.
- K. Miyazaki, N. Narita, R. Sakuta, T. Miyahara, H. Naruse, N. Okado and M. Narita, Brain Dev., 2004, 26, 292–295 CrossRef PubMed.
- S.-H. Shim, Y. Hwangbo, Y.-J. Kwon, H.-Y. Jeong, B.-H. Lee, H.-J. Lee and Y.-K. Kim, Prog. Neuro-Psychopharmacol. Biol. Psychiatry, 2008, 32, 1824–1828 CrossRef CAS PubMed.
- A. H. El-Gharbawy, D. C. Adler-Wailes, M. C. Mirch, K. R. Theim, L. Ranzenhofer, M. Tanofsky-Kraff and J. A. Yanovski, J. Clin. Endocrinol. Metab., 2006, 91, 3548–3552 CrossRef CAS PubMed.
- E. Gajewska, M. Sobieska, D. Lojko, K. Wieczorowska-Tobis and A. Suwalska, Eur. Rev. Med. Pharmacol. Sci., 2014, 18, 3246–3250 CAS.
- K. Sakata and S. M. Duke, Neuroscience, 2014, 260, 265–275 CrossRef CAS PubMed.
- J. A. Siuciak, C. Boylan, M. Fritsche, C. A. Altar and R. M. Lindsay, Brain Res., 1996, 710, 11–20 CrossRef CAS PubMed.
- C. A. Altar, C. B. Boylan, C. Jackson, S. Hershenson, J. Miller, S. J. Wiegand, R. M. Lindsay and C. Hyman, Proc. Natl. Acad. Sci. U. S. A., 1992, 89, 11347–11351 CrossRef CAS.
- F. Fumagalli, G. Racagni, E. Colombo and M. A. Riva, Mol. Psychiatry, 2003, 8, 898–899 CrossRef CAS PubMed.
- C. Rendeiro, D. Vauzour, M. Rattray, P. Waffo-Teguo, J. M. Merillon, L. T. Butler, C. M. Williams and J. P. Spencer, PLoS One, 2013, 8, e63535 CAS.
- C. Rendeiro, D. Vauzour, R. J. Kean, L. T. Butler, M. Rattray, J. P. Spencer and C. M. Williams, Psychopharmacology, 2012, 223, 319–330 CrossRef CAS PubMed.
- K. Blum, P. K. Thanos and M. S. Gold, Front. Psychol., 2014, 5, 919 Search PubMed.
- C. M. Adler, I. Elman, N. Weisenfeld, L. Kestler, D. Pickar and A. Breier, Neuropsychopharmacology, 2000, 22, 545–550 CrossRef CAS PubMed.
- M. Meireles, F. Martel, J. Araujo, C. Santos-Buelga, S. Gonzalez-Manzano, M. Duenas, V. de Freitas, N. Mateus, C. Calhau and A. Faria, J. Membr. Biol., 2013, 246, 669–677 CrossRef CAS PubMed.
- G. D. Norata, S. Raselli, L. Grigore, K. Garlaschelli, E. Dozio, P. Magni and A. L. Catapano, Stroke, 2007, 38, 2844–2846 CrossRef CAS PubMed.
- M. C. Toufektsian, P. Salen, F. Laporte, C. Tonelli and M. de Lorgeril, J. Nutr., 2011, 141, 37–41 CrossRef CAS PubMed.
- D. Vauzour, N. Tejera, C. O'Neill, V. Booz, B. Jude, I. M. Wolf, N. Rigby, J. M. Silvan, P. J. Curtis, A. Cassidy, S. de Pascual-Teresa, G. Rimbach and A. M. Minihane, J. Nutr. Biochem., 2015, 26, 211–218 CrossRef CAS PubMed.
- M. Kniazeva, Q. T. Crawford, M. Seiber, C. Y. Wang and M. Han, PLoS Biol., 2004, 2, E257 Search PubMed.
- A. Faria, I. Fernandes, S. Norberto, N. Mateus and C. Calhau, J. Agric. Food Chem., 2014, 62, 6898–6902 CrossRef CAS PubMed.
- E. J. Calabrese and R. B. Blain, Regul. Toxicol. Pharmacol., 2011, 61, 73–81 CrossRef CAS PubMed.
- A. Murakami, Arch. Biochem. Biophys., 2014, 557, 3–10 CrossRef CAS PubMed.
- S. Reagan-Shaw, M. Nihal and N. Ahmad, FASEB J., 2008, 22, 659–661 CrossRef CAS PubMed.
- X. Wu, G. R. Beecher, J. M. Holden, D. B. Haytowitz, S. E. Gebhardt and R. L. Prior, J. Agric. Food Chem., 2006, 54, 4069–4075 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |