Reduced energy demand for municipal wastewater recovery using an anaerobic floating filter membrane bioreactor
M. D.
Seib
*,
K. J.
Berg
and
D. H.
Zitomer
Department of Civil, Construction and Environmental Engineering, Marquette University, P.O. Box 1881, Milwaukee, WI 53201-1881, USA. E-mail: matthew.seib@marquette.edu
First published on 23rd December 2015
Abstract
Anaerobic membrane bioreactor (AnMBR) technology for municipal wastewater has shown great promise for achieving low energy, low carbon footprint treatment. However, one potential drawback to fixed-film systems such as fluidized-bed reactors (FBR) is the need for high recycle flow rates to fluidize biocarrier. These high recycle rates could diminish the significant energy savings achieved from eliminating activated sludge aeration. In this study 3.3 L FBR and downflow floating media filter (DFF) AnMBRs were fed a synthetic primary effluent at 10 and 25 °C and evaluated on the basis of organic removal and energy demands. Both FBR and DFF configurations achieved BOD5 < 8 mg L−1 at bioreactor hydraulic retention times (HRT) ranging from 4.2–9.8 h. The DFF bioreactor required 60–75% less recycle energy than the FBR bioreactor while achieving organic removal similar to FBR and conventional aerobic treatment at 10 and 25 °C. Additionally, DFF AnMBR biotechnology coupled with nutrient and dissolved methane removal technologies are estimated to require between 30 and 50% less energy compared to an activated sludge process.
Water impactAn anaerobic biotechnology is described to improve the sustainability of municipal wastewater reclamation. The unique anaerobic membrane bioreactor (AnMBR) configuration described required 60–75% less energy than similar technology and achieved high organic removal at temperatures as low as 10 °C. |
Introduction
Sustainable scenarios for municipal wastewater management typically involve replacing aerobic systems with anaerobic biotechnology.1,2 Wastewater management scenarios for cities of the future emphasize water, energy, and nutrient (nitrogen, phosphorous, and potassium) recovery3,4 with reduced biosolids production and energy usage. For this, anaerobic treatment can be superior to aerobic processes.1,2,5 Furthermore, wastewater treatment can be decentralized to reuse water locally without the need for extensive conveyance systems. This can be done by constructing water reclamation facilities within self-contained eco-blocks or dense urban areas.6,7 Again, anaerobic systems may offer an advantage by requiring less footprint area than aerobic systems.Although anaerobic systems have benefits, challenges must be overcome before they can be widely employed for municipal water recovery in cold climates. For example, anaerobic biotechnology traditionally has been only applied to high-strength wastewater, manure, and biosolids.8,9 Further, anaerobic processes are traditionally performed at mesophilic or thermophilic temperatures (25–50 °C) which are cost prohibitive for municipal wastewater treatment if heating is required.10,11 Anaerobic biotechnology for municipal wastewater treatment must be feasible at low temperatures without reactor heating in order to be more sustainable for widespread application in cold/temperate climates.12,13
Low temperature operation, however, creates organic removal challenges for anaerobic systems. Low temperature decreases microorganism growth and metabolism rates, potentially leading to poor organic removal,9 especially at the short hydraulic residence times (HRTs) necessary for low energy and small footprint applications. Also, low strength municipal wastewater does not contain sufficient organic pollutant concentrations to produce enough methane to be practically useful if heating is necessary for effective treatment.10,11,14 Lastly, anaerobic processes convert a portion of the nitrogen and phosphorus to soluble ammonia and phosphate rather than removing them via nitrification/denitrification and biological accumulation as is done in aerobic processes. Therefore, additional nutrient removal steps often will be required after anaerobic treatment to achieve effluent quality sufficient for discharge to receiving waters.
Progress has recently been made to overcome the organic removal challenges faced by anaerobic municipal wastewater treatment. Over the past decade, anaerobic membrane bioreactor (AnMBR) technology has gained much attention to accomplish anaerobic treatment of municipal wastewater. Several reviews have summarized laboratory and pilot scale studies examining AnMBRs for both industrial and municipal applications.14–19 These studies focused on operational parameters such as HRT, solids retention time (SRT), temperature, membrane flux, transmembrane pressure (TMP), reactor design, and membrane configuration. Recent municipal wastewater low temperature (as low as 6 °C) AnMBR studies by Ho and Sung,20 Smith et al.,13 Smith et al.,21 and Shin et al.22 have all successfully demonstrated low organic concentrations in effluents (<20 mg L−1 five day biochemical oxygen demand (BOD5), <40 mg L−1 chemical oxygen demand (COD)) while employing different bioreactor and membrane configurations.
Existing AnMBR studies reveal two main strategies for bioreactor selection. In the first strategy, complete-mix stirred tank reactors (CSTRs) are used to maintain flocculent biomass.23 AnMBR studies employing CSTRs with submerged membranes have showed promising results with energy demands competitive to those required for organic removal with conventional activated sludge aeration.11,13,21 However, if operated with high suspended solids concentrations, membrane fouling potential is increased in these systems.24 In the second strategy, attached growth technologies such as the upflow anaerobic sludge blanket (UASB) or fluidized bed reactors (FBRs) are used to maintain granules or biofilms and reduce bulk liquid suspended solids, thus reducing bulk liquid foulants seen by the membrane.22 Biofilm technologies are often more efficient than flocculent systems because biofilm formation enhances interspecies substrate degradation and mass transfer and may allow for direct electron transfer between individual cells;25,26 making biofilm technologies a promising option.2,15,27–29
Advancement of AnMBR technology is dependent upon a reliable bioreactor and design that minimizes energy demands both for bioreactor and membrane operation. While biofilm technologies may demonstrate high substrate conversion rates, there are drawbacks such as difficulty forming granular biomass and retaining biosolids in UASB reactors30 and high energy requirements for fluidizing recycle flow in FBRs. Additionally, some reactors have been coupled to external crossflow membrane configurations that have historically required 3.0 to 7.3 kW h m−3 for membrane operation.16 This energy consumption is well above the 0.3 to 0.6 kW h m−3 typically required for activated sludge,31 and is also above the energy that can be gained from the CH4 produced. However, in recent years new methods of external membrane operation have been developed that drastically reduce energy demands. For example, Kim et al.29 operated a two-stage fluidized-bed AnMBR and indicated energy demands for the first-stage FBR accounted for 52% of total energy demand. In order to minimize energy needed to operate AnMBRs, bioreactor recycle pumping rates should be reduced.
The objectives of this study were to develop an AnMBR using a biological downflow floating media filter (DFF) that required less energy than a FBR to achieve effluent BOD5 concentrations less than 10 mg L−1 for municipal wastewater management and demonstrate the feasibility of implementing anaerobic biotechnology as a viable alternative to activated sludge. Alternative attached growth bioreactor configurations have been developed in the past including the anaerobic packed bed (APB) or anaerobic filter (AF),9,32 which require significantly lower recycle pumping rates than an FBR. However, these configurations have historically not been widely adopted.3 While membrane incorporation has been shown to improve organic removal in other bioreactor configurations, no reports were found describing membranes coupled to a DFF for low strength municipal wastewater recovery.
Materials and methods
AnMBR configurations
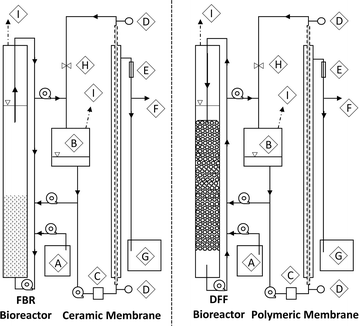
Two different AnMBR configurations, having different biofilm carrier materials, recycle flows and membrane types, were employed (Fig. 1). The first AnMBR configuration was a DFF utilizing buoyant media coupled to an external polymeric cross-flow membrane. The DFF bioreactor contained 165 g of buoyant media (Aqwise, Herzliya, Israel) and was operated with a downflow recycle velocity of 11 m h−1. The DFF polymeric tubular membrane consisted of two 750 mm long, 12.5 mm diameter polyvinylidene fluoride (PVDF) tubes (surface area = 0.059 m2) with nominal molecular weight cutoff of 100 kDa (∼0.018 μm nominal pore size) encased in a stainless steel housing (FP100, PCI Membranes, Fareham, UK). The second was a FBR using granular activated carbon (GAC) media coupled to an external ceramic cross-flow membrane. The FBR bioreactor contained 300 g of 12 × 30 mesh GAC (TIGG 5 DC 1230, TIGG Corp, Oakdale, PA) fluidized at an upflow velocity of 30 m h−1. The FBR ceramic tubular membrane was a single, 100 cm long, 16 mm diameter aluminum oxide tube (surface area = 0.05 m2) with a 0.05 μm nominal pore size encased in a stainless steel housing (Type 1/16, atech innovations, Gladbeck, Germany).Each bioreactor consisted of an 80 cm tall, 6.35 cm diameter clear polyvinylchloride tube with a working volume of 2.3 L. Each external membrane system consisted of an equalization tank, pulse dampener, and membrane unit with combined working volume of 1 L (Fig. 1). A recycle line was used to transfer retentate from the membrane equalization tanks back to the bioreactors. All membranes were mounted vertically and TMP was recorded at the top and bottom of each module using gauges (NOSHOK Inc., Berea, OH). Peristaltic pumps (Masterflex, Vernon Hills, IL) were used for bioreactor recycle, fluid transfer, and membrane cross-flow. Recycle head losses were determined using a digital manometer (EXTECH Instruments, Nashua, NH).
Bioreactor inocula and operation
Each bioreactor was inoculated with 2 g VSS of a biomass mix from five sources including two different mesophilic upflow anaerobic sludge blanket (UASB) reactors treating brewery wastewaters, a mesophilic municipal anaerobic digester treating primary and waste activated sludges, an ambient-temperature industrial anaerobic lagoon treating sugar beet waste, and a laboratory, mesophilic anaerobic propionate enrichment culture previously described by Tale et al.33Bioreactors were fed a synthetic primary effluent (SPE) wastewater that was modeled after primary effluent at the South Shore Water Reclamation Facility (Oak Creek, WI). SPE was formulated with constituents adapted from the SYNTHES recipe developed by Aiyuk and Verstraete34 and an inorganic nutrient media developed by Speece5 (Table 1). SPE contained the following average constituent concentrations in deionized water: 235 mg L−1 BOD5, 480 mg L−1 total chemical oxygen demand (TCOD), 18 mg L−1 ammonia nitrogen (NH3–N), 43 mg L−1 organic nitrogen (Norg) 2.5 mg L−1 phosphate–phosphorus (PO4−3–P), 5 mg L−1 total phosphorus (TP), 120 mg L−1 total suspended solids (TSS), and 115 mg L−1 volatile suspended solids (VSS).
| Constituent | mg L−1 |
|---|---|
| a The concentration of each compound was this value. | |
| Organic | |
| Non-fat dry milk | 133 |
| Soluble potato starch | 133 |
| Yeast extract | 67 |
| Casein peptone | 67 |
| CH3COONa·3H2O | 75 |
| Cysteine | 10 |
| Inorganic | |
| NaHCO3 | 510 |
| MgCl2·6H2O | 260 |
| CaCl2·2H2O | 275 |
| NaCl | 140 |
| NH4Cl | 64 |
| MgSO4 | 36 |
| FeSO4·7H2O | 23 |
| KCl | 12 |
| KI | 10 |
| MgHPO4·3H2O | 7 |
| (NaPO3)6 | 4 |
| CoCl2·6H2O; NiCl2·6H2O; ZnCl2 | 1 |
| MnCl2·4H2O; NH4VO3; CuCl2·2H2O; AlCl3·6H2O; NaMoO4·2H2O; H2BO3; NaWO4·2H2O; Na2SeO3 | 0.5a |
Each AnMBR configuration was evaluated at both 10 and 25 °C, for a total of four systems (FBR10, FBR25, DFF10, DFF25) to simulate temperatures typical for municipal wastewaters.31 During start-up, all AnMBRs were acclimated for 45 days at 25 °C with a total system HRT of 18 h (12.5 h bioreactor, 5.5 h membrane compartment). After day 45, the temperature in FBR10 and DFF10 AnMBRs was reduced to 10 °C. The AnMBRs were allowed to acclimate until day 79; during this time no performance data were collected. From day 80 to 145, total system HRT for all AnMBRs was reduced to 9 h. On day 146, HRT for each system was adjusted to the lowest value required to achieve membrane permeate BOD5 < 10 mg L−1. During acclimation, the influent flowrate to the AnMBRs was less than the membrane permeate flow rate and a portion of membrane permeate was returned to each membrane equalization tank. Once HRT was adjusted on day 146, the influent flow rate to some AnMBRs was greater than the membrane permeate flow, so any excess bioreactor flow to membrane equalization tanks was directly removed from the system before it passed through the membrane.
Membrane operation
The membranes were operated at target fluxes of 5.9 to 7.4 L m−2 h−1 by manually controlling TMP. The ceramic and polymeric membranes were operated at cross-flow velocities of 0.27 to 0.30 m s−1, respectively. Membranes were considered fouled when the average TMP increased above 0.9 bar. Once a membrane fouled, it was removed and cleaned by spraying the inside of the membrane tube with a water jet to remove the fouling cake layer then chemically cleaned by soaking in a high pH bath for 60 minutes and then with an acidic bath for 25 minutes. For the ceramic membrane, the high pH bath consisted of a solution of NaClO (200 ppm free chlorine) adjusted to a pH of 11 using 6 N NaOH. For the polymeric membrane, the high pH bath consisted of a solution of NaClO (200 ppm free chlorine) with a pH of 10. The acidic bath for both membranes consisted of distilled water adjusted to a pH of 2 using HNO3. Solids removed during cleaning were collected and quantified along with liquid wasted from equalization tanks to determine VSS mass wasting rate from each system.Analytical procedures
Influent and effluent BOD5, COD, NH3–N, Norg, PO4−3–P, TP, TSS, and VSS concentrations were determined by standard methods.35 Volatile fatty acid concentrations were determined by gas chromatography with a flame ionization detector (FID) (Agilent 7890A, Santa Clara, CA). Sulfate concentrations were determined using an ion chromatograph (Dionex ICS-1100, Sunnyvale, CA) and packed column (Ionpac AS22, Dionex, Sunnyvale, CA). Biogas methane and permeate dissolved methane content were determined using gas chromatography with a thermal conductivity detector (TCD) (Agilent 7890A, Santa Clara, CA). Biogas was collected in 2 L Tedlar bags and the volume quantified using a 140 mL syringe. Dissolved methane in membrane permeate was quantified using the method of Kim et al.29 Briefly, permeate samples were collected in 60 mL serum bottles that were previously dried and weighed. Each serum bottle contained 0.2 mL of 6 N NaOH. Serum bottles were filled with approximately 50 mL of permeate and immediately sealed with rubber stoppers. The sealed bottle was then weighed to determine the exact volume of liquid in the bottle. Bottles were then incubated at 35 °C and shaken at 200 rpm using an orbital shaker table for one hour. Serum bottle headspace gas was sampled and analyzed for methane content using gas chromatography and the initial dissolved methane concentration was calculated based on Henry's law and measured headspace methane content.Energy estimate
An energy estimate was performed to determine the overall energy requirements to treat 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3 per day municipal wastewater using either aerobic or anaerobic processes, both with primary sedimentation and anaerobic solids digestion. Energy inputs/outputs for different unit processes including BOD5 removal, nutrient recovery/removal, solids processing, anaerobic solids digestion, and energy generated from methane were determined from literature values. Activated sludge aeration energy required for BOD5 removal was reported by Speece.36 Energies required for conventional biological nutrient removal and solids processing as well as produced from methane in anaerobic solids digestion were obtained from previous literature.37 AnMBR flow normalized energy requirements for each bioreactor used in this study were determined using the power equation for pumping,38P = (QγE)/(Qiη), where P is power requirement per cubic meter treated (kW h m−3), Q is recycle flow rate (m3 s−1), γ is specific weight of water (kN m−3), E is headloss (m H2O), Qi is influent flow to that portion of the system (m3 h−1), and η is pump efficiency (assumed 66%). Recycle headlosses were determined for the FBR and DFF bioreactors using a manometer.
000 m3 per day municipal wastewater using either aerobic or anaerobic processes, both with primary sedimentation and anaerobic solids digestion. Energy inputs/outputs for different unit processes including BOD5 removal, nutrient recovery/removal, solids processing, anaerobic solids digestion, and energy generated from methane were determined from literature values. Activated sludge aeration energy required for BOD5 removal was reported by Speece.36 Energies required for conventional biological nutrient removal and solids processing as well as produced from methane in anaerobic solids digestion were obtained from previous literature.37 AnMBR flow normalized energy requirements for each bioreactor used in this study were determined using the power equation for pumping,38P = (QγE)/(Qiη), where P is power requirement per cubic meter treated (kW h m−3), Q is recycle flow rate (m3 s−1), γ is specific weight of water (kN m−3), E is headloss (m H2O), Qi is influent flow to that portion of the system (m3 h−1), and η is pump efficiency (assumed 66%). Recycle headlosses were determined for the FBR and DFF bioreactors using a manometer.
To compare nutrient removal in aerobic and anaerobic systems, ion exchange was assumed for recovery of N and P in the anaerobic system and the energy requirement for ion exchange systems (both N and P) was reported by Howe et al.39 Energies for anaerobic biosolids digestion for the AnMBR systems were considered to be 75% of that of activated sludge systems; this assumes a 25% reduction in the overall dry mass of waste biosolids that need to be processed from combining primary sludge with solids removed from AnMBR primary effluent treatment versus primary sludge combined with activated sludge treatment of primary effluent. Energy needed for dissolved methane stripping/recovery was reported by McCarty et al.2 AnMBR energy generation from methane production was estimated based on COD reduction assuming 0.28 m3 CH4 per kg COD removed (1 atm, 0 °C) and 37 MJ m−3 CH4.40 From estimated AnMBR produced methane, electrical energy production was estimated assuming 33% conversion of methane energy to electricity.29
Results and discussion
AnMBR performance and organic removal comparison
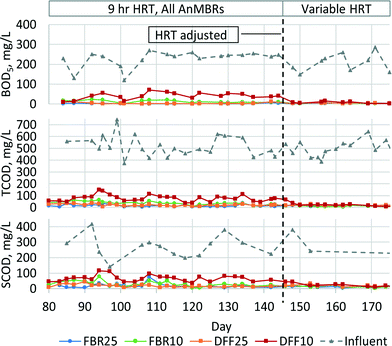
All AnMBRs produced high quality effluent based on BOD5, with average permeate concentration less than 5 mg L−1 for the FBR25, FBR10, and DFF25 systems and less than 8 mg L−1 for the DFF10 after day 146 (Fig. 2). It should be noted that HRT in all systems was adjusted on day 146 to achieve average permeate BOD5 concentration less than 10 mg L−1, resulting in bioreactor HRT values for the FBR25, DFF25, FBR10 and DFF10 systems of 4.2 h, 4.2 h, 5.6 h, and 9.8 h, respectively. Permeate BOD5 consistently remained low once HRT values were adjusted.All AnMBRs achieved greater than 95% TCOD removal. Average permeate TCOD concentrations were less than 14 mg L−1 for DFF25 and DFF10 systems and less than 25 mg L−1 TCOD for the FBR25 and FBR10 systems after day 145. Permeate TCOD and soluble chemical oxygen demand (SCOD) were similar, as was expected since the membrane nominal pore sizes were smaller than the standard 0.45 μm filter used for SCOD analysis.41 Influent organics were converted to CH4, which was detected in biogas and dissolved in membrane permeate (Table 2). Average CH4 production was low because of poor capture due to suspected leaking from system headspace. Influent SPE sulfate concentration was 35 mg L−1 SO4−2 and, if reduced to sulfide, would account for removal of 23 mg L−1 TCOD. Average VSS production ranged from 0.01–0.07 VSS g−1 CODr.
| FBR25 | FBR10 | DFF25 | DFF10 | |
|---|---|---|---|---|
| Gaseous | 119 | 45 | 109 | 37 |
| Dissolved | 37 | 30 | 36 | 28 |
| Total | 156 | 75 | 145 | 65 |
Average influent phosphorus to each system was 40% phosphate, whereas effluent from each system was approximately 100% phosphate, indicating essentially all phosphorus leaving each AnMBR had been fully converted to phosphate. Approximately 0.9 and 1.2 mg L−1 of influent total phosphorus to the 25 °C and 10 °C systems, respectively, was apparently incorporated into biomass. Average influent nitrogen to each system was 40% NH3–N, whereas effluent from each AnMBR was 85% NH3–N. Approximately 6.5 mg L−1 of influent total nitrogen was apparently incorporated into biomass.
AnMBRs were able to achieve the same organic removal as conventional activated sludge technology under similar hydraulic loading conditions. Both the FBR25 and DFF25 systems achieved the same organic removal efficiency while operating at a 4.2 h bioreactor HRT, indicating little difference in BOD5 removal based on the type of fixed-film media or membrane material selected under the same temperature and hydraulic conditions. Results from all four AnMBRs in this study were comparable to results found in other recent AnMBR studies describing different configurations (Table 3). Permeate BOD5 concentrations observed herein were within typical values reported for conventional activated sludge treatment with biological nutrient removal (5–20 mg L−1 BOD5).31 Additionally, AnMBR solids production rates (0.01–0.07 g VSS g−1 CODr) were much lower than typical solids yields of 0.4–0.7 g VSS g−1 BODr for aerobic activated sludge.31 Therefore, energy for solids processing is expected to be lower for anaerobic versus activated sludge systems. It should also be noted that while this study lasted 175 days, this may not have been sufficient time for complete biomass adaptation, especially in the 10 °C AnMBRs. Additional operation time may have resulted in the ability to further reduce system HRTs while maintaining the same target effluent quality.
| This study | Ho and Sung20 | Smith et al.13 | Shin et al.22 | ||
|---|---|---|---|---|---|
| Bioreactor | FBR | DFF | Complete mix | Complete mix | FBR |
| Membrane | External tubular, ceramic | External tubular, polymeric | External tubular, polymeric | Internal flat sheet, polymeric | Internal hollow fiber, polymeric |
| Waste type | Synthetic primary effluent | Synthetic primary effluent | Synthetic primary effluent | Synthetic wastewater | Real wastewater |
| Scale | Bench | Bench | Bench | Bench | Pilot |
| HRT (h) | 6–8 | 6–14 | 6–12 | 16 | 4.5–6.8 |
| Temperature (°C) | 10–25 | 10–25 | 25 | 15 | 8–30 |
| Inf. COD (mg L−1) | 500 | 500 | 500 | 440 | 198–362 |
| Eff. COD (mg L−1) | <14 | <25 | <40 | 36 | <25 |
| Eff. BOD5 (mg L−1) | <4 | <8 | — | 18 | <10 |
The decrease in BOD5 and TCOD permeate concentration seen in the DFF10 AnMBR when HRT was increased on day 146 (Fig. 2) demonstrated that permeate from this system contained readily biodegradable BOD5 when operated at a 9 h total system HRT. The required HRT increase for DFF10 AnMBR was consistent with expectations of reduced biomass activity at lower temperature. The relatively longer HRT necessary to achieve permeate BOD5 less than 10 mg L−1 from DFF10 is likely due to a lower biomass concentration on the DFF media compared to FBR media and/or due to substrate diffusion limitations with thicker biofilm layers expected on the DFF media.42,43 If the longer required HRT for DFF10 was due to lower biomass concentration, then these results indicate that at low temperature additional time may be needed to grow sufficient biomass to reduce HRT. In contrast, if the longer HRT for DFF10 was due to substrate diffusion limitations, then efforts to maintain a thin biofilm on the DFF media may be beneficial to reduce HRT.
Energy requirements
Flow normalized recycle energy requirements for the DFF bioreactor were 60–75% lower compared to the FBR bioreactor (Fig. 3). Membrane recycle energy requirements ranged between 1.9 and 2.2 kW h m−3 for the ceramic systems and 3.3 to 3.8 kW h m−3 for the polymeric membranes, depending on temperature.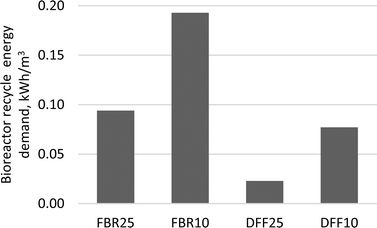 | ||
| Fig. 3 AnMBR flow normalized bioreactor recycle energy requirements to achieve permeate <10 mg L−1 BOD5. | ||
Anaerobic biotechnology can offer significant energy savings compared to activated sludge for BOD5 removal by eliminating the need for aeration and offsetting internal energy needs by producing methane that can be used as fuel. However, these savings may be diminished by the pumping demands or membrane biogas sparging required for various membrane bioreactor configurations. Configurations such as the FBR require recycle pumping at rates much higher than influent flow to fluidize the biocarrier. Since recycle pumping is fixed relative to hydraulic loading, it is imperative to minimize HRT, not simply to keep bioreactor volume to a minimum, but also to minimize the amount of energy needed per unit of flow treated. For example, the energy requirements for FBR10 and DFF10 bioreactors were higher relative to the FBR25 and DFF25 bioreactors due to the formers' increased HRT and increased headloss from the viscosity increase due to lower temperature. Therefore, special attention should be given to minimize headlosses from piping and unnecessary pumping in order to optimize hydraulic efficiency. The DFF bioreactors in this study required between 60 and 75% less energy than the FBRs due to significantly lower recycle pumping requirements. The DFF systems were also able to achieve the same organic removal as the FBR systems, which demonstrates fixed-film bioreactor technology does not necessarily require high recycle rates to produce low effluent BOD5.
Recovery needs for converted substrates
Nutrient removal remains a challenge when using AnMBRs. Aerobic processes can successfully remove nitrogen and accumulate phosphorus in wasted biosolids. Anaerobic biotechnology, on the other hand, converts nitrogen to soluble ammonia and phosphorus to soluble phosphate. Both of these products typically must be recovered or removed in order to prevent environmental degradation in the form of eutrophication.44,45Most of the N and P entering the AnMBRs was converted to ammonia and phosphate in membrane permeate. In order for AnMBRs to become more widely applicable, nutrients must be removed or recovered before they enter receiving waters. Since the AnMBR permeate in this study was virtually free of organic carbon and oxygen, conventional aerobic biological nutrient removal processes after AnMBR treatment may not be suitable. Partial nitritation/nitrification coupled with Anammox18,46 has been suggested as an autotrophic biological process to remove nitrogen with an energy demand of 1.2 kW h kg−1 N removed,47 but process control is challenging for mainstream applications, whereas it is more easily applied to digested sludge filtrate with high ammonia concentration at mesophilic temperatures.14
In contrast, physical/chemical processes such as ion exchange48 or struvite precipitation4 may be more sustainable than biological methods. Ion exchange may be appropriate because AnMBR permeate contains no suspended solids that can clog ion exchange beds and most of the N and P exiting AnMBRs is in the form of ammonia and phosphate that can be captured using ion exchange resins. Struvite precipitation, on the other hand, requires the addition of magnesium and can only remove a portion of the nitrogen since the maximum extent of struvite formation from municipal wastewater is typically phosphate limited when excess magnesium is added. Nutrient recovery and concentration using ion exchange may be particularly attractive since concentrated nutrients in ion exchange regeneration brine could be utilized in agricultural applications to offset new fertilizer production.49,50
Dissolved methane lost in AnMBR permeate poses a concern as a greenhouse gas, especially at lower temperature operation when methane solubility is higher.19,51 Dissolved methane lost in membrane permeate can also result in lost renewable energy available from biogas. Air stripping has been proposed to recover dissolved methane from AnMBR permeate,2 with the off-gas blended with primary sludge anaerobic digester biogas for energy production in internal combustion engines. Air stipping would also help aerate AnMBR permeate to increase dissolved oxygen concentration prior to discharge. This also may be achieved simply by cascading the effluent or with a small aeration basin, but special attention should be given to greenhouse gas collection as well as potential concerns with sulfurous gasses52,53 and odors.54
Recovery needs for converted substrates
While energy reduction in bioreactor operation is important, it is clear that the high energy demand for traditional external cross-flow membrane operation is not economical compared to the 0.3–0.6 kW h m−3 typically required for activated sludge.31 The cross-flow tubular membranes used in this study were operated at cross-flow velocities significantly lower than traditionally used velocities of 2 to 3 m s−1,16 but still the energy demand was 2 to 3 kW h m−3. It should be noted, however, that the AnMBRs were not optimized to minimize head losses and membranes were operated at relatively low fluxes. Estimates conducted by Le-Clech et al.55 on previous AnMBR studies showed that a cross-flow membrane operated at low cross-flow velocity and flux of 30 L m−2 h−1 was expected to require 0.23 kW h m−3. This demonstrates that hydraulic optimization and proper membrane selection can significantly reduce membrane energy requirements. The membrane energy estimate of Le-Clech et al.55 along with DFF energy results from this study result in a total AnMBR system energy demand of approximately 0.25–0.31 kW h m−3. This significant result shows that AnMBRs can be energy competitive with the activated sludge process for BOD5 removal, even without considering the renewable energy gains made from utilizing produced methane.Overall, AnMBR treatment coupled with ion exchange for nutrient recovery and air stripping for dissolved methane recovery is expected to require 30–50% less energy than current aerobic treatment with biological nutrient removal (Table 4). The wide range in energy reduction for AnMBRs is due to the large variability of required HRT values and head losses observed in this study. Previous estimates that municipal wastewater anaerobic treatment can result in an energy positive process2,36 are challenging to achieve based on requirements for recycle flow, nutrient removal and/or dissolved methane removal. Nutrient removal and dissolved methane processes are expected to account for one third of the total energy demand for municipal water recovery by anaerobic treatment. Energy potential from AnMBR biogas production may be enough to offset energy demands for ion exchange nutrient recovery and effluent dissolved methane recovery, but is not estimated herein to satisfy all energy demands. More research is required to optimize systems and reduce total energy requirements for AnMBR systems.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3 per day municipal wastewater
000 m3 per day municipal wastewater
| Treatment process | Aerobic treatment with nitrification kW h d−1 | Anaerobic treatment with ion exchange kW h d−1 |
|---|---|---|
| a From Speece.36 b From WEF.37 c From this study and Le-Clech et al.55 d From Howe et al.39 e From McCarty et al.2 | ||
| Aeration (diffused air)a | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— |
| Biological nitrificationb | 3400 | — |
| AnMBRc | — | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100–12 100–12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
| Ion exchange nutrient removald | — | 4800 |
| Anaerobic digestionb | 1700 | 1300 |
| Belt filter pressb | 500 | 350 |
| Dissolved methane recoverye | — | 2000 |
| Energy recovered from AnMBR biogas | — | (8900) |
| Energy from primary digester biogasb | (3500) | (2600) |
| Total (kW h d−1) | 14100 | 7050–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
| kW h m−3 treated | 0.35 | 0.18–0.25 |
Future work
DFF AnMBRs achieved the same organic removal as conventional activated sludge technology and were estimated to require between 30 and 50% less energy than currently required for activated sludge. Future work should focus on hydraulic optimization to reduce pumping and head losses and on optimal membrane selection to maximize hydraulic loading while minimizing energy demands. Additionally, low energy processes for dissolved methane and nutrient removal should be identified for the anaerobic permeate from an AnMBR.Conclusions
Bench scale AnMBRs utilizing different fixed-film media were operated to treat synthetic primary effluent municipal wastewater at 10 and 25 °C. Effluent BOD5 less than 8 mg L−1 were observed for all AnMBR systems, even at 10 °C, indicating the AnMBRs are able to achieve high organic removal rates greater than 95% while treating low-strength municipal wastewater. The DFF bioreactor in this study required 60–75% less energy for recycle pumping than the FBR configuration, demonstrating that low energy alternatives to high recycle fixed-film anaerobic systems are possible. Additionally, a DFF AnMBR coupled with additional steps to remove nutrients and dissolved methane was estimated to require 30–50% less energy than currently required for activated sludge. Further investigation is needed to understand hydraulic loading limitations, optimal selection of cross-flow membranes, and strategies to minimize headlosses to reduce energy demands. Additionally, dissolved methane and nutrient removal requires additional study in order to identify low energy processes well-suited for the low carbon, anaerobic permeate from an AnMBR.Acknowledgements
The authors wish to thank Xylem, Inc. for assisting with project development and for providing materials, funding, and technical support throughout this study. This article was also developed under STAR Fellowship Assistance Agreement no. FP-91766901-0 awarded by the U.S. Environmental Protection Agency (EPA). It has not been formally reviewed by EPA. The views expressed in this article are solely those of the authors and EPA does not endorse any products or commercial services mentioned in this article.References
- W. Verstraete and S. Vlaeminck, Int. J. Sustain. Dev. World Ecol., 2011, 18, 253–264 CrossRef.
- P. L. McCarty, J. Bae and J. Kim, Environ. Sci. Technol., 2011, 45, 7100–7106 CrossRef CAS.
- J. B. van Lier, Water Sci. Technol., 2008, 57, 1137–1148 CrossRef CAS PubMed.
- W. Mo and Q. Zhang, J. Environ. Manage., 2013, 127, 255–267 CrossRef CAS PubMed.
- R. Speece, Anaerobic Biotechnology and Odor/Corrosion Control for Municipalities and Industries, Archae Press, Nashville, TN, 2008 Search PubMed.
- V. Novotny, Water Sci. Technol., 2011, 63, 184–190 CrossRef CAS PubMed.
- J. B. van Lier and G. Lettinga, Water Sci. Technol., 1999, 40, 171–183 CrossRef.
- A. van Haandel, M. T. Kato, P. F. F. Cavalcanti and L. Florencio, Rev. Environ. Sci. Bio/Technol., 2006, 5, 21–38 CrossRef CAS.
- M. S. Switzenbaum, Enzyme Microb. Technol., 1983, 5, 242–250 CrossRef CAS.
- G. Lettinga, S. Rebac and G. Zeeman, Trends Biotechnol., 2001, 19, 363–370 CrossRef CAS.
- I. Martin, M. Pidou, A. Soares, S. Judd and B. Jefferson, Environ. Technol., 2011, 32, 921–932 CrossRef CAS.
- G. Collins, S. McHugh, S. Connaughton, A.-M. Enright, A. Kearney, C. Scully, T. Mahony, P. Madden and V. O'Flaherty, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2006, 41, 881–895 CrossRef CAS.
- A. L. Smith, S. J. Skerlos and L. Raskin, Water Res., 2013, 47, 1655–1665 CrossRef CAS PubMed.
- A. L. Smith, L. B. Stadler, N. G. Love, S. J. Skerlos and L. Raskin, Bioresour. Technol., 2012, 122, 149–159 CrossRef CAS PubMed.
- P. M. Sutton, P. Berube and E. R. Hall, Membrane bioreactors for anaerobic treatment of wastewaters, Water Environment Research Foundation, Alexandria, VA, 2004 Search PubMed.
- B. Q. Liao, J. T. Kraemer and D. M. Bagley, Crit. Rev. Environ. Sci. Technol., 2006, 36, 489–530 CrossRef CAS.
- G. Skouteris, D. Hermosilla, P. López, C. Negro and Á. Blanco, Chem. Eng. J., 2012, 198–199, 138–148 CrossRef CAS.
- D. C. Stuckey, Bioresour. Technol., 2012, 122, 137–148 CrossRef CAS PubMed.
- H. Lin, W. Peng, M. Zhang, J. Chen, H. Hong and Y. Zhang, Desalination, 2013, 314, 169–188 CrossRef CAS.
- J. Ho and S. Sung, Water Environ. Res., 2009, 81, 922–928 CrossRef CAS.
- A. L. Smith, S. J. Skerlos and L. Raskin, Environ. Sci.: Water Res. Technol., 2015, 1, 56–64 CAS.
- C. Shin, P. L. McCarty, J. Kim and J. Bae, Bioresour. Technol., 2014, 159, 95–103 CrossRef CAS PubMed.
- J. Ho and S. Sung, Bioresour. Technol., 2010, 101, 2191–2196 CrossRef CAS PubMed.
- F. Meng, S.-R. Chae, A. Drews, M. Kraume, H.-S. Shin and F. Yang, Water Res., 2009, 43, 1489–1512 CrossRef CAS PubMed.
- P. L. McCarty and D. P. Smith, Environ. Sci. Technol., 1986, 20, 1200–1206 CrossRef CAS.
- M. Morita, N. S. Malvankar, A. E. Franks, Z. M. Summers, L. Giloteaux, A. E. Rotaru, C. Rotaru and D. R. Lovleya, mBio, 2015, 2(4), e00159 Search PubMed.
- S. McHugh, G. Collins, T. Mahony and V. O'Flaherty, Water Sci. Technol., 2005, 52, 107–113 CAS.
- B. E. Rittmann and P. L. McCarty, Environmental Biotechnology: Principles and Applications, McGraw-Hill, 2001 Search PubMed.
- J. Kim, K. Kim, H. Ye, E. Lee, C. Shin, P. L. McCarty and J. Bae, Environ. Sci. Technol., 2011, 45, 576–581 CrossRef CAS PubMed.
- V. O'Flaherty, G. Collins and T. Mahony, Rev. Environ. Sci. Bio/Technol., 2006, 5, 39–55 CrossRef.
- Metcalf & Eddy, Wastewater Engineering, Treatment and Reuse, 4th edn, 2003 Search PubMed.
- R. Speece, Environ. Sci. Technol., 1983, 17, 416A–427A CrossRef CAS.
- V. P. Tale, J. S. Maki, C. A. Struble and D. H. Zitomer, Water Res., 2011, 45, 5249–5256 CrossRef CAS.
- S. Aiyuk and W. Verstraete, Bioresour. Technol., 2004, 93, 269–278 CrossRef CAS.
- APHA, AWWA and WEF, Standard Methods for the Examination of Water and Wastewater, American Water Works Association, 20th edn, 1999 Search PubMed.
- R. Speece, Anaerobic Biotechnology for Industrial Wastewaters, Archae Press, Nashville, TN, 1996 Search PubMed.
- WEF, Energy Conservation in Water and Wastewater Treatment Facilities, WEF Manual of Practice No. 32, WEF Press, Alexandria, VA, 2009 Search PubMed.
- R. Yoo, J. Kim, P. L. McCarty and J. Bae, Bioresour. Technol., 2012, 120, 133–139 CrossRef CAS.
- K. J. Howe, D. W. Hand, J. C. Crittenden, R. R. Trussell and G. Tchobanoglous, Principles of Water Treatment, John Wiley & Sons, Inc., Hoboken, NJ, 2012 Search PubMed.
- N. Khartchenko, N. V. Khartchenko and V. M. Kharchenko, Advanced Energy Systems, Taylor & Francis, Washington DC, 1997 Search PubMed.
- H. Melcer, Methods for wastewater characterization in activated sludge modeling, IWA Publishing, 2004 Search PubMed.
- B. E. Rittmann and J. Manem, Biotechnol. Bioeng., 1992, 39, 914–922 CrossRef CAS PubMed.
- Environmental Microbiology, ed. R. Mitchell and J.-D. Gu, Wiley-Blackwell, Hoboken, NJ, 2nd edn, 2010 Search PubMed.
- L. Sala and R. Mujeriego, Water Sci. Technol., 2001, 43, 109–116 CAS.
- WEF, Nutrient Removal, WEF Manual of Practice No. 34, WEF Press, Alexandria, VA, 2010 Search PubMed.
- A. A. van de Graaf, P. de Bruijn, L. A. Robertson, M. S. M. Jetten and J. G. Kuenen, Microbiology, 1996, 142, 2187–2196 CrossRef CAS.
- D. J. Batstone, T. Hülsen, C. M. Mehta and J. Keller, Chemosphere, 2015, 140, 2–11 CrossRef CAS.
- S. Aiyuk, I. Forrez, D. K. Lieven, A. van Haandel and W. Verstraete, Bioresour. Technol., 2006, 97, 2225–2241 CrossRef CAS PubMed.
- B. E. Rittmann, B. Mayer, P. Westerhoff and M. Edwards, Chemosphere, 2011, 84, 846–853 CrossRef CAS.
- A. T. Williams, D. H. Zitomer and B. K. Mayer, Environ. Sci.: Water Res. Technol., 2015, 1, 832–838 CAS.
- M. Hatamoto, H. Yamamoto, T. Kindaichi, N. Ozaki and A. Ohashi, Water Res., 2010, 44, 1409–1418 CrossRef CAS PubMed.
- M. von Sperling, Wastewater characteristics, treatment and disposal, Volume 1, IWA Publishing, New York, 2007 Search PubMed.
- A. van Haandel and J. G. M. van der Lubbe, Handbook of Biological Wastewater Treatment: Design and Optimisation of Activated Sludge Systems, IWA Publishing, 2012 Search PubMed.
- M. S. Switzenbaum, Bioresour. Technol., 1995, 53, 255–262 CrossRef CAS.
- P. Le-Clech, V. Chen and T. A. G. Fane, J. Membr. Sci., 2006, 284, 17–53 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |