Pretreatment of natural organic matter to control biological stability
Mahdi
Bazri
and
Madjid
Mohseni
*
Department of Chemical and Biological Engineering, University of British Columbia, 2360 East Mall Vancouver, BC V6T 1Z3, Canada. E-mail: madjd.mohseni@ubc.ca; Fax: +1 604 822 6003; Tel: +1 604 822 0047
First published on 16th December 2015
Abstract
Application of UV/H2O2 process for degradation of micropollutants in surface waters could deteriorate the biological stability of treated water. This is because of the partial oxidation of natural organic matter under the applied UV/H2O2 conditions that in turn leads to an increase in assimilable organic carbon (AOC). To address this issue, alum coagulation was investigated as a NOM pretreatment alternative prior to the UV/H2O2 process in order to improve the treatment efficacy and water quality. A recently developed technique was utilized to rapidly assess the AOC of the treated water at various stages. Alum was effective in removing a substantial portion of large to medium molecular weight organic molecules leading to a considerable reduction in AOC. However, the fractions not removed by coagulation were shown to promote some levels of bacterial regrowth after undergoing subsequent UV/H2O2 treatment. That said, alum pretreatment was found to be an effective strategy for reducing the formation of AOC by 14 to 40% depending on the water used and UV dose applied. The findings of this study are of interest for utilities that already have coagulation in use and seek to comply with more upcoming stringent regulations by incorporating advanced oxidation processes (e.g., UV/H2O2) in their treatment train.
Water impactThe biostability of surface waters potentially deteriorates as a result of the partial oxidation of natural organic matter under UV/H2O2 treatment that is applied to the removal of micropollutants. This research highlights the impact of using coagulation to remove natural organic matter prior to the UV/H2O2 process by utilizing a robust and rapid technique for gauging the changes in the biostability. |
1. Introduction
Advanced oxidation processes (AOPs, e.g., UV/H2O2) are one of the most effective alternatives for the elimination of organic and trace-level micropollutants in water.1–4 However, the efficacy of UV/H2O2 for the abatement of target contaminants is diminished by the presence of natural organic matter (NOM) in water. During UV-based AOPs (e.g., UV/H2O2 treatment), NOM shields UV and scavenges OH radicals (HO˙) generated during the process, thereby reducing the efficacy of the treatment. The UV dose and H2O2 concentration applied are designed to degrade micropollutants and therefore NOM is only partially oxidized and is broken down into smaller, more biodegradable molecules.5,6 These smaller organics are reported to be one of the major contributors to bacterial regrowth and biofilm formation within distribution systems and are usually referred to as assimilable organic carbon (AOC).7–9 Therefore, removing NOM (i.e., completely or partially) via a pretreatment process could potentially enhance the performance of UV/H2O2 at removing micropollutants and also improving the biostability of treated water.Several processes have been proposed and examined in the literature for the removal of NOM under various water loadings and qualities.10–22 Among the options proposed, coagulation processes such as those using alum, ferric chloride and PACl are well established and commonly applied in large-scale applications.12,13,17,23 Moreover, they could serve as viable pretreatment strategies prior to the UV/H2O2 process because of the recognized ability of coagulants (e.g., alum, ferric chloride, PACl) to remove a considerable portion of medium to high molecular weight NOM, and their relatively straightforward operation.24–27 Therefore, the main objective of this research was to gauge the impact of coagulation (e.g., alum) on the degradation of NOM and its subsequent effect on the biological stability (i.e., AOC) of UV/H2O2-treated water.
Two natural water sources were selected and preliminary coagulation tests were conducted to assess the optimum alum dose for NOM removal. Changes in the physicochemical properties (such as UV254, total organic carbon (TOC) content and NOM molecular weight distribution) of raw, alum-treated, UV/H2O2-treated and alum–UV/H2O2-treated water samples were carefully assessed and monitored. A recently developed AOC bioassay using flow cytometry29 that was previously modified for UV/H2O2-treated waters28 was utilized to quantify AOC in all stages. The findings of this research are potentially of interest for those utilities that already use coagulation processes (e.g., alum, ferric chloride, PACl) and hence could readily implement advanced oxidation processes (e.g., UV/H2O2) in their treatment train to meet more stringent guidelines in future.
2. Materials and methods
2.1. Source water characteristics
Raw waters for this study were collected from Bowen Island (BI, UV254 = 0.183 cm−1, TOC ~4.81 ppm, pH ~6.7) and Capilano Reservoir (CR, TOC ~1.45 ppm, UV254 = 0.061 cm−1, pH ~6.3), both located in British Columbia, Canada.2.2. UV/H2O2 treatment
A collimated beam set-up utilizing a low-pressure (LP) high-output amalgam lamp was used to conduct UV/H2O2 treatment experiments. The LP UV lamp was selected because of its wider application due to lower cost and energy use as well as lower potential of forming hazardous by-products.1,3,30–33 Water samples were initially filtered (0.45 μm) and spiked to an initial H2O2 concentration of ∼10 ppm (H2O2 30%, Fisher Scientific). Next, the samples were irradiated to the desired UV fluence (i.e., up to 2000 mJ cm−2) as determined by chemical actinometry.34 Experimental conditions (e.g., UV dose and H2O2 concentration) were selected based on previous research studies1,5,6,16,30,35,36 and detailed description of the collimated beam set-up and the experimental procedure is also described elsewhere.6,352.3. Coagulation-UV/H2O2 treatment
Alum (Al2(SO4)3·18H2O, ACS reagent +98%, Sigma Aldrich) was used as the coagulation reagent for NOM removal. The jar test applied involved two minutes of rapid mixing at 150 rpm followed by 30 minutes of slow mixing at 60 rpm. Flocs formed were then allowed to settle for an hour. Preliminary results indicated 5 and 15 ppm as the most effective doses of alum in terms of TOC and UV254 reduction for CR and BI waters, respectively (data not shown). The alum-treated samples were then filtered (pre-rinsed 0.45 μm filter) to remove any potential particles/flocs (that may not have settled) and then underwent UV/H2O2 treatment as described earlier.2.4. Analytical methods
Several water quality parameters (e.g., UV254, total organic carbon (TOC) and molecular weight distribution) were monitored to study the fate of NOM during the treatment. The concentration of H2O2 was measured using the triiodide method.37 A UV-Vis spectrophotometer (Shimadzu UV-Mini 1240, cell path length of 1 cm) was used to conduct all the spectrophotometric measurements (e.g., UV254, [H2O2]). A Shimadzu TOC-VCPH analyser was used to measure the total and dissolved organic carbon (TOC, DOC) content of water. High-performance size-exclusion chromatography (HPSEC) was used to analyse the apparent molecular weight (AMW) distribution of NOM with a similar procedure described by Sarathy and Mohseni (2007).5 A WATERS 2695 HPLC system equipped with a 2998 photodiode detector, set to detection at 260 nm, served as the instrument for HPSEC analysis. The calibration curve correlating the AMW with retention time was obtained from polysulfonate standards (7 kDa PSS7K, 4 kDa PSS4K, 2 kDa PSS2K, American Polymer Standards Corporation), acetone and benzoic acid (10 ppm).52.5. AOC bioassay
To eliminate any potential for cross-contamination, all glassware materials were thoroughly cleansed and baked at 550 °C for 5 h. All other tools (e.g., pipette tips) were autoclaved to ensure that the risk of contamination was minimized. Residual H2O2 (after UV/H2O2 treatment) has been shown to have a detrimental effect on the growth of microorganisms and AOC bioassay;28 hence, it was removed using catalase from bovine liver immobilized on a polymeric substrate (SEPABEADS™, Resindion, Italy). Details on the preparation and validation of this analytical method are described elsewhere.28,38 Then, AOC bioassay was performed according to the protocol proposed by Hammes and Egli (2005)29 with some modifications as described elsewhere.28 In brief, a natural indigenous inoculum, made from the source water, was utilized instead of the conventionally used pure strains i.e., P17 and NOX. Ultrapure (Milli-Q) water spiked with various levels of sodium acetate (>99.99%), all filtered through pre-rinsed 0.22 μm filters (γ-irradiated), served as the control throughout all experiments. Samples quenched from H2O2 were seeded with the natural inoculum and incubated for 72 h at 30 °C in amber vials. Flow cytometry in combination with fluorescence cell staining was used to count the cells grown, providing more accurate and reliable data. A flow cytometer unit (FACSCalibur system, Becton, Dickinson and Company) located in the Biomedical Research Centre at the University of British Columbia (UBC) was used to carry out the cell analysis. The detailed procedure on cell enumeration and AOC data analysis is extensively described elsewhere.283. Results and discussion
3.1. Changes in physicochemical characteristics
Alum coagulation alone resulted in 50% and 73% reduction of TOC and UV254, respectively, for CR water. A similar impact was observed on BI water as the TOC and UV254 were reduced by 63% and 80%, respectively. Control experiments involved the treatment of raw water using UV/H2O2 with 10 ppm peroxide and UV fluences of up to 2000 mJ cm−2 (in the absence of alum). Table 1 shows the TOC and UV254 reductions for the raw and alum-treated waters irradiated under the UV fluence of 2000 mJ cm−2. As a result of the extensive UV/H2O2 treatment (i.e., 2000 mJ cm−2, control experiments), UV254 was reduced between 35–67% while considerably lower TOC reductions of 9–26% were recorded for the waters tested. The larger fractional decrease of chromophoric NOM (CNOM, represented by UV254) in both treatment cases (i.e., UV/H2O2 and UV/H2O2 after coagulation) implies the preference of generated OH radicals to react with UV254-absorbing compounds and partial oxidation of the organic matter. Similar findings have also been reported by other researchers.5,35| CR water | BI water | |||
|---|---|---|---|---|
| Raw water (control) | Alum-treated | Raw water (control) | Alum-treated | |
| UV254 | 52.5% (0.032) | 66.7% (0.010) | 34.4% (0.063) | 52.9% (0.018) |
| TOC | 25.9% (0.384) | 18.0% (0.131) | 12.1% (0.58) | 8.7% (0.151) |
Moreover, alum is known to be effective in removing large to medium range molecular weight organics.12,13,39 Therefore, downstream UV/H2O2 treatment was expected to cause larger fractional CNOM reductions due to the absence of larger organics that preferentially react with HO˙.5,35,40 It is noteworthy that lower absolute reductions in UV254 and TOC were observed for the alum-treated water samples as a result of the UV/H2O2 treatment (Table 1). This could be mainly explained by the pseudo-first order reaction of TOC and CNOM with OH radicals generated during the UV/H2O2 process.35 That is, with lower amounts of initial organic matter, lower reaction rates are expected; however, higher UV transmittance would result in a higher amount of generated OH radicals, thereby compensating for the organic concentration term.35
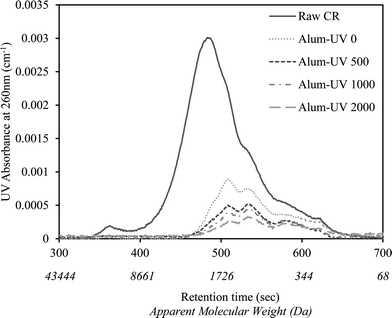
Fig. 1 shows the changes in the apparent molecular weight distribution of alum-pretreated CR water that has undergone various UV/H2O2 treatment extents. As demonstrated, the use of alum alone (i.e., Alum-UV 0) was effective in removing a substantial portion of NOM, mainly larger molecular weight fractions. Consistent with the literature, alum was shown to preferentially remove organics of high to medium molecular weight range.12 Application of UV/H2O2 after alum resulted in further decrease in the AMW of UV-absorbing NOM, up to the UV fluence of 500 mJ cm−2. However, no considerable change was observed upon extending the UV dose beyond 500 mJ cm−2.
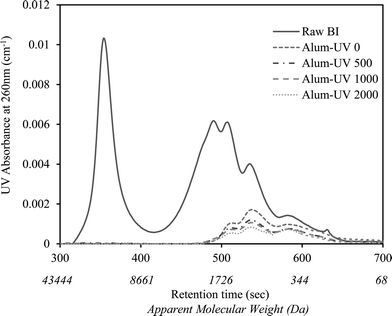
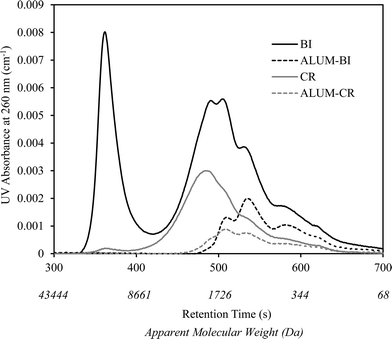
A similar observation was recorded for the changes in the NOM molecular weight distribution of BI water as shown in Fig. 2. As is illustrated, alum coagulation eliminated the first large eluting peak, often associated with colloidal organic matter, as well as considerable portions of other organic molecules mainly from the large to medium weight range (i.e., >500 Da). However, further downstream UV/H2O2 treatment resulted in small reductions in the remainder of the chromophoric organic molecules.
3.2. Impact on biostability and AOC
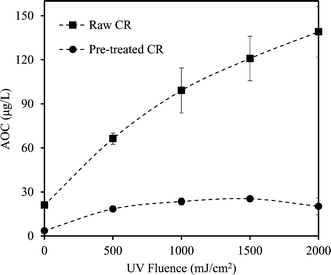
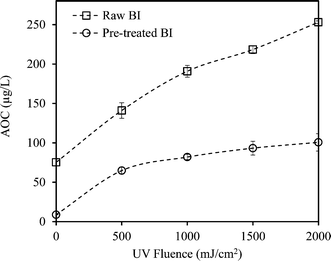
Coagulation noticeably reduced the AOC of CR and BI waters. The reduction was more significant for BI water (88.2% compared to 83% for CR), likely attributed to the elimination of the large single eluting peak corresponding to high molecular weight organics in the HPSEC chromatogram (Fig. 2). This is also consistent with the greater TOC and UV254 reductions observed for BI water (compared to CR water) as it underwent alum coagulation.Fig. 3 and 4 compare the AOC profiles of raw and alum-treated CR and BI waters under various UV doses. As is shown, UV/H2O2 advanced oxidation resulted in a significant increase in AOC for raw waters. This is because of the partial oxidation and breakdown of (larger) organic molecules (i.e., into smaller ones) as a result of the reaction with OH radicals. Fig. 3 and 4 also depict the ability of the organics not removed via coagulation to promote bacterial growth even though their observed initial AOC values were very low. Interestingly, the AOC of the alum-treated waters still increased (and then plateaued) under UV/H2O2 treatment, supporting the fact that further structural breakdown of NOM molecules (even though mostly of lower molecular weight nature) took place. That said, using the HPSEC technique was not sufficient to capture all the changes in the molecular structure of NOM. Nonetheless, as expected, the absolute increase in AOC was noticeably lower for the waters pretreated with alum. This was because alum removed a considerable portion of large to medium organic molecules which are the most susceptible ones towards reaction with OH radicals. As a result of alum treatment, the assimilable percentage of NOM (i.e., AOC/TOC × 100) decreased from 1.42% and 1.56% (for raw waters) to 0.49% and 0.51% for alum-treated CR and BI waters, respectively. This confirms that a considerably lower assimilable fraction remained after coagulation. The observations made here are also in agreement with the observation of Chong Soh et al. (2008),13 who also found that the remaining NOM fractions after coagulation were able to support bacterial regrowth.
Both alum-treated waters showed greater fractional AOC increase (7 and 12 folds, respectively, under UV/H2O2 treatment) in comparison with the raw waters (5 and 3.5 folds, respectively). Also, as previously shown in Table 1, greater fractional UV254 reduction was observed for the alum-treated waters. Therefore, this can be mainly attributed to the more effective interactions between the OH radicals and smaller organic molecules (i.e., in the absence of high MW OH-scavenging dissolved organics), leading to higher enhancement in the biodegradability of NOM. That is, lower levels of organic matter would result in smaller UV254 absorbance consequently leading to a higher UV absorption rate of H2O2.35,41 Therefore, higher fractional AOC increase of the pretreated waters would be expected as less shielding and scavenging effects of NOM exist. As a result, a more effective number of interactions/reactions between the OH radicals and organic matter would be expected.6,35
The behaviour of the AOC profiles of pretreated CR and BI waters over the course of UV/H2O2 treatment is also noteworthy (Fig. 3 and 4). After the UV fluence of 1000 mJ cm−2, the AOC profiles begin to plateau, indicating a possible equilibrium with respect to the formation of smaller (biodegradable) organics and their subsequent degradation with OH radicals. Moreover, the AOC of the pretreated CR starts to decrease slightly after the UV fluence of 1500 mJ cm−2 (Fig. 3). A likely and plausible explanation is that, at this fluence, the degradation rate of organic molecules was dominant and greater than the rate of formation; hence, an overall decrease in the amount of small biodegradable organic molecules was observed.
Moreover, the AOC of pretreated CR water increased by about 7 times at the UV fluence of 2000 mJ cm−2; however, the AOC of pretreated BI water showed an increase of about 12 times from its initial value with the same level of treatment (Fig. 3 and 4). This could be attributed to the higher organic content (i.e., TOC) as well as the nature of NOM in the source water. That is, differences in the nature and characteristics of NOM in BI and CR waters resulted in different behaviours and responses with respect to their reaction with OH radicals and consequently biodegradability increase under identical AOP treatment. Also, alum-treated BI water contained higher amount of organic molecules in the range of low to medium molecular weights (AMW < 1000 Da) in comparison with alum-treated CR water (Fig. 5). As a result, the remaining organics in BI water were likely more biodegradable to start with and underwent further partial oxidation, leading to a greater amount and percentage of AOC generated during UV/H2O2 treatment (i.e., UV fluence of 2000 mJ cm−2). Supporting either of these hypotheses require further investigations using more analytical techniques such as liquid chromatography equipped with organic carbon detection, use of isolation techniques (e.g., nanofiltration) and also evaluation of different source waters. This will eventually lead to better understanding of the fate of NOM during various treatments and the potential for AOC formation.
Even though the AOC of alum-treated water still increased over the course of the UV/H2O2 process, it is important to note that the final AOC was comparable to that of raw water (with no treatment). This means that the combined treatment strategy did not significantly change the biostability characteristics of the water. This is an important consideration because it indicates that the application of combined alum and UV/H2O2 may not deteriorate the biostability of the treated water to a level that downstream biological treatment (e.g., biological activated carbon) would be required. On the other hand, standalone UV/H2O2 treatment with the resultant significant increase of AOC could not be implemented without the application of downstream biological treatment to remove the generated AOC.
Data obtained from the standalone UV/H2O2 treatment along with those of the combined treatment clearly indicated that the application of alum significantly reduces the concentration of high molecular weight NOM. A lower concentration of NOM, in particular from the higher molecular weight fractions, leads to the more effective use of UV photons and lower competition for the OH radicals in the water matrix. The lower scavenging of UV and OH radicals, in turn, leads to more effective removal of target contaminants (i.e., micropollutants) which are the primary reason for the application of UV-based AOPs. More importantly, this will help to conserve a considerable amount of electrical energy used for delivering the necessary UV fluence (to achieve the degradation of target contaminants).30
One important note to consider is that the findings here would be of interest for those facilities that already have coagulation processes in place. Otherwise, incorporating coagulation (coagulation, flocculation and sedimentation) into a new treatment plant may not be a feasible pretreatment alternative since coagulation is a relatively expensive process.
4. Conclusions
Application of the UV/H2O2 process to remove micropollutants has been shown to adversely affect the biological stability of treated water. This is because of the breakdown of large natural organic matter molecules (as a result of reaction with HO˙ and UV) into smaller ones that can be readily consumed by bacteria (i.e., AOC). To address this issue, alum coagulation was used as a NOM pretreatment alternative to eliminate medium to high molecular weight fractions upstream of the oxidation process, hence mitigating the formation of smaller, more biodegradable organic molecules (i.e., AOC). A rapid and novel technique, modified for UV/H2O2 applications, was utilized to monitor the AOC profile (i.e., concentration of biodegradable molecules) through coagulation and UV/H2O2 treatment. AOC was reduced by 80–85% as a result of alum coagulation. However, the downstream UV/H2O2 process raised the amount of biodegradable organic molecules (AOC), with the extent of the AOC increase dependent on the nature of the NOM and the treatment conditions (e.g., treatment time).Overall, application of a pretreatment process (e.g., alum) prior to UV/H2O2 treatment can potentially reduce the risk of deteriorating the biostability while saving a considerable amount of electrical energy to achieve the same level of target contaminant removal.
Acknowledgements
The authors acknowledge the support from the RES’EAU-WaterNET Small Water System Strategic Network and Natural Science and Engineering Research Council of Canada (NSERC). Also, we are grateful to Drs. Siva Sarathy and Mihaela Stefan of Trojan Technologies for the technical support and input.References
- S. R. Sarathy and M. Mohseni, IUVA NEWS, 2006, pp. 16–27 Search PubMed.
- J. Kruithof, P. Kamp and M. Belosevic, Water Supply, 2002, 2, 113–122 CAS.
- J. C. Kruithof, P. C. Kamp and B. J. Martijn, Ozone: Sci. Eng., 2007, 29, 273 CrossRef CAS.
- S. Vilhunen, M. Vilve, M. Vepsäläinen and M. Sillanpää, J. Hazard. Mater., 2010, 179, 776–782 CrossRef CAS PubMed.
- S. R. Sarathy and M. Mohseni, Environ. Sci. Technol., 2007, 41, 8315–8320 CrossRef CAS PubMed.
- M. M. Bazri, B. Barbeau and M. Mohseni, Water Res., 2012, 46, 5297–5304 CrossRef CAS PubMed.
- I. C. Escobar and A. A. Randall, Water Res., 2001, 35, 4444–4454 CrossRef CAS PubMed.
- L. J. Hem and H. Efraimsen, Water Res., 2001, 35, 1106–1110 CrossRef CAS PubMed.
- D. Van der Kooij, J. – Am. Water Works Assoc., 1992, 84, 57–65 CAS.
- J. G. Jacangelo, J. DeMarco, D. M. Owen and S. J. Randtke, J. - Am. Water Works Assoc., 1995, 87, 64–77 CAS.
- H. Humbert, H. Gallard, H. Suty and J.-P. Croué, Water Res., 2008, 42, 1635–1643 CrossRef CAS PubMed.
- C. W. K. Chow, J. A. Van Leeuwen, M. Drikas, R. Fabris, K. M. Spark and D. W. Page, Water Sci. Technol., 1999, 40, 97–104 CrossRef CAS.
- Y. Chong Soh, F. Roddick and J. van Leeuwen, Water Sci. Technol., 2008, 58, 1173 CrossRef PubMed.
- T. H. Boyer and P. C. Singer, Water Sci. Technol.: Water Supply, 2008, 8, 167 CrossRef CAS.
- D. Fearing, J. Banks, D. Wilson, P. H. Hillis, A. T. Campbell and S. A. Parsons, Water Sci. Technol.: Water Supply, 2004, 4, 139–145 Search PubMed.
- A. Matilainen and M. Sillanpää, Chemosphere, 2010, 80, 351–365 CrossRef CAS PubMed.
- A. Matilainen, M. Vepsäläinen and M. Sillanpää, Adv. Colloid Interface Sci., 2010, 159, 189–197 CrossRef CAS PubMed.
- R. Lamsal, M. E. Walsh and G. A. Gagnon, Water Res., 2011, 45, 3263–3269 CrossRef CAS PubMed.
- S. Gur-Reznik, I. Katz and C. G. Dosoretz, Water Res., 2008, 42, 1595–1605 CrossRef CAS PubMed.
- H. Huang, K. Schwab and J. G. Jacangelo, Environ. Sci. Technol., 2009, 43, 3011–3019 CrossRef CAS PubMed.
- G. Huang, F. Meng, X. Zheng, Y. Wang, Z. Wang, H. Liu and M. Jekel, Appl. Microbiol. Biotechnol., 2011, 90, 1795–1803 CrossRef CAS PubMed.
- M. Drikas, M. Dixon and J. Morran, Water Res., 2011, 45, 1539–1548 CrossRef CAS PubMed.
- R. Fabris, C. W. K. Chow and M. Drikas, Water Sci. Technol.: Water Supply, 2004, 4, 89–94 CAS.
- C. Volk, K. Bell, E. Ibrahim, D. Verges, G. Amy and M. LeChevallier, Water Res., 2000, 34, 3247–3257 CrossRef CAS.
- D. A. Fearing, J. Banks, S. Guyetand, C. Monfort Eroles, B. Jefferson, D. Wilson, P. Hillis, A. T. Campbell and S. A. Parsons, Water Res., 2004, 38, 2551–2558 CrossRef CAS PubMed.
- E. L. Sharp, P. Jarvis, S. A. Parsons and B. Jefferson, Colloids Surf., A, 2006, 286, 104–111 CrossRef CAS.
- Y. Song, B. Dong, N. Gao and Y. Deng, Int. J. Environ. Res. Public Health, 2015, 12, 6700–6709 CrossRef CAS PubMed.
- M. M. Bazri and M. Mohseni, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2013, 48, 1086–1093 CrossRef CAS PubMed.
- F. A. Hammes and T. Egli, Environ. Sci. Technol., 2005, 39, 3289–3294 CrossRef CAS PubMed.
- B. Martijn, A. Fuller, J. Malley and J. Kruithof, Ozone: Sci. Eng., 2010, 32, 383–390 CrossRef CAS.
- Q. Zhao, C. Shang, X. Zhang, G. Ding and X. Yang, Water Res., 2011, 45, 6545–6554 CrossRef CAS PubMed.
- W. Buchanan, F. Roddick, N. Porter and M. Drikas, Water Sci. Technol.: Water Supply, 2004, 4, 103–111 CAS.
- J. Thomson, A. Parkinson and F. A. Roddick, Environ. Sci. Technol., 2004, 38, 3360–3369 CrossRef CAS PubMed.
- R. O. Rahn, Photochem. Photobiol., 1997, 66, 450–455 CrossRef CAS.
- S. R. Sarathy, M. M. Bazri and M. Mohseni, J. Environ. Eng., 2011, 137, 903–912 CrossRef CAS.
- A. J. Martijn and J. C. Kruithof, Ozone: Sci. Eng., 2012, 34, 92–100 CrossRef CAS.
- N. V. Klassen, D. Marchington and H. C. McGowan, Anal. Chem., 1994, 66, 2921–2925 CrossRef CAS.
- S. R. Sarathy and M. I. Stefan, in Ozone and UV: Leading-edge science and technologies, Paris, France, 2011 Search PubMed.
- M. Drikas, C. W. K. Chow and D. Cook, J. Water Supply: Res. Technol.--AQUA, 2003, 52, 475–487 CAS.
- S. Sarathy and M. Mohseni, Can. J. Civ. Eng., 2009, 36, 160–169 CrossRef CAS.
- C. M. Sharpless and K. G. Linden, Environ. Sci. Technol., 2003, 37, 1933–1940 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |