Triclosan adsorption using wastewater biosolids-derived biochar†
Yiran
Tong
,
Brooke K.
Mayer
and
Patrick J.
McNamara
*
Department of Civil, Construction and Environmental Engineering, Marquette University, 1637 W. Wisconsin Ave., Milwaukee, WI 53233, USA. E-mail: Patrick.McNamara@marquette.edu; Tel: +414 288 2188
First published on 14th June 2016
Abstract
Organic micropollutants are ubiquitous in the environment and stem from municipal wastewater treatment plant discharges. Adsorption can be used as a tertiary treatment to complement the conventional activated sludge process to remove micropollutants prior to discharge. This research evaluated the performance of wastewater biosolids-derived biochar as an adsorbent to remove triclosan from water. Pre-conditioning of the biochar using hydrochloric acid (HCl) was an essential step for triclosan adsorption. Using acid-conditioned biochar, maximum adsorption of 872 μg triclosan per g biochar was achieved with biochar produced at 800 °C. Biochar produced at higher pyrolysis temperatures tended to have higher triclosan sorption capacity using initial triclosan concentrations of 200 μg L−1 levels. However, pyrolysis temperature had less impact on triclosan sorption at lower, environmentally relevant concentrations. Low solution pH (3) enhanced adsorption and high pH (11) inhibited adsorption. Effective triclosan sorption was observed between pH 5 and 9, with little variation, which is positive for practical applications operated at near-neutral solution pH. In wastewater, acid-treated biochar also effectively sorbed triclosan, albeit at a decreased adsorption capacity and removal rate due to competition from other organic constituents. This study indicated that adsorption may occur mainly due to high surface area, hydrophobicity, and potential interaction between biochar and triclosan functional groups including hydrogen bonding and π-stacking. This work demonstrated that acid-conditioned biosolids-derived biochar could be a suitable sorbent to remove triclosan from wastewater as a final polishing treatment step.
Water impactThe biochar produced by pyrolysis of wastewater biosolids can be used as a sorbent to remove organic micropollutants from water. Micropollutants are ubiquitous in water and cause adverse ecological impacts. Use of biochar to sorb micropollutants not only produces higher quality water, but also provides an alternative approach to biosolids management via on-site production of an effective adsorbent. |
1. Introduction
Organic micropollutants including hormones, pharmaceuticals and personal care products, flame-retardants, artificial sweeteners, and antimicrobials, are widely used in consumer products. As population increases, so does use of these consumer products, which inevitably end up in wastewater treatment systems.1 Municipal wastewater treatment plants (WWTPs) are major sources of organic micropollutant discharges into the environment.2 Many micropollutants are not degraded or are partially degraded in conventional WWTPs.3 Consequently, micropollutants are ubiquitous in natural waters and are increasingly detected in industrialized and remote environments.4 Although they are found in waters at low concentrations (ng L−1), micropollutants cause adverse ecological impacts such as feminization of fish.5,6Triclosan (structure shown in ESI,† section S1) is an antimicrobial that is widely used for personal hygiene and disinfection products including hand soap, oral care products, and lotion7 and is widely found in human urine and WWTP effluents.8 Each year, US WWTPs release approximately 1.1 × 105 to 4.2 × 105 kg triclosan to the environment.9 Exposure to triclosan might also select for spread of antibiotic resistance, which is an emerging public health issue.7,10
Conventional activated sludge processes are not designed to remove micropollutants, although a large fraction is removed in settling tanks due to sorption to biosolids. The removal rate of triclosan via sorption to biosolids can vary substantially, ranging from 15% to 100%.11–14 Even though a substantial fraction of triclosan may be removed with the solids, triclosan is still discharged into receiving waters. For example, Blair et al.11 detected 54 ng L−1 triclosan in WWTP effluent discharged to Lake Michigan. Advanced tertiary treatment techniques have been investigated for increased removal of micropollutants in WWTPs. Advanced oxidation, UV treatment, and membrane filtration can be effective techniques for micropollutant removal.15 These methods can have high infrastructure and operational costs.16,17 Activated carbon can also achieve substantial removal of a broad spectrum of micropollutants from water by sorption,18 but it has high environmental impacts that arise from activated carbon production and feedstock supply.17
Alternative sorbents capable of effectively removing micropollutants are of interest to WWTPs. Biochar, which is the carbonaceous residual solid product produced by pyrolysis (a process that involves heating biomass in the absence of oxygen), may have potential as an effective, low-cost sorbent for the capture of micropollutants. Biochar can be produced using a wide range of biomass feedstock sources, including wood wastes, plant residuals and animal wastes.19–21 Biochar products have attracted increased attention in agronomy as a stable soil amendment to enhance soil fertility and plant growth.22,23 In addition to agronomy applications, biochar has been evaluated as a low-cost sorbent to capture inorganic and organic contaminants.
Biochar derived from pyrolysis of wood wastes has been applied for removal of inorganic contaminants from water. The maximum adsorption capacity was 4.25 mg g−1 and 7.51 mg g−1 for lead and chromium, respectively, which exceeds performance for some activated carbon.24,25 Biochar can also be utilized to retain nutrients. Yao et al.26 used biochar produced from sugar beet tailings to remove 73% phosphate from water. Carey et al.23 used biosolids-derived biochar to remove ammonium from wastewater. The ammonium-saturated biochar subsequently improved growth of Kentucky Bluegrass.
In addition to removal of inorganic compounds, biochar produced from a wide range of feedstocks has also been found to adsorb organic contaminants such as catechol, humic acid, and endocrine disrupting chemicals.27,28 No research yet exists describing the use of wastewater biosolids-derived biochar to capture micropollutants. This waste-to-resource process would be implemented by pyrolyzing wastewater-derived biosolids to produce a readily renewable sorbent onsite. Furthermore, pyrolysis removes organic micropollutants such as triclosan from biochar,29 indicating that the biochar could be re-pyrolyzed to remove any sorbed micropollutants.
The objective of this study was to determine if biosolids-derived biochar could be used to adsorb triclosan, a pervasive micropollutant, in water and wastewater. Bench-scale batch tests were conducted to explore adsorption capacities under a range of physical conditions (pre-conditioning of biochar, solution pH, and pyrolysis temperature). Isotherm modeling and characterization of the biochar surface were performed to better understand the mechanism of interaction between triclsoan and the biochar surface.
2. Materials and methods
2.1 Biochar production and pre-conditioning for sorption
Milorganite®, a heat-dried blend of anaerobically digested primary solids and waste activated sludge biosolids produced by the Milwaukee Metropolitan Sewage District (MMSD), was used as feedstock. The feedstock was pyrolyzed to produce biochar by placing 30 g of heat-dried biosolids in a 250 ml flask and purging with argon gas for 15 minutes. The flask opening was wrapped with aluminum foil and the flask was heated in a muffle furnace at 300 °C, 500 °C, 600 °C, 700 °C or 800 °C for 60 minutes and cooled down in a desiccator before conditioning.All biochar was washed with Milli-Q® (Billerica, MA) water to remove residual surface impurities. To produce acid-treated biochar, 1 N HCl was used to pretreat the biochar, while base-treated biochar was conditioned with 1 N NaOH, both at dosages of 1 g biochar per 10 ml solution. The mixtures were agitated on a shaker table at 200 rpm for 12 hours. The biochar slurry was filtered with Whatman® (Ann Arbor, MI) 0.7 μm glass fiber filters via vacuum filtration, and the recovered biochar was rinsed with deionized water. The Milli-Q, acid, or base-conditioned biochar was dried at 90 °C and stored in a desiccator prior to use in sorption experiments.
2.2 Characterization of biochar properties
2.3 Adsorption tests
Batch adsorption tests were conducted to determine the sorption capacity of triclosan on biochar. Glass serum bottles (60 mL) were silanized using 5% by volume dichlorodimethylsilane (99.5%, Sigma Aldrich, St. Louis, MO, USA) and 95% by volume heptane (99%, Sigma-Aldrich, St. Louis, MO, USA) solution to prevent chemicals from adsorbing onto the glass. Triclosan (97%, Sigma Aldrich, St. Louis, MO) was pre-dissolved in HPLC-grade methanol (99%, Sigma-Aldrich, St. Louis, MO, USA) for use as stock solution. The volumetric ratio of methanol stock to water was below 0.5% for all tests, which negates co-solvent effects.31 All adsorption tests were conducted in triplicate in 50 mL of solution.To determine the effect of pre-conditioning, triclosan stock solution was spiked to produce a final concentration of approximately 200 μg L−1 TCS in Milli-Q water. Acid (HCl), base (NaOH), or Milli-Q water-treated biochar (prepared at 600 °C) was dosed at 0.4 g L−1.
The impact of bulk solution pH on triclosan adsorption was tested using 0.4 g L−1 of 600 °C HCl-treated biochar (selected based on previous pre-conditioning experiments). The pH of the Milli-Q water was adjusted to 3, 5, 7, 9, or 11 using HCl and NaOH. Triclosan was added at a concentration of approximately 300 μg L−1 for all pH experiments.
Adsorption isotherm experiments were conducted in serum bottles by spiking approximately 300 μg L−1 of TCS in Milli-Q water (initial pH approximately 6.5). Biochar pyrolyzed at different temperatures (300 °C, 500 °C, 600 °C, 700 °C and 800 °C) was dosed at 0.2 g L−1, 0.4 g L−1, 0.6 g L−1, 0.8 g L−1, and 1 g L−1. Filtrasorb® 400 granular activated carbon (GAC, Calgon Carbon, IL, USA) was used as a comparison to biochar adsorption performance.
A municipal secondary-treated wastewater effluent sample from Jones Island Water Reclamation Facility, Milwaukee, WI, was used to test triclosan adsorption to biochar in complex matrices. Water quality parameters including pH, chemical oxygen demand (COD), total organic carbon (TOC), turbidity, and total suspended solids (TSS) were measured according to standard methods,32 results of which are provided in Table S2 of the ESI,† section S7. Triclosan stock solution was injected into wastewater effluent at approximately 300 μg L−1. Each bottle was dosed with 0.4 g L−1 of 600 °C HCl biochar. To investigate the adhesion of triclosan to suspended solids, solutions injected with TCS without adding biochar were used as a control. The background triclosan concentration in the wastewater was below detection.
The serum bottle reactors were mixed end-over-end using a Cole-Parmer (IL, USA) Roto-Torque Variable Speed Rotator for 24 hours (which provided sufficient time to reach equilibrium, as determined by the kinetic tests described in the ESI,† section S2). Water samples were collected from the serum bottles and filtered with 0.45 μm PTFE syringe filters (Agela Technologies, Wilmington, DE) prior to subsequent analysis.
2.4 Analysis of triclosan with liquid chromatography-mass spectrometry (LC-MS)
Aqueous-phase triclosan concentrations were measured with liquid chromatography-mass spectrometry (LCMS-2020, Shimadzu Corporation, MD, USA). Method details are provided in the ESI,† section S3. The triclosan quantification limit (based on a signal-to-noise ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was 5 μg L−1.
1) was 5 μg L−1.
2.5 Sorption calculations and statistical analysis
The adsorption capacity of triclosan on biochar (Qe, μg TCS g−1 biochar) was calculated using eqn (1):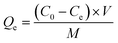 | (1) |
Isotherm modeling (linear, Langmuir and Freundlich) and statistical analyses (t-test and ANOVA, α level = 5%) were performed using GraphPad Prism 6 (La Jolla. CA, USA).
3. Results and discussion
3.1 The impact of biochar preconditioning on adsorption performance
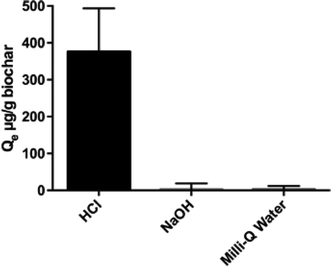
Preconditioning the biochar with HCl significantly enhanced triclosan sorption onto biochar relative to biochar preconditioned with NaOH or Milli-Q water (Fig. 1; ANOVA, p = 0.0094). The initial bulk solution pH was approximately 6.5, and it decreased approximately 1 pH unit over the course of testing, likely due to the intrinsic HCl-biochar surface acidity.33,34 Based on these data, acid pre-conditioning is necessary for biosolids-derived biochar to be effectively used as a triclosan adsorbent.There are several possible reasons why HCl pre-conditioning might enhance adsorption. As shown in the SEM images presented in Fig. 2, HCl appeared to more effectively clean the biochar surface than the Milli-Q water or NaOH. Acid-treated biochar also appeared to have fewer granular impurities and be more porous than both base- and Milli-Q-conditioned biochar. These visual differences suggest that HCl-biochar may offer more surface area for sorption reactions. Surface area analysis by BET verified that HCl substantially increased the specific surface area of the biochar, as shown in Table 1. The HCl-biochar specific surface area was an order of magnitude greater than Milli-Q-biochar. As shown in Fig. 2B, the NaOH-treated biochar surface was smoother and had fewer pores than acid-treated biochar. Other researchers have observed that NaOH conditioning of activated carbon can decrease the specific surface area because pores and cracks swell in the presence of aqueous base.35,36 For carbon-based adsorbents such as activated carbon and biochar, the functionality as a sorbent is partially due to the highly porous surface of the solid and the extremely high surface area to volume ratio.37
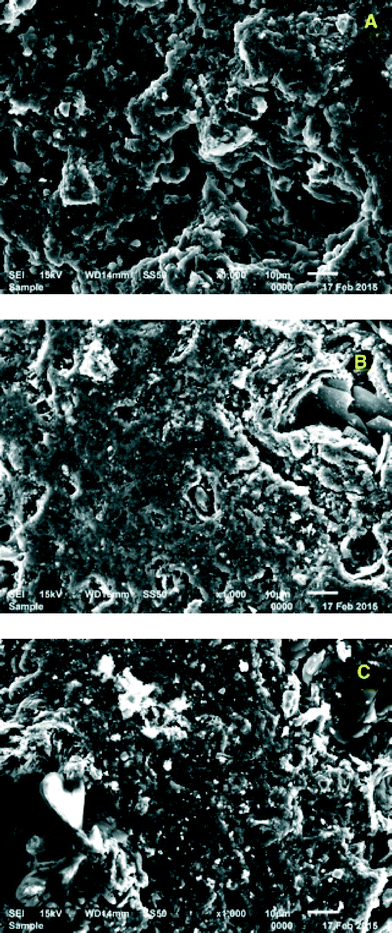 | ||
| Fig. 2 SEM images of biochar produced at 600 °C conditioned with A) 1 N HCl, B) 1 N NaOH, and C) Milli-Q water. Surface porosity and impurities vary with pre-conditioning. | ||
| Sample name | C [%] | H [%] | N [%] | S [%] | O [%] | Fixed carbon [%] | Volatiles [%] | Ash [%] | BET surface area [m3 g−1] |
|---|---|---|---|---|---|---|---|---|---|
| 600 °C Milli-Q | 30 | 1 | 4 | 0.8 | 4.2 | 16 | 24 | 60 | 21 |
| 600 °C HCl | 35 | 2 | 5 | 0.9 | 16.1 | 37 | 22 | 41 | 141 |
| Activated carbon | 82 | 0.9 | 0.5 | 0.8 | 5.8 | 87 | 3 | 10 | 755 |
| Heat-dried biosolids | 37 | 5 | 7 | 1 | 24 | 8 | 67 | 26 | 1 |
In addition to specific surface area, the fraction of fixed carbon and ash content can influence sorption. The ash content was lower in HCl-biochar than in Milli-Q-biochar (Table 1). The removal of ash during acid conditioning likely increased the porous carbon structure available for adsorption and increased the specific surface area. Thus, the cleaning and eroding effect of HCl conditioning makes it a suitable pre-conditioning step for enhancing the sorption capacity of biochar.
Previous research has shown that the surface chemistry of carbon-based adsorbents can be altered using inorganic acid modification. On carbon-based adsorbents, HCl conditioning increased weak or strong acidic oxygen functional groups and single-bonded oxygen functional groups such as phenols, ethers and lactones.38,39 For Calgon Carbon® Filtrasorb® 400 activated carbon, conditioning with 2 N HCl significantly affected functional group composition, as shown by FT-IR spectrum data indicating that the hydroxyl functional groups on the carbon were transformed into carboxylic, carbonyl, or ether groups after acid washing.40 These changes in surface chemistry enhanced phenol adsorption. Since the backbone structure and surface chemistry of HCl-biochar is similar to activated carbon, it is likely that similar chemical behavior occurs on the surface of biochar following acid conditioning.41,42 Indeed, the FT-IR spectra (Fig. S3†) shows differences among the three types of preconditioned biochar in this study. For HCl-treated biochar, the presence of broad bands at 1200 cm−1 and 600 cm−1 indicates that acid treatment increased carboxylic C–O bonds, such as phenol and aromatic C–H bonds, on the biochar surface. These shifts in chemical composition can alter H-bonding and π-interaction between the sorbent and solutes in water.38 The phenyl groups on triclosan molecules likely interact with phenol groups on HCl-biochar via hydrogen bonding, and aromatic groups on both adsorbate and adsorbent are able to form non-covalent π–π stacking,43 which supports the finding of increased adsorption on the HCl-biochar.
3.2 The impact of bulk solution pH on adsorption performance
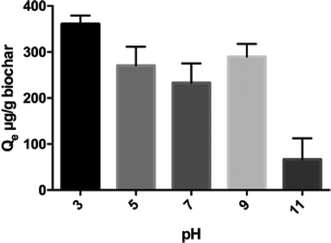
Changes in adsorption as a function of bulk solution pH are important not only from a practical standpoint, i.e., near-neutral pH is preferable in water/wastewater applications to avoid drastic pH adjustments, but also from a mechanistic perspective. Exploring the relationship between pH and adsorption helps to understand which mechanisms of adsorption play major roles in removal, e.g., electrostatic or non-electrostatic interactions, which enable science-based process design and operation.The bulk solution pH (tested from pH 3 to pH 11) significantly impacted the adsorption capacity of triclosan on biochar, as shown in Fig. 3 (ANOVA, p < 0.0001). While there was no statistical difference in sorption capacity at pH 5, 7, and 9, the overall trend from pH 3 to 11 suggests that triclosan adsorption increased as pH dropped. Protonated triclosan molecules dominate as pH drops below 7.9 (pKa of triclosan), and because they are more hydrophobic than the deprotonated anions, increased sorption is likely to occur on the biochar at lower water pH. The triclosan sorption capacity at pH 3 was greater than all other pH values (p < 0.05).
The bulk solution pH also affects the surface charge of the biochar. The point of zero charge (PZC) is the pH at which the number of negative charges are exactly offset by the number of positive charges on the surface, i.e., the net surface charge is zero.44 When solution pH is above the PZC, the biochar surface will carry a net negative charge, thus repulsing anions. Zeta potential measurements of the biochar in this study indicate a PZC below 4, where the PZC was approximately 3.28–3.5, 3–3.28, and <3 for 600 °C HCl, NaOH, and MilliQ-treated biochar, respectively (data shown in ESI,† section S5). When the biochar surface is positively charged (pH < PZC), essentially no deprotonated triclosan is present. Thus, direct electrostatic attraction cannot account for increased sorption at pH 3, and is unlikely to contribute to triclosan adsorption on biosolids-derived biochar.
Covalent bonding may lend itself to triclosan sorption as triclosan has both hydrogen donor and acceptor moieties, facilitating hydrogen bonding. As pH drops below the PZC, additional protonated functional groups may be present on the biochar surface, offering greater potential for hydrogen bonding, and perhaps contributing to the increase in triclosan sorption at pH 3.
Enhanced triclosan adsorption at pH 3 may also be attributed to the increased ionic strength when adjusting the solution pH with HCl. When not driven by electrostatic interactions, the adsorption of organic compounds has been shown to increase with bulk solution ionic strength, potentially due to shrinkage or aggregation of sorbates.45–47 Although ionic strength impacts could also be relevant at high pHs due to NaOH addition, the strong electrostatic repulsion between the negatively charged biochar surface and the deprotonated triclosan and the relative increase in hydrophilicity of the protonated triclosan are likely to dominate, leading to decreased adsorption, as observed in Fig. 3. The impact of ionic strength on triclosan adsorption should be investigated in future research.
When used for wastewater treatment applications, biosolids-derived biochar would most likely be used in near-neutral pH solutions. For practical usage, wastewater effluent pH is unlikely to be adjusted to acidic levels in order to achieve higher adsorption capacity, and it is possible that extreme acidic conditions might not be favorable for adsorption of other micropollutants. Accordingly, neutral pH is sufficient for practical use.
3.3 Isotherm modeling and the impact of pyrolysis temperature
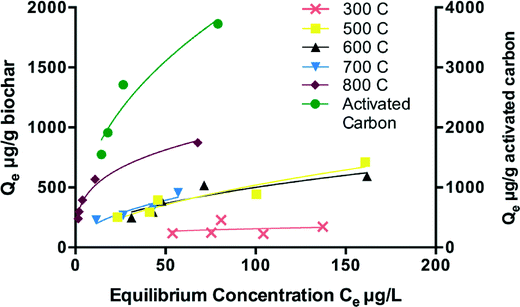
The sorption capacity as a function of equilibrium concentration was modeled using linear, Langmuir, and Freundlich isotherms, the complete results of which are shown in Table S1 in section S6 of the ESI.† Overall, the Freundlich model provided the best fit, which suggests that many layers of triclosan may adsorb to the biochar surface.31 With the exception of 300 °C biochar, the KF (capacity factor) generally increased while 1/n (n is an indicator of strength of bonding between sorbents and sorbates) decreased as pyrolysis temperature increased (Fig. 4 and Table 2). These results suggest that triclosan adhesion to the biochar surface increases with pyrolysis temperature. Generally, for the same feedstock, as pyrolysis temperature increases, biochar will have higher residual carbon content and higher aromaticity, which strengthens the bonding between organic compounds and char surface.43,48 Surface area also increases with pyrolysis temperature, likely due to loss of volatiles.49 Changes in these biochar surface properties with pyrolysis temperature suggest that 800 °C biochar will experience stronger interaction and higher adsorption capacity, thus supporting the observed increase in triclosan adsorption.| Isotherm model | Equation | Parameter | Sorbent | |||||
|---|---|---|---|---|---|---|---|---|
| 300 °C | 500 °C | 600 °C | 700 °C | 800 °C | Activated carbon | |||
| Freundlich | Q e = KFC1/ne | K F | 56.5 | 43.2 | 62.0 | 62.9 | 254 | 554 |
| 1/n | 0.22 | 0.54 | 0.45 | 0.46 | 0.30 | 0.44 | ||
| R 2 | 0.0593 | 0.912 | 0.835 | 0.85 | 0.977 | 0.928 | ||
Biochar acidity is also affected by pyrolysis temperature. Biochar produced at low temperature is usually acidic.33,34 This can greatly affect biochar's ability to remove acidic organic molecules in the deprotonated form. While surface acidity is relevant in some scenarios, it is important to note that, at neutral pH, the majority of triclosan molecules are protonated. Therefore, sorption mechanisms may rely more on hydrophobic interactions and partitioning, whereas biochar surface acidity could have less relative impact.
The adsorption behavior of activated carbon was similar to the 800 °C HCl-biochar (Fig. 4). The Freundlich 1/n value indicated that the bonding between the activated carbon and triclosan was weaker than the bonding between 800 °C biochar and triclosan, which could be attributed to the intrinsic difference between the different feedstocks. However, activated carbon offers greater triclosan adsorption capacity compared to all types of biochar tested. According to Table 1, activated carbon has a much lower ash content, higher carbon content, and higher BET surface area than biochar, which explains why commercialized activated carbon is a more effective adsorbent than the wastewater-derived biochar.
None of the isotherm models provided a good fit for the 300 °C biochar. Pyrolysis temperature can affect physical and chemical properties related to adsorption, resulting in differences in the biochar's triclosan adsorption capacity. The poor isotherm fits for the 300 °C biochar may be due to the lack of sorption caused by heterogeneity or low specific surface area (3.87 m2 g−1). Volatiles, such as py-oil, might be present at higher levels in biochar pyrolyzed at lower temperatures and may clog pores, thereby limiting available sorption sites. For 300 °C HCl-biochar, there was no change in capacity as equilibrium concentration increased. Thus, the adsorption sites on the 300 °C HCl-biochar were likely initially saturated with residual organic matter, thereby severely limiting the triclosan adsorption capacity. This suggests that pyrolysis temperatures above 300 °C are needed to produce biochar for use as a micropollutant adsorbent.
3.4 Adsorption performance using low chemical concentrations
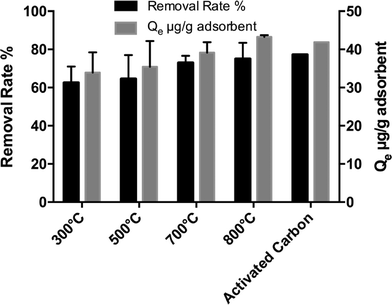
While activated carbon has higher adsorption capacity compared to biochars at high equilibrium concentrations, it does not differ significantly from the biochars at low equilibrium concentrations (ANOVA, p = 0.0748). Fig. 4 shows that at lower equilibrium concentrations, the isotherms appear to converge, which indicates that similar capacities may be observed for all of the biochars as well as activated carbon. Although testing with high triclosan concentrations in Milli-Q water gives an idea of the influence of solution pH and pyrolysis temperature on adsorption mechanisms, concentrations in actual WWTP effluents would likely be in the range of 0.02 μg L−1 to 20 μg L−1.50,51 As shown in Fig. 5, when an initial concentration of approximately 20 μg L−1 triclosan was used, there was no significant difference between sorption capacity of the HCl-biochars produced at 300–800 °C or activated carbon (ANOVA, p = 0.07). This result is significant in that for practical use at environmental levels of triclosan, biochar produced on-site at lower temperatures could perform as well as activated carbon.3.5 Triclosan adsorption on biochar in treated secondary effluent
Acid-treated biochar was tested in secondary treated municipal wastewater effluent to investigate the feasibility of triclosan adsorption in a complex matrix. For the same amounts of sorbent and triclosan, the triclosan removal rate decreased from 70 ± 10% in Milli-Q water to 32 ± 5.0% in wastewater effluent. The triclosan adsorption capacity in wastewater effluent was 239 ± 42 μg g−1 biochar, as compared to 518 ± 49 μg g−1 biochar in Milli-Q water (Fig. 4). The suppression effect of the wastewater matrix was expected due to the co-existence of TSS and organic constituents, which were present in higher concentrations relative to the triclosan (Table S2, ESI,† section S7). In control experiments where no biochar was added, triclosan concentrations were similar before and after the experiment, indicating minimal sorption to the wastewater solids. It is likely that the organic matter in the wastewater sorbed to the biochar and reduced available sites for triclosan to sorb on the biochar. Future work should focus on developing a mechanistic understanding of sorption competition due to complex wastewater matrices, thereby providing a means to improve the selectivity of biochar for target micropollutants.Conclusions
This work demonstrated that acid-conditioned (HCl) biosolids-derived biochar could be a suitable alternative to activated carbon for removing triclosan, a pervasive micropollutant, from water at near-neutral pH. Preconditioning of the biochar using acid was essential for triclosan adsorption. One practical limitation of using HCl to condition the biochar as a sorbent may be the cost of chemical inputs. Therefore, more work must be conducted to determine if less expensive acids, such as sulfuric acid, can be used as effectively as HCl for conditioning biochar. While acid preconditioning was necessary for triclosan adsorption, high pyrolysis temperatures do not appear to be necessary for production considering the low triclosan concentrations commonly encountered in environmental applications.Biochar characterization indicated that adsorption may occur mainly due to high surface area, hydrophobicity, and potential interaction between biochar and triclosan functional groups including hydrogen bonding, and π-stacking. Additional research should be conducted to evaluate biosolids-derived biochar as a sorbent for other compounds with varied pKa values and hydrophobicity.
Acknowledgements
Y. T. was supported by the National Science Foundation (NSF) Water Equipment and Policy Center (WEP) at Marquette University under Grant No. 0968844 and by the Lafferty Family Foundation. All opinions expressed in the paper are the authors' and do not necessarily reflect the views of NSF or WEP. We would like to express sincere thanks to Mr. Matthew Magruder from MMSD for his thoughtful guidance and insight throughout the project. Dr. Fournelle at Marquette University is greatly appreciated for his help with SEM imaging.References
- D. J. de Ridder, J. Q. J. C. Verberk, S. G. J. Heijman, G. L. Amy and J. C. van Dijk, Sep. Purif. Technol., 2012, 89, 71–77 CrossRef CAS.
- M. R. Servos, D. T. Bennie, B. K. Burnison, A. Jurkovic, R. McInnis, T. Neheli, A. Schnell, P. Seto, S. A. Smyth and T. A. Ternes, Sci. Total Environ., 2005, 336, 155–170 CrossRef CAS PubMed.
- L. Rossi, P. Queloz, A. Brovelli, J. Margot and D. A. Barry, PLoS One, 2013, 8(3), e58864 CAS.
- R. P. Schwarzenbach, B. I. Escher, K. Fenner, T. B. Hofstetter, C. A. Johnson, U. von Gunten and B. Wehrli, Science, 2006, 313, 1072–1077 CrossRef CAS PubMed.
- K. A. Kidd, P. J. Blanchfield, K. H. Mills, V. P. Palace, R. E. Evans, J. M. Lazorchak and R. W. Flick, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 8897–8901 CrossRef CAS PubMed.
- A. M. Vajda, L. B. Barber, J. L. Gray, E. M. Lopez, J. D. Woodling and D. O. Norris, Environ. Sci. Technol., 2008, 42, 3407–3414 CrossRef CAS PubMed.
- D. E. Carey and P. J. Mcnamara, Front. Microbiol., 2015, 5 Search PubMed.
- B. F. G. Pycke, L. A. Geer, M. Dalloul, O. Abulafia, A. M. Jenck and R. U. Halden, Environ. Sci. Technol., 2014, 48, 8831–8838 CrossRef CAS PubMed.
- J. Heidler and R. U. Halden, Chemosphere, 2007, 66, 362–369 CrossRef CAS PubMed.
- P. J. McNamara, T. M. Lapara and P. J. Novak, Environ. Sci. Technol., 2014, 48, 7393–7400 CrossRef CAS PubMed.
- B. D. Blair, J. P. Crago, C. J. Hedman, R. J. F. Treguer, C. Magruder, L. S. Royer and R. D. Klaper, Sci. Total Environ., 2013, 444, 515–521 CrossRef CAS PubMed.
- N. Lozano, C. P. Rice, M. Ramirez and A. Torrents, Water Res., 2013, 47, 4519–4527 CrossRef CAS PubMed.
- D. C. McAvoy, B. Schatowitz, M. Jacob, A. Hauk and W. S. Eckhoff, Environ. Toxicol. Chem., 2002, 21, 1323–1329 CrossRef CAS PubMed.
- H. Singer, S. Müller, C. Tixier and L. Pillonel, Environ. Sci. Technol., 2002, 36, 4998–5004 CrossRef CAS PubMed.
- K. Kimura, G. Amy, J. E. Drewes, T. Heberer, T.-U. Kim and Y. Watanabe, J. Membr. Sci., 2003, 227, 113–121 CrossRef CAS.
- M. Carballa, F. Omil, T. Ternes and J. M. Lema, Water Res., 2007, 41, 2139–2150 CrossRef CAS PubMed.
- B. M. K. Manda, E. Worrell and M. K. Patel, J. Cleaner Prod., 2014, 72, 153–166 CrossRef CAS.
- J. Margot, C. Kienle, A. Magnet, M. Weil, L. Rossi, L. F. de Alencastro, C. Abegglen, D. Thonney, N. Chèvre, M. Schärer and D. A. Barry, Sci. Total Environ., 2013, 461–462, 480–498 CrossRef CAS PubMed.
- W. A. W. A. K. Ghani, A. Mohd, G. da Silva, R. T. Bachmann, Y. H. Taufiq-Yap, U. Rashid and A. H. Al-Muhtaseb, Ind. Crops Prod., 2013, 44, 18–24 CrossRef.
- X. Cao and W. Harris, Bioresour. Technol., 2010, 101, 5222–5228 CrossRef CAS PubMed.
- M. Keiluweit, P. S. Nico, M. G. Johnson and M. Kleber, Environ. Sci. Technol., 2010, 44, 1247–1253 CrossRef CAS PubMed.
- J. L. Gaunt and J. Lehmann, Environ. Sci. Technol., 2008, 42, 4152–4158 CrossRef CAS PubMed.
- D. E. Carey, P. J. McNamara and D. H. Zitomer, Water Environ. Res., 2015, 87(12), 2098–2106 CrossRef CAS PubMed.
- Z. Liu and F.-S. Zhang, J. Hazard. Mater., 2009, 167, 933–939 CrossRef CAS PubMed.
- D. Mohan, S. Rajput, V. K. Singh, P. H. Steele and C. U. Pittman, J. Hazard. Mater., 2011, 188, 319–333 CrossRef CAS PubMed.
- Y. Yao, B. Gao, M. Inyang, A. R. Zimmerman, X. Cao, P. Pullammanappallil and L. Yang, Bioresour. Technol., 2011, 102, 6273–6278 CrossRef CAS PubMed.
- G. N. Kasozi, A. R. Zimmerman, P. Nkedi-Kizza and B. Gao, Environ. Sci. Technol., 2010, 44, 6189–6195 CrossRef CAS PubMed.
- C. Jung, J. Park, K. H. Lim, S. Park, J. Heo, N. Her, J. Oh, S. Yun and Y. Yoon, J. Hazard. Mater., 2013, 263(2), 702–710 CrossRef CAS PubMed.
- J. J. Ross, D. H. Zitomer, T. R. Miller, C. A. Weirich and P. J. McNamara, Environ. Sci.: Water Res. Technol., 2016, 2, 282–289 CAS.
- A. Sluiter, B. Hames, R. O. Ruiz, C. Scarlata, J. Sluiter, D. Templeton and D. of Energy, Biomass Anal. Technol. Team Lab. Anal. Proced., 2004, pp. 1–6 Search PubMed.
- R. P. Schwarzenbach, P. M. Gschwend and D. M. Imboden, Environmental organic chemistry, John Wiley & Sons, 2005 Search PubMed.
- APHA, AWWA and WEF, Standard Methods for the Examination of Water and Wastewater, 1998 Search PubMed.
- B. Singh, B. P. Singh and A. L. Cowie, Aust. J. Soil Res., 2010, 48, 516–525 CrossRef CAS.
- M. K. Hossain, V. Strezov, K. Y. Chan, A. Ziolkowski and P. F. Nelson, J. Environ. Manage., 2011, 92, 223–228 CrossRef CAS PubMed.
- S.-J. Park and Y.-S. Jang, J. Colloid Interface Sci., 2002, 249, 458–463 CrossRef CAS PubMed.
- J.-W. Shim, S.-J. Park and S.-K. Ryu, Carbon, 2001, 39, 1635–1642 CrossRef CAS.
- S. Wang, Z. H. Zhu, A. Coomes, F. Haghseresht and G. Q. Lu, J. Colloid Interface Sci., 2005, 284, 440–446 CrossRef CAS PubMed.
- J. P. Chen and S. Wu, Langmuir, 2004, 20, 2233–2242 CrossRef CAS PubMed.
- A. Dandekar, R. T. K. Baker and M. A. Vannice, Carbon, 1998, 36, 1821–1831 CrossRef CAS.
- P. Cañizares, M. Carmona, O. Baraza, A. Delgado and M. A. Rodrigo, J. Hazard. Mater., 2006, 131, 243–248 CrossRef PubMed.
- S. P. Sohi, E. Krull, E. Lopez-Capel and R. Bol, A review of biochar and its use and function in soil, 2010, vol. 105 Search PubMed.
- J. Lehmann and S. Joseph, Biochar for Environmental Management, Earthscan, 2009 Search PubMed.
- G. B. McGaughey, M. Gagné and A. K. Rappé, J. Biol. Chem., 1998, 273, 15458–15463 CrossRef CAS PubMed.
- A. Silber, I. Levkovitch and E. R. Graber, Environ. Sci. Technol., 2010, 44, 9318–9323 CrossRef CAS PubMed.
- M. Campinas and M. J. Rosa, J. Colloid Interface Sci., 2006, 299, 520–529 CrossRef CAS PubMed.
- Y. Aldegs, M. Elbarghouthi, A. Elsheikh and G. Walker, Dyes Pigm., 2008, 77, 16–23 CrossRef CAS.
- A. Delle Site, J. Phys. Chem. Ref. Data, 2001, 30, 187–439 CrossRef CAS.
- G. Newalkar, K. Iisa, A. D. D'Amico, C. Sievers and P. Agrawal, Energy Fuels, 2014, 28, 5144–5157 CrossRef CAS.
- G. Q. Lu, J. C. F. Low, C. Y. Liu and A. C. Lua, Fuel, 1995, 74, 344–348 CrossRef CAS.
- G.-G. Ying and R. S. Kookana, Environ. Int., 2007, 33, 199–205 CrossRef CAS PubMed.
- C. M. Foran, E. R. Bennett and W. H. Benson, Mar. Environ. Res., 2000, 50, 153–156 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ew00127k |
| This journal is © The Royal Society of Chemistry 2016 |