Demonstrating organic contaminant removal in an ozone-based water reuse process at full scale†
Judy
Blackbeard
a,
James
Lloyd
a,
Mirela
Magyar
a,
John
Mieog
a,
Karl G.
Linden
b and
Yaal
Lester
*c
aMelbourne Water, Melbourne, Victoria, 3001 Australia
bDepartment of Civil, Environmental, and Architectural Engineering, University of Colorado Boulder, Boulder, CO 80309, USA
cThe Water Research Center, Tel Aviv University, 69978 Tel Aviv, Israel. E-mail: lester.yaal@gmail.com
First published on 11th December 2015
Abstract
The 350 ML per d Eastern Treatment Plant (ETP) tertiary facility produces “Class A” water for the city of Melbourne, Australia, which is used for irrigation, dual reticulation and fire fighting. The ETP process utilizes ozone and biological media filtration as part of the advanced treatment train for secondary treated wastewater. An extensive sampling campaign was carried out to evaluate the removal of hundreds of contaminants of concern through various steps in the treatment train, as well as identify the formation of any byproducts of the treatment process. Degradation of contaminants throughout the treatment was mainly controlled by the ozone process. Chemicals with moderate to high reaction rates with ozone and/or ˙OH radical (10 M−1 s−1 ≤ kO3 or kO3 < 10 M−1 s−1 and k˙OH ≥ 5 × 109 M−1 s−1) were removed by the tertiary treatment to below the detection limit. However, the advanced treatment train resulted in some contaminants increasing in concentration (i.e. NDMA and desisopropyl atrazine) and other newly forming contaminants, such as trihalomethanes and chloral hydrate. The resulting levels of contaminants of concern remaining in the ETP final effluent were below levels of relevance based on guidelines for reuse in irrigation or discharge to the environment.
Water ImpactCurrently, trace organic contaminants are repeatedly detected in the aquatic environment. Primary sources for these contaminants are municipal wastewater treatment facilities, which mostly employ (secondary) biological treatment, not originally designed to remove modern-day contaminants of concern. The current situation has led scientists and regulators to consider tertiary treatment processes, such as ozone, as potential solutions for contaminant removal. In this study we demonstrate the removal of a large number of organic contaminants by a full-scale tertiary wastewater treatment facility in Melbourne, Australia. The treatment is based on ozone and biological media filtration, and produces high-quality effluent suitable for irrigation and other environmental applications. |
1. Introduction
Melbourne Water in Melbourne, Australia, operates the Eastern Treatment Plant tertiary facility for the production of “Class A” wastewater effluent. The treatment train has been in operation since December 2012 and consists of conventional activated sludge treatment (secondary treatment) followed by pre-ozonation, biological filtration, post-ozonation, UV disinfection and chlorination. Unique to this facility is the production of high quality reclaimed water without the use of membranes. This water is recycled post-treatment for uses such as irrigation, dual reticulation and fire fighting, with the remainder being discharged to a marine outfall.Concern surrounding the presence of emerging chemicals in wastewater effluents, the natural environment and drinking waters has grown over the past three decades. This concern has accelerated recently as improvements in analytical methods, coupled with larger scale surveys, revealed the broad range of apparently persistent contaminants that remain through the urban water cycle of wastewater-to-drinking water,1–7 which becomes especially critical for reclamation of water for beneficial reuse. Although the overall risk associated with chronic exposure for humans remains to be seen,8 the ecological impacts are better documented.9–11 Furthermore, international public awareness of the potential problem of emerging contaminants has brought this issue to the forefront in the water reuse industry. In reuse situations, improvements in the efficiency of treating emerging contaminants at wastewater treatment plants represents an essential line of defence against the proliferation of these chemicals in our environment and reuse applications.
Monitoring studies around the world have shown that various classes of pharmaceuticals are present in surface waters receiving WWTP effluents,2–4,12–16 indicating that current treatment technologies are not optimized or capable of effectively eliminating these emerging contaminants. Literature reports on efficiency of removing pharmaceuticals from wastewater via biological treatment vary widely, from 10% to 90% depending on the physical/chemical properties of the compounds and the treatment process employed.5,16–19 For example, while 64% to 78% of ethinyl estradiol (EE2) was eliminated from WWTPs,6 the concentration of iopromide (a pharmaceutical contrast agent) remained relatively unchanged.20
Oxidation processes at WWTPs (e.g. chlorination, ozonation) have not typically been implemented with the intention of achieving removal of contaminants of emerging concern (CECs); rather, they are applied for disinfection. Furthermore, little consideration has been given to the application point of an oxidation processes in the WWTP process train, relative to other potentially complementary processes such as biological treatment. Recent research has indicated that ozonation of biologically treated wastewater (secondary effluent) can effectively degrade a variety of pharmaceuticals (e.g. those containing unsaturated double bonds and/or electron donating properties) while others remain relatively unaffected (i.e. iodinated contrast media).21–26 Ultraviolet (UV) irradiation at doses applicable for disinfection (i.e. 40 mJ cm−2) proved to be ineffectual for pharmaceutical transformation, but increased dosages (i.e. >400 mJ cm−2) were more effective, and addition of an advanced oxidation process (i.e. UV/hydrogen peroxide) significantly increased degradation.27,28
Although oxidation methods appear to be effective at removing many CECs, they often do not fully mineralize the contaminants, producing large number of transformation products,29,30 and if oxidation is the last step in wastewater treatment then these products remain in plant effluents. The ecological and human health implications of this reality are not well studied for the vast array of contaminants, making the combination of treatment processes more attractive. Recent work has shown the lack of complete mineralization for numerous pharmaceuticals.31–37 Both ozonation and UV-oxidation have been shown to reduce or eliminate the estrogenic activity in water via destruction of pharmaceuticals such as EE2 without achieving mineralization.23,38 While the removal of the endocrine disrupting properties is important from a natural systems perspective, the inability to mineralize pharmaceuticals yields potential risks to water supplies (i.e. direct or indirect potable reuse) via subsequent reactions upon chemical disinfection typical of water treatment processes.
Advanced oxidation has been used to achieve biodegradation enhancement for years for certain industrial wastewaters that are particularly recalcitrant.39–41 However, there are not many field applications in the domestic wastewater treatment industry where oxidation processes have been coupled with a polishing bio-treatment step designed specifically to remove CECs. Biological media filtration is a promising treatment downstream of a coupled activated sludge plus oxidation process train because the low carbon content of the effluent will minimize biological flocculation, reducing the effectiveness of suspended culture systems.42 Additionally, biofiltration systems can retain a larger biomass density over time than suspended systems. In drinking waters with low carbon (i.e. natural organic matter-NOM) content, biofiltration has been employed to eliminate disinfection byproducts and disinfection byproduct precursors.43,44 The molecular size of NOM is typically much larger than the trace organic compounds present in wastewater effluents.45 Nevertheless, the limited success with oxidation-enhanced biodegradation and biofiltration of NOM suggest this as a cost-effective solution to enhanced removal of CECs and their transformation products from wastewater effluents.
The objectives of this study were to:
1. Determine the concentration of a wide range of CECs in the influent and effluent of the Melbourne Eastern Treatment Plant (ETP), to demonstrate the degradation of key contaminants that are of widespread interest.
2. Assess the contribution of different process steps (pre-ozonation, biological filtration, post-ozonation, UV disinfection, and chlorination) to both the degradation of contaminants and the potential formation of reaction products of concern.
2. Experimental
The ETP treats a volume of 350 ML per d comprising around 13% industrial sewage and 87% domestic sewage. The primary and secondary processes consist of de-gritting, screening, primary settling followed by a step feed nitrifying/denitrifying activated sludge plant. Samples for the present CECs degradation study were taken from the end of the secondary treatment process onwards.A total of 26 samples representing 4 sampling periods over 6 locations across the full scale ETP treatment processes, along with controls, were taken. The samples were analysed for a large suite of contaminants described below.
2.1 Location of sampling points at ETP
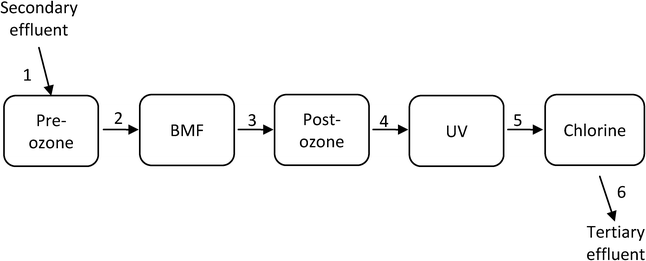
Fig. 1 illustrates the ETP process steps investigated and the sampling points for the study.The treatment processes include pre-ozone, biological media filtration (BMF), post-ozone, UV disinfection and chlorination. The primary objectives of the pre-ozone step were to satisfy the ozone demand of the effluent, reduce color and odor, and improve UVT. The BMF treatment aimed to assist with satisfying ozone demand. The post-ozone step was designed mainly for the inactivation of protozoa (≥0.6-log), virus (4-log) and bacteria (4-log). UV provided 4-log reduction of Cryptosporidium; whereas chlorination provided residual disinfectant for the system. Samples were taken before and after all the major processes.
2.2. Sampling protocol
Samples were collected with 24 h automatic samplers, set up for sampling between 9:00 am to 9:00 am the following day, representing 24 h composites. The 24 h composite samples were collected in 10 L glass containers (at 417 mL h−1 sampling flow) refrigerated during the 24 h sampling period. Analyses were performed on all samples that the correct total volume of 10 L was collected and for which the ETP tertiary processes had no interruptions during sampling. The 10 L containers were rinsed with process water between the samples.Each 10 L volume of sample was distributed into 20 × 500 mL bottles (amber glass or polyethylene), washed with ethanol, then with acetone and then dried prior to use. Preservatives were added to the bottles as specifically required for each analyte), for individual analytical tests. The bottles were labelled for the selected analyte tests, including date, sampling point identifier and were delivered to Queensland Forensic and Scientific Services (QFSS, Queensland Australia) at 4 °C with an overnight courier. The trip blanks (deionized water) were carried together with the other bottles during the sampling and packing and then delivered to QFSS for analysis of the entire range of analytes.
Sampling only occurred when the following ETP plant characteristics were satisfied:
■ The five ETP processes investigated here (pre-ozonation, BMF, post ozonation, UV and chlorination) worked uninterruptedly and within all the validated operational envelopes over the 24 h sampling period;
■ Flow into the ETP plant was representative of the overall ETP average inflow of 350 ML per day.
2.3. List of analytes
Chemicals analysed were chosen to enable a comparison of the removal efficiencies across specific processes in the ETP. The groups of chemicals analysed are listed below (Table 1).| Groups of chemicals analysed | Number of specific analytes |
|---|---|
| Nitrosamines | 5 |
| Endocrine disrupting compounds | 20 |
| Pharmaceuticals | 58 |
| Phenoxy herbicides | 11 |
| Haloacetic acids | 9 |
| Herbicides by GCMS | 26 |
| Organochlorine pesticides | 33 |
| Herbicides and other compounds by LCMS | 25 |
| Organo-phosphorus pesticides | 46 |
| Other pesticides | 25 |
| Synthetic pyrethroids | 12 |
| Other GCMS compounds | 20 |
| Phenolics | 18 |
| Polycyclic aromatic hydrocarbons (PAHs) | 30 |
| Trihalomethanes | 5 |
| Iodinated halomethanes | 5 |
| Perfluorinated compounds | 17 |
| Other compounds (aldehydes) | 4 |
| Other disinfection by-products | 11 |
The complete list of analytes, their limit of reporting and details of the preservatives used for preparation of the sampling bottles are included in the ESI (Tables SI1 and SI2†).
2.4. Statistical analyses
Concentration data of minimum, maximum, average and standard deviation for each analyte, as well as percentage removals through the treatment process train were determined. For analytes with all 5 samples below the level of reporting (LOR), the mean result was reported as <LOR. Average of results was calculated for all analytes that had at least two results above the LOR. In cases of highly effective removal, data were filtered to ignore any removal calculations where the concentrations were less than 5 times the LOR before and after the process. Some parameters were below the LOR at the plant influent but were recorded above the LOR for subsequent locations in the treatment train (e.g. disinfection by-products). For these parameters the percentage removals appear as a negative value, because they represent a gain in that product.2.5. Operating conditions and water quality parameters
During the testing period, key operating data were recorded for each specific treatment process. The pre-ozone system, which consisted of two parallel contactors (620 m3 each) with side stream injection and static mixers, had a baffling factor (T10/T50) of 0.9 and an average ozone dose of 9.7 ± 0.5 mg L−1. The post-ozone reactor consisted of two parallel rectangular concrete contactors of 1600 m3 each, with baffling factor and average ozone dose of 0.7 and 4.7 (±0.5) mg L−1, respectively. The chlorination system was composed of two parallel contactors of 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3 each, with baffling factor of 0.8 and chlorine dose of 3.29 (±0.07) mg Cl2 L−1. The UV system included 1000 W low-pressure high-output UV reactors, dosing approximately 23 mJ cm−2. Detailed operating data is provided in Tables SI3–SI7.†
000 m3 each, with baffling factor of 0.8 and chlorine dose of 3.29 (±0.07) mg Cl2 L−1. The UV system included 1000 W low-pressure high-output UV reactors, dosing approximately 23 mJ cm−2. Detailed operating data is provided in Tables SI3–SI7.†
The system operated under typical and steady state conditions for the plant. To document this operation, several water quality parameters were monitored during the sampling investigation, including: inflow to ETP, outflow from the tertiary plant, pH, alkalinity, COD and suspended solids (SS) of the secondary effluent supplied to the treatment plant (Table 2). Total organic carbon (TOC) was not monitored during the sampling period; however, for the purpose of the kinetics analysis, recent TOC data of ~15 mg C L−1 was used (resulting in specific ozone doses of 0.65 and 0.31 mg O3 mg−1 TOC, for the pre-ozone and post-ozone steps respectively).
| Date | IPS flow rate | TSPS flow rate | Alkalinity | COD | SS | Temperature | |
|---|---|---|---|---|---|---|---|
| Units | kL s−1 | kL s−1 | pH | mg L−1 as CaCO3 | mg L−1 | mg L−1 | °C |
| IPS – Inflow Pump Station flow (pumped into ETP from the sewer); TSPS – Tertiary Supply Pump Station flow. | |||||||
| 22-May-13 | 4.14 | 4.03 | 7.5 | 71 | 90 | 6 | 19.6 |
| 27-May-13 | 4.11 | 3.86 | 7.2 | 108 | 71 | 10 | 18.6 |
| 28-May-13 | 3.94 | 4.07 | 7.1 | 74 | 67 | 9 | 19.0 |
| 30-May-13 | 4.11 | 4.06 | 6.9 | 86 | 55 | 5 | 19.4 |
| Average | 4.07 | 4.00 | 7.2 | 85 | 71 | 7.5 | 19.1 |
3. Results and discussion
3.1. Contaminants influent concentration
The influent concentration of the investigated contaminants was measured for each sampling event and monitored over time to assess the variation in concentration that the treatment plant needs to process and safely discharge.The average concentration of main analytes at the inflow into the ETP (location 1 in Fig. 1) is presented in Table 3 (the complete data is provided in Table SI8 of the ESI†). There were a large number of contaminants consistently detected in the wastewater influent (91 of 380 were detected in at least two samples). While some concentrations were fairly consistent across the sampling period, others varied widely at the inflow into the ETP for each day of sampling. These variations depend on a wide range of factors, including for example the release pattern of the contaminants within the sewer catchment. Analysis of the coefficient of variation (COV – ratio of the standard deviation to the mean), which represents the relative variability in the concentrations of compounds, showed that several compounds were consistently present in the ETP influent at similar levels (COV < 0.2), including acesulfame K (sweetener), diclofenac, frusemide, hydrochlorthiazide, indomethacin, galaxolide, tris(chloro) phosphates, primidone, propranolol, triclosan and diuron. This implies that these compounds have a relatively unflixable patern of use.
| Group | Analyte | Units | LOR | Concentration in second. eff. | STDV |
|---|---|---|---|---|---|
| Nitrosamines | NDMA | ng L−1 | 5.00 | 15.27 | 10.64 |
| NMOR | ng L−1 | 10.0 | 209.12 | 205.74 | |
| Endocrine disrupting chemicals EDCs | 4-t-Octylphenol | ng L−1 | 10.0 | 128.34 | 151.07 |
| Nonylphenol | ng L−1 | 100.0 | 229.12 | 244.70 | |
| Bisphenol A | ng L−1 | 10.0 | 25.94 | 23.66 | |
| Cholesterol (not an EDC) | ng L−1 | 100.0 | 321.06 | 366.36 | |
| Pharmaceuticals | Acesulfame K (sweetener) | μg L−1 | 0.01 | 6.69 | 1.31 |
| Atenolol | μg L−1 | 0.01 | 0.29 | 0.12 | |
| Caffeine | μg L−1 | 0.02 | 0.10 | 0.11 | |
| Carbamazepine | μg L−1 | 0.01 | 0.76 | 0.21 | |
| Diclofenac | μg L−1 | 0.01 | 0.46 | 0.09 | |
| Frusemide | μg L−1 | 0.01 | 1.23 | 0.16 | |
| Gabapentin | μg L−1 | 0.05 | 2.09 | 0.97 | |
| Hydro-chlorthiazide | μg L−1 | 0.01 | 1.37 | 0.04 | |
| Iopromide | μg L−1 | 0.20 | 0.94 | 0.27 | |
| Metoprolol | μg L−1 | 0.01 | 0.28 | 0.74 | |
| Oxazepam | μg L−1 | 0.01 | 0.46 | 0.06 | |
| Salicylic acid | μg L−1 | 0.10 | 0.11 | 0.19 | |
| Sulfamethoxazole | μg L−1 | 0.01 | 0.15 | 0.07 | |
| Temazepam | μg L−1 | 0.01 | 0.39 | 0.04 | |
| Tramadol | μg L−1 | 0.01 | 0.78 | 0.08 | |
| Triclosan | μg L−1 | 0.01 | 0.02 | 0.42 | |
| Trimethoprim | μg L−1 | 0.01 | 0.22 | 0.00 | |
| Venlafaxine | μg L−1 | 0.01 | 0.88 | 0.07 | |
| Herbicides | Atrazine | μg L−1 | 0.01 | 0.17 | 0.2 |
| Desethyl atrazine | μg L−1 | 0.01 | — | ||
| Diuron | μg L−1 | 0.01 | 0.13 | 0.02 | |
| Metolachlor | μg L−1 | 0.01 | 0.19 | 0.23 | |
| Simazine | μg L−1 | 0.01 | 0.34 | 0.14 | |
| Total diuron | μg L−1 | 0.03 | 0.17 | 0.03 | |
| MCPA | μg L−1 | 0.01 | 0.59 | 0.5 | |
| 2,4-D | μg L−1 | 0.01 | 1.10 | 1.27 | |
| Metolachlor | μg L−1 | 0.10 | 0.15 | 0.16 | |
| Other GCMS compounds | 1H-Benzotriazole | μg L−1 | 0.50 | 0.57 | 0.33 |
| 1H-Benzotriazole, 5-methyl | μg L−1 | 0.20 | 0.52 | 0.19 | |
| Galaxolide | μg L−1 | 0.10 | 0.99 | 0.08 | |
| N-Butyl benzene | μg L−1 | 0.20 | 0.15 | 0.09 | |
| Tris(chloroethyl) phosphate | μg L−1 | 0.10 | 0.28 | 0.08 | |
| Perfluorinated compounds | Perfluorohexanoic acid | μg L−1 | 0.01 | 0.02 | 0.01 |
| Perfluorooctanoic acid | μg L−1 | 0.01 | 0.03 | 0.01 |
3.2. Contaminants removal across the ETP tertiary treatment train
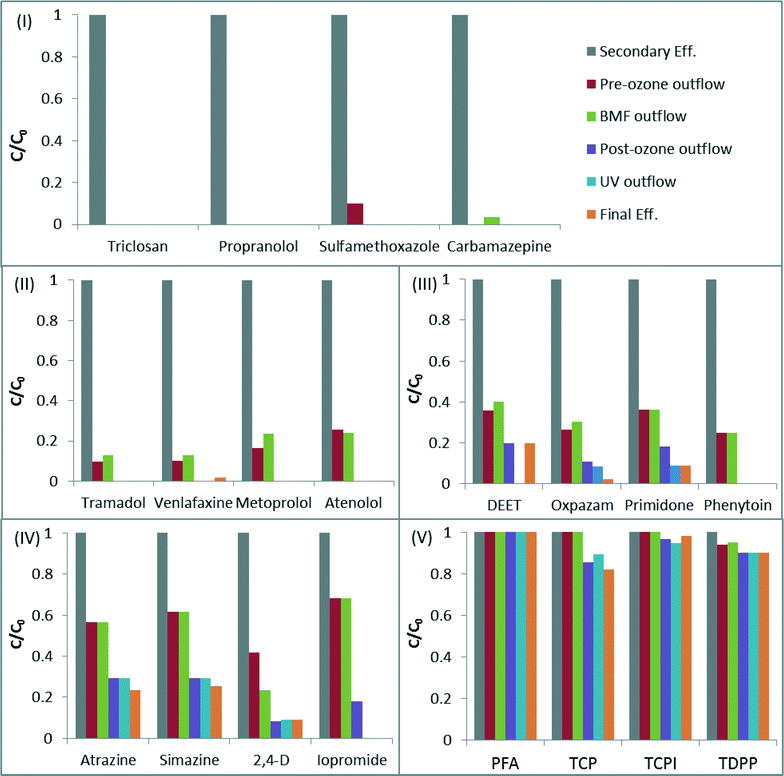
Of the 91 chemicals reported in the secondary effluent feed into the advanced process train, 38 were degraded to below the detection limit after the pre-ozone step and only 36 remained detectable in the effluent after the post-ozone step (Table SI9 in the ESI†). The UV system only marginally contributed to CECs degradation, which was expected in view of the relatively low UV dose applied (i.e. 23 mJ cm−2).27,28 Contribution of chlorine was also negligible, as the vast majority of reactive compounds were already oxidized by ozone (typically exhibiting much higher reaction rate constants with CECs than chlorine46). The BAF system was generally ineffective in removing CECs, with the exception of NDMA, which was removed by 91%. BAF is known to efficiently remove NDMA, both from drinking water and wastewater effluent,47 and is frequently applied post-ozone to mitigate the in situ generation of this carcinogenic transformation product. Removal of CECs by the individual processes is presented in detail in Tables SI10–SI14.†Following the above results it is safe to say that the efficiency of CECs degradation in the ETP treatment is primarily controlled by the ozone processes. To model CECs degradation we adopted an approach recently proposed for ozone-based systems; where, CECs are classified into five groups, based on their reaction rate constants with ozone and ˙OH radical:25 (I) compounds with kO3 > 1 × 105 M−1 s−1, (II) compounds with 10 M−1 s−1 ≤ kO3 < 1 × 105 M−1 s−1, (III) compounds with kO3 < 10 M−1 s−1 and k˙OH ≥ 5 × 109 M−1 s−1, (IV) compounds with 1 × 109 M−1 s−1 ≤ k˙OH < 5 × 109 M−1 s−1 and (V) compounds with k˙OH < 1 × 109 M−1 s−1. Fig. 2 illustrates the degradation of selected compounds from each group (reaction rate constants of the compounds are given in Table 4).
| Compound | k O3 M−1 s−1 (pH 7)a | k ˙OH M−1 s−1 (109)a | CDPH Classificationd |
|---|---|---|---|
| a Rate constants adapted from references 23, 25 and 47; b DB – Double bond; c Rate constants estimated based on von Gunten and von Sonntag 2012; d CDPH – California Groundwater Replenishment Reuse regulations 2011. | |||
| Group 'I' | |||
| Triclosan | 4 × 107 | 10 | A. Hydroxy aromatic |
| Sulfamethoxazole | 3 × 106 | 6 | B. Amino/acylamino aromatic |
| Carbamazepine | 3 × 105 | 8.8 | C. Nonaromatic with carbon DBb |
| Propranolol | 1 × 105 | 7.6 | E. Alkoxy polyaromatic |
| Group 'II' | |||
| Venlafaxine | 3.3 × 104 | 10 | F. Alkoxy aromatic |
| Metoprolol | 2 × 103 | 7.3 | D. Deprotonated amine |
| Atenolol | 2 × 103 | 8 | D. Deprotonated amine |
| Gemfibrozil | 5 × 104 | 10 | F. Alkoxy aromatic |
| Group 'III' | |||
| DEET | <10 | 5 | G. Alkyl aromatic |
| Oxapazam | 1 | 9.1 | G. Alkyl aromatic |
| Primidone | 1 | 7 | G. Alkyl aromatic |
| Phenytoin | <10 | 6 | G. Alkyl aromatic |
| Group 'IV' | |||
| Atrazine | 6 | 2.4 | D. Deprotonated amine |
| Simazine | 4.3 (pH 5) | 2.9 | D. Deprotonated amine |
| 2,4-D | 5.3 (pH 2) | 3.2 | F. Alkoxy aromatic |
| Iopromide | 0.8 | 3.1 | H. Saturated aliphatic |
| Group 'V'c | |||
| Perfluorooctanoic acid | <1 | <1 | Halogenated aliphatic |
| Tris (chloroethyl) phosphate | <1 | <1 | Halogenated aliphatic |
| Tris (chloropropyl) phos. isomers | <1 | <1 | Halogenated aliphatic |
| Tris (dichloro-propyl) phosphate | <1 | <1 | Halogenated aliphatic |
Compounds from group I (e.g. triclosan, trimethoprim) were degraded to below their limit of detection already at the pre-ozonation step (thus after an ozone dose of 0.65 mg O3 mg TOC−1), while compounds from group II were removed completely after the post-ozonation step (after a combined ozone dose of 0.96 mg O3 mg−1 TOC). These results are in excellent agreement with previous studies, which demonstrated more than 98% elimination of compounds from groups I and II at ozone doses of 0.5 and 1 mg O3 mg−1 DOC respectively.25
The degradation level of group III compounds was lower than that of groups I and II, and many of them could be detected in the final effluent (e.g. primidone). This is consistent with their lower kO3 values (<10 M−1 s−1), implying that reaction with ˙OH is the major pathway for their elimination. The average elimination levels of oxpazam, phenytoin, DEET and primidone were 69% and 88% after the pre-ozonation and post-ozonation steps respectively (Fig. 2-III), which agree well with the data published earlier by Lee25 for compounds of similar reactivity (i.e. 63% and 93%, for mg O3 mg−1 DOC of 0.5 and 1.0). The degradation efficiency of compounds from groups IV and V is typically controlled by their reaction rate with ˙OH, with only marginal contribution from direct ozone reaction.25 The average degradation level of selected compounds from these groups after post-ozonation was 83% (group IV) and 7% (group V), comparable to the results published by Lee et al.25
The above data suggest that the removal of CECs in the ETP water will be most effective for compounds that exhibit moderate to high ozone and ˙OH reaction rate constants (groups I–III). Removal of specific CECs across each step of the treatment train is presented in more detail in the ESI† (Tables SI9–SI13).
In addition to the above reactivity-based model, the detected CECs were classified according to the recently revised California DPH Groundwater Replenishment Reuse regulations (CDPH, 2011). The revised regulation outlines required removals of indicator compounds by a full advanced treatment (i.e. reverse osmosis and an oxidation treatment), based on the compounds chemical structures and functional groups (last column in Table 4). According to the revised regulations, an oxidation process in a full-advanced-treatment facility must demonstrate at least 0.5-log removal (69%) for indicators from groups A–G, and 0.3-log removal (50%) for indicators from groups H–I (at least one indicator in each group).
In the context of Fig. 2, CDPH regulations were achieved by demonstrating more than 69% total removal of all compounds from groups I–IV (with the exception of iopromide) and more than 50% removal of iopromide. Group V (Fig. 2-V) is not addressed by the CDPH regulations, as these compounds (halogenated aliphatic) are known to resist oxidation processes. Compounds from CDPH group I (nitro-aromatics) were not detected throughout the ETP treatment. It is noteworthy that the new CDPH regulations only apply to advanced oxidation in full-advanced-treatment facilities; however, this approach may be adopted in the future to monitor CECs removal by other treatment alternatives.
3.3. Formation of transformation products in the ETP
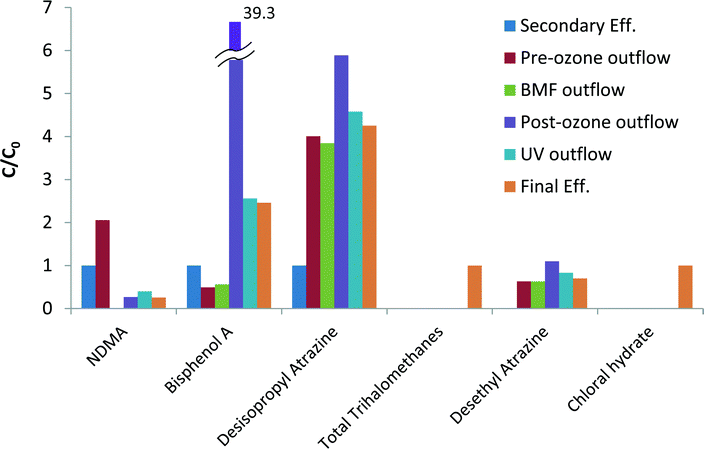
Transformation based processes (such as oxidation via ozone) may result in the formation of products that are of concern and considered CECs, or in direct increased of CECs already present in the influent. The generation path of products usually consists of oxidants reaction with either CECs or undefined dissolved organic matter (DOM). Indeed, several increases in contaminants concentrations (formation or increased product concentration from inlet to outlet) were observed across the tertiary treatment steps. Fig. 3 summarizes the main products that increased in concentration at various points throughout the treatment train. The complete data is presented in the ESI† (Tables SI16 and SI17).Contaminants that were formed as a result of oxidants reaction with DOM mostly include chlorination by-products such as chloroform, bromodichloromethane and dibromochloromethane, as well as chloral hydrate. NDMA doubled in concentration across the pre-ozonation step from 15.3 ng L−1 to 31.4 ng L−1. Hollender et al.47 reported that ozonation of WW effluent led to an increase in NDMA concentration, likely due to ozone reaction with NDMA precursors such as compounds containing dimethylamino moieties.48
Contaminants resulting from oxidant reactions with known CECs were mainly detected following the ozonation processes. Atrazine decreased through the treatment process; however, its ozonation products desisopropyl atrazine and desethyl atrazine increased, as previously observed by Adams and Randtke.49 Concentration of BPA dramatically increased during the post-ozonation step from 14.6 to 1024.4 ng L−1, which was unexpected as BPA readily reacts with ozone (kO3,BPA > 106 M−1 s−1 at pH 7 (ref. 50)) and does not typically accumulate during the process.51,52 The increase in BPA level might due to its leaching into the effluent from polycarbonate elements in the ozone system, as was previously found by Mercea53 though this was not verified.
Comparing the levels of contaminants detected in the effluent of the ETP with relevant Australian environmental discharge or irrigation guidelines,54 none of the chemicals listed in these guidelines were detected at levels above either guidelines values, indicating the water quality is appropriate for the intended reuse.
4. Conclusions
The data presented above represent the results of an investigation of a large suite of chemicals at various steps in the ETP tertiary treatment process. The ETP process utilizes ozone, biological media filtration, UV and chlorination as advanced treatment technologies, post secondary treatment of wastewater, to produce high quality effluent for various reuse options. Of the applied processes, ozone was shown to have the highest influence on contaminants' degradation. Chemicals that are known to have moderate to high reaction rates with ozone were removed to below the detection limit through the ozonation process. However, the advanced treatment train resulted in some contaminants increasing in concentration due to ozonation, and other newly forming contaminants due to chlorination. The resulting levels of contaminants of concern remaining in the ETP final effluent were below levels of relevance based on current Australian guidelines for reuse in irrigation or discharge to the environment.References
- D. B. Huggett, I. A. Khan, C. M. Foran and D. Schlenk, Determination of beta-adrenergic receptor blocking pharmaceuticals in United States wastewater effluent, Environ. Pollut., 2003, 121, 199–205 CrossRef CAS PubMed.
- D. W. Kolpin, E. T. Furlong, M. T. Meyer, E. M. Thurman, S. T. Zaugg, L. B. Barber and H. T. Buxton, Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: a national reconnaissance, Environ. Sci. Technol., 2002(36), 1202–1211 Search PubMed.
- N. Lindqvist, T. Tuhkanen and L. Kronberg, Occurrence of acidic pharmaceuticals in raw and treated sewages and in receiving waters, Water Res., 2005, 39, 2219–2228 CrossRef CAS PubMed.
- C. D. Metcalfe, B. G. Koenig, D. T. Bennie, M. Servos, T. A. Ternes and R. Hirsch, Occurrence of neutral and acidic drugs in the effluents of Canadian sewage treatment plants, Environ. Toxicol. Chem., 2002, 22, 2872–2880 CrossRef.
- T. A. Ternes, P. Kreckel and J. Mueller, Behaviour and occurrence of estrogens in municipal sewage treatment plants - II. aerobic batch experiments with activated sludge, Sci. Total Environ., 1999, 225, 91–99 CrossRef CAS PubMed.
- T. A. Ternes, M. Stumpf, J. Mueller, K. Haberer, R. D. Wilken and M. Servos, Behavior and occurrence of estrogens in municipal sewage treatment plants - I. investigations in Germany, Canada and Brazil, Sci. Total Environ., 1999, 225, 81–90 CrossRef CAS PubMed.
- T. A. Ternes, A. Joss and H. Siegrist, Scrutinizing pharmaceuticals and personal care products in wastewater treatment, Environ. Sci. Technol., 2004, 38, 392A–399A CrossRef CAS PubMed.
- NERL- National Exposure Research Laboratory, 2014, Environmental Sciences Pharmaceutical and Personal Care Products as Environmental Pollutants. http://www.epa.gov/esd/chemistry/pharma/index.html Search PubMed.
- T. S. Coe, P. B. Hamilton, D. Hodgson, G. C. Paull, J. R. Stevens, K. Sumner and C. R. Tyler, An environmental estrogen alters reproductive hierarchies, disrupting sexual selection in group-spawning fish, Environ. Sci. Technol., 2008, 42, 5020–5025 CrossRef CAS PubMed.
- K. A. Kidd, P. J. Blanchfield, K. H. Mills, V. P. Palace, R. E. Evans, J. M. Lazorchak and R. W. Flick, Collapse of a fish population after exposure to a synthetic estrogen, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 8897–8901 CrossRef CAS PubMed.
- D. Pascoe, W. Karntanut and C. T. Müller, Do pharmaceuticals affect freshwater invertebrates? A study with the cnidarian Hydra vulgaris, Chemosphere, 2003, 51, 521–528 CrossRef CAS PubMed.
- H. Andersen, H. Siegrist, B. H. Sørensen and T. A. Ternes, Fate of estrogens in a municipal sewage treatment plant, Environ. Sci. Technol., 2003, 37, 4021–4026 CrossRef CAS PubMed.
- L. Lishman, S. A. Smyth, K. Sarafin, S. Kleywegt, J. Toito, T. Peart, B. Lee, M. Servos, M. Beland and P. Seto, Occurrence and reductions of pharmaceuticals and personal care products and estrogens by municipal wastewater treatment plants in Ontario, Canada, Sci. Total Environ., 2006, 367, 544–558 CrossRef CAS PubMed.
- X. S. Miao, F. Bishay, M. Chen and C. D. Metcalfe, Occurrence of antimicrobials in the final effluents of wastewater treatment plants in Canada, Environ. Sci. Technol., 2004, 38, 3533–3541 CrossRef CAS PubMed.
- P. J. Phillips, S. G. Smith, D. W. Kolpin, S. D. Zaugg, H. T. Buxton, E. T. Furlong, K. Esposito and B. Stinson, Pharmaceutical formulation facilities as sources of opioids and other pharmaceuticals to wastewater treatment plant effluents, Environ. Sci. Technol., 2010, 44, 4910–4916 CrossRef CAS PubMed.
- T. A. Ternes, Occurrence of drugs in german sewage treatment plants and rivers, Water Res., 1998, 32, 3245–3260 CrossRef CAS.
- M. R. Servos, D. T. Bennie, B. K. Burnison, A. Jurkovic, R. McInnis, T. Neheli, A. Schnell, P. Seto, S. A. Smyth and T. A. Ternes, Distribution of estrogens, 17 beta-estradiol and estrone, in Canadian municipal wastewater treatment plants, Sci. Total Environ., 2005, 336, 155–170 CrossRef CAS PubMed.
- A. Svenson, A. S. Allard and M. Ek, Removal of estrogenicity in Swedish municipal sewage treatment plants, Water Res., 2003, 37, 4433–4443 CrossRef CAS PubMed.
- U.S. Environmental Protection Agency, 2010, Treating Contaminants of Emerging Concern: A Literature Review Database, Washington DC, p. 100 Search PubMed.
- T. A. Ternes and R. Hirsch, Occurrence and behavior of X-ray contrast media in sewage facilities and the aquatic environment, Environ. Sci. Technol., 2000, 34, 2741–2748 CrossRef CAS.
- M. O. Buffle, J. Schumacher, E. Salhi, M. Jekel and U. von Gunten, Measurement of the initial phase of ozone decomposition in water and wastewater by means of a continuous quench-flow system: Application to disinfection and pharmaceutical oxidation, Water Res., 2006, 40, 1884–1894 CrossRef CAS PubMed.
- S. A. Snyder, S. Adham, A. M. Redding, F. S. Cannon, J. DeCarolis, J. Oppenheimer, E. C. Wert and Y. Yoon, Role of membranes and activated carbon in the removal of endocrine disruptors and pharmaceuticals, Desalination, 2007, 202, 156–181 CrossRef CAS.
- M. M. Huber, A. Gobel, A. Joss, N. Hermann, D. Loffler, C. S. Mcardell, A. Ried, H. Siegrist, T. A. Ternes and U. von Gunten, Oxidation of pharmaceuticals during ozonation of municipal wastewater effluents: A pilot study, Environ. Sci. Technol., 2005, 39, 4290–4299 CrossRef CAS PubMed.
- R. I. L. Eggen, J. Hollender, A. Joss, M. Schärer and C. Stamm, Reducing the discharge of micropollutants in the aquatic environment: the benefits of upgrading wastewater treatment plants, Environ. Sci. Technol., 2014, 15, 7683–7689 CrossRef PubMed.
- Y. Lee, D. Gerrity, M. Lee, A. E. Bogeat, E. Salhi, S. Gamage, R. A. Trenholm, E. C. Wert, S. A. Snyder and U. von Gunten, Prediction of micropollutant elimination during ozonation of municipal wastewater effluents: Use of kinetic and water specific information, Environ. Sci. Technol., 2013, 47, 5872–5881 CrossRef CAS PubMed.
- U. Hübner, U. Miehe and M. Jekel, Optimized removal of dissolved organic carbon and trace organic contaminants during combined ozonation and artificial groundwater recharge, Water Res., 2012, 46, 6059–6068 CrossRef PubMed.
- V. J. Pereira, K. G. Linden and H. S. Weinberg, Evaluation of UV irradiation for photolytic and oxidative degradation of pharmaceutical compounds in water, Water Res., 2007, 41, 4413–4423 CrossRef CAS PubMed.
- E. J. Rosenfeldt and K. G. Linden, Degradation of endocrine disrupting chemicals bisphenol A, ethinyl estradiol, and estradiol during UV photolysis and advanced oxidation processes, Environ. Sci. Technol., 2004, 38, 5476–5483 CrossRef CAS PubMed.
- D. Vogna, R. Marotta, A. Napolitano, R. Andreozzi and M. d'Ischia, Advanced oxidation of the pharmaceutical drug diclofenac with UV/H2O2 and ozone, Water Res., 2004, 38, 414–422 CrossRef CAS PubMed.
- D. C. McDowell, M. M. Huber, M. Wagner, U. von Gunten and T. A. Ternes, Ozonation of carbamazepine in drinking water: Identification and kinetic study of major oxidation products, Environ. Sci. Technol., 2005, 39, 8014–8022 CrossRef CAS PubMed.
- M. M. Huber, T. A. Ternes and U. von Gunten, Removal of estrogenic activity and formation of oxidation products during ozonation of 17 alpha-ethinylestradiol, Environ. Sci. Technol., 2004, 38, 5177–5186 CrossRef CAS PubMed.
- O. S. Keen, S. Baik, K. G. Linden, D. S. Aga and N. G. Love, Enhanced biodegradation of carbamazepine after UV/H2O2 advanced oxidation, Environ. Sci. Technol., 2012, 46, 6222–6227 CrossRef CAS PubMed.
- O. S. Keen, E. M. Thurman, I. Ferrer, A. D. Dotson and K. G. Linden, Dimer formation during UV photolysis of diclofenac, Chemosphere, 2013, 93, 1948–1956 CrossRef CAS PubMed.
- O. S. Keen, E. M. Thurman, I. Ferrer and K. G. Linden, Degradation pathways of lamotrigine under advanced treatment by direct UV photolysis, hydroxyl radicals, and ozone, Chemosphere, 2014, 117, 316–323 CrossRef CAS PubMed.
- V. J. Pereira, H. S. Weinberg, K. G. Linden and P. C. Singer, UV degradation kinetics and modeling of pharmaceutical compounds in laboratory grade and surface water via direct and indirect photolysis at 254 nm, Environ. Sci. Technol., 2007, 41, 1682–1688 CrossRef CAS PubMed.
- U. Hübner, U. von Gunten and M. Jekel, Evaluation of the persistence of transformation products from ozonation of trace organic compounds - A critical review, Water Res., 2015, 68, 150–170 CrossRef PubMed.
- H. Shemer, Y. K. Kunukcu and K. G. Linden, Degradation of the pharmaceutical Metronidazole via UV, Fenton and photo-Fenton processes, Chemosphere, 2006, 63, 269–276 CrossRef CAS PubMed.
- E. J. Rosenfeldt, P. J. Chen, S. Kullman and K. G. Linden, Destruction of estrogenic activity in water using UV advanced oxidation, Sci. Total Environ., 2007, 377, 105–113 CrossRef CAS PubMed.
- C. D. Adams, P. A. Scanlan and N. D. Secrist, Oxidation and biodegradability enhancment of 1,4-dioxane using hydrogen-peroxide and ozone, Environ. Sci. Technol., 1994, 28, 1812–1818 CrossRef CAS PubMed.
- C. D. Adams, S. Spitzer and R. M. Cowan, Biodegradation of nonionic surfactants and effects of oxidative pretreatment, J. Environ. Eng., 1996, 122, 477–483 CrossRef CAS.
- I. A. Balcioglu, I. A. Alaton, M. Otker, R. Bahar, N. Bakar and M. Ikiz, Application of advanced oxidation processes to different industrial wastewaters, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2003, 38, 1587–1596 CrossRef.
- C. P. L. J. Grady, G. T. Daigger, N. G. Love and C. Filipe, Biological Wastewater Treatment, 2011, 3rd edn., Taylor and Francis Search PubMed.
- N. J. D. Graham, Removal of humic substances by oxidation/biofiltration processes - a review, Water Sci. Technol., 1999, 40, 141–148 CrossRef CAS.
- R. M. Hozalski, E. J. Bouwer and S. Goel, Removal of natural organic matter (NOM) from drinking water supplies by ozone-biofiltration, Water Sci. Technol., 1999, 40, 157–163 CrossRef CAS.
- H. K. Shon, S. Vigneswaran and S. A. Snyder, Effluent organic matter (EfOM) in wastewater: constituents, effects and treatments, Crit. Rev. Environ. Sci. Technol., 2006, 36, 327–374 CrossRef CAS.
- V. K. Sharma, Oxidative transformations of environmental pharmaceuticals by Cl2, ClO2, O3, and Fe(VI): Kinetics assessment, Chemosphere, 2008, 73, 1379–1386 CrossRef CAS PubMed.
- J. Hollender, S. G. Zimmermann, S. Koepke, M. Krauss, C. S. McArdell, C. Ort, H. Singer, U. von Gunten and H. Siegrist, Elimination of organic micropollutants in a municipal wastewater treatment plant upgraded with a full-scale post-ozonation followed by sand filtration, Environ. Sci. Technol., 2009, 43, 7862–7869 CrossRef CAS PubMed.
- P. Andrzejewski, B. Kasprzyk-Hordern and J. Nawrocki, N-nitrosodimethylamine (NDMA) formation during ozonation of dimethylamine-containing waters, Water Res., 2008, 42, 863–870 CrossRef CAS PubMed.
- C. D. Adams and S. J. Randtke, Ozonation byproducts of atrazine in synthetic and natural waters, Environ. Sci. Technol., 1992, 26, 2218–2227 CrossRef CAS.
- M. Deborde, S. Rabouan, J. Duguet and B. Legube, Kinetics of aqueous ozone-induced oxidation of some endocrine disruptors, Environ. Sci. Technol., 2005, 39, 6086–6092 CrossRef CAS PubMed.
- S. Irmak, O. Erbatur and A. Akgerman, Degradation of 17β-estradiol and bisphenol A in aqueous medium by using ozone and ozone/UV techniques, J. Hazard. Mater., 2005, B126, 54–62 CrossRef PubMed.
- E. C. Wert, F. L. Rosario-Ortiz and S. E. Snyder, Effect of ozone exposure on the oxidation of trace organic contaminants in wastewater, Water Res., 2009, 43, 1005–1014 CrossRef CAS PubMed.
- P. Mercea, Physicochemical processes involved in migration of bisphenol A from polycarbonate, J. Appl. Polym. Sci., 2009, 112, 579–593 CrossRef CAS.
- Australian and New Zealand Environment and Conservation Council - ANZECC and Agriculture and Resource Management Council of Australia and New Zealand – ARMCANZ, Australian and New Zealand Guidelines for Fresh and Marine Water Quality, 2000, http://www.environment.gov.au/resource/australian-and-new-zealand-guidelines-fresh-and-marine-water-quality-volume-3-primary.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ew00186b |
| This journal is © The Royal Society of Chemistry 2016 |