Water-compatible surface molecularly imprinted polymers with synergy of bi-functional monomers for enhanced selective adsorption of bisphenol A from aqueous solution†
Feifei
Duan
abc,
Chaoqiu
Chen
b,
Xiaofeng
Zhao
ac,
Yongzhen
Yang
ad,
Xuguang
Liu
*ac and
Yong
Qin
*b
aKey Laboratory of Interface Science and Engineering in Advanced Materials (Taiyuan University of Technology), Ministry of Education, Taiyuan 030024, China. E-mail: liuxuguang@tyut.edu.cn
bState Key Laboratory of Coal Conversion, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001, China. E-mail: qinyong@sxicc.ac.cn
cCollege of Chemistry and Chemical Engineering, Taiyuan University of Technology, Taiyuan 030024, China
dResearch Center on Advanced Materials Science and Technology, Taiyuan University of Technology, Taiyuan 030024, China
First published on 4th January 2016
Abstract
Water-compatible molecularly imprinted polymers (MIPs) with dual monomer–template interactions were synthesized via the synergy of bi-functional monomers of water-soluble 2-acrylamido-2-methylpropanesulfonic acid (AMPS) and styrene (St) for the selective adsorption of bisphenol A (BPA) from aqueous media using porous graphene oxide as a support. Both hydrogen bonds and π–π interactions are responsible for the adsorption of BPA on the synthesized MIPs. The formation and structure of the MIPs are verified by Fourier transform infrared spectroscopy, thermogravimetric analysis, transmission electron microscopy and dispersion analysis in water. The adsorption results show that the adsorption capacity of MIPs is greatly enhanced by virtue of the synergy of AMPS and St. The MIPs prepared with a molar ratio (AMPS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) St) of 2.5
St) of 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 exhibit the highest adsorption capacity (up to 85.7 mg g−1 at 293 K) toward BPA in aqueous media. The kinetics and isotherm data can be well fitted with the pseudo-second-order kinetic model and the Freundlich isotherm, respectively. Competitive adsorption experiments demonstrate that the synthesized MIPs display excellent selectivity toward BPA against analogue molecules. The MIPs show good recoverability and exhibit excellent adsorption affinity toward BPA even in complex river water. This work provides a versatile approach for the fabrication of high performance MIPs for application in aqueous environments.
2.5 exhibit the highest adsorption capacity (up to 85.7 mg g−1 at 293 K) toward BPA in aqueous media. The kinetics and isotherm data can be well fitted with the pseudo-second-order kinetic model and the Freundlich isotherm, respectively. Competitive adsorption experiments demonstrate that the synthesized MIPs display excellent selectivity toward BPA against analogue molecules. The MIPs show good recoverability and exhibit excellent adsorption affinity toward BPA even in complex river water. This work provides a versatile approach for the fabrication of high performance MIPs for application in aqueous environments.
Nano impactDetection, separation and treatment of endocrine disrupting chemicals (EDCs) are an increasing concern for public health due to their toxicity, low biodegradability and emission in various aquatic environments. Molecularly imprinted polymers (MIPs) are promising sorbent materials to remove EDCs because of their high affinity and selectivity toward target molecules. In this work, water-compatible MIPs/porous graphene oxide nanomaterials with dual monomer–template interactions were synthesized using water-soluble 2-acrylamido-2-methylpropanesulfonic acid (AMPS) and styrene (St) as bi-functional monomers and were demonstrated to be effective adsorbent materials for the selective removal of bisphenol A from water. The MIPs prepared with a molar ratio (AMPS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) St) of 2.5 St) of 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 exhibit the highest adsorption capacity (up to 85.7 mg g−1 at 293 K) toward BPA in aqueous media, which is the highest reported BPA adsorption capacity for MIP-based sorbents in water to date. 2.5 exhibit the highest adsorption capacity (up to 85.7 mg g−1 at 293 K) toward BPA in aqueous media, which is the highest reported BPA adsorption capacity for MIP-based sorbents in water to date.
|
1. Introduction
Bisphenol A (BPA) has been widely used in the production of plastics, pesticides, coatings and antioxidants. However, prolonged utilization and abuse of BPA in industry and households lead to its release and accumulation in the surrounding environment, especially in aquatic environments because of its strong polarity and low volatility.1–3 Moreover, because of its weak estrogen-like effect, BPA is harmful to organisms even at low doses.4,5 Therefore, many methods such as membrane separation,6,7 photocatalytic degradation8,9 and adsorption4,10–12 have been developed to remove BPA from environmental water. Among these methods, adsorption has attracted much attention because of its low cost, easy operation and low environmental impact. Regarding the adsorption technique, the key point is the design of adsorbents. Although a number of conventional adsorbents such as active carbon, zeolites and resins have shown excellent adsorption ability and have been widely used in practical wastewater treatment, their poor selectivity is still a large defect. Therefore, the development of adsorption materials with high adsorption capacity and selectivity is very important for removing low-level BPA from aquatic environments.Molecularly imprinted polymers (MIPs) are promising and facile adsorption materials for separation procedures and chemical analyses because of their characteristic molecular recognition based on memory effects of the shape, size and functional groups of the template/target molecules.13,14 Due to their highly selective adsorption by molecular recognition, various MIPs have been developed to remove or monitor BPA.2,3,15–19 Although molecular imprinting technology has been developed for many years and great progress has been achieved, many challenges remain to be addressed. In particular, it has been demonstrated that MIPs synthesized in organic solvents show excellent selective adsorption ability toward targeted molecules in organic solvents but poor molecular recognition performance in aqueous environments, which severely limits their practical application in areas such as wastewater treatment and biomimetic sensors.13,20 One of the main reasons for the incompatibility of MIPs in aqueous solutions is that the presence of a great excess of water can weaken the non-covalent interactions between the template molecules and MIPs such as hydrogen bonds and electrostatic forces.13,21 Furthermore, MIPs synthesized in organic solutions show poor dispersion and a different swelling effect in aqueous solution, which impede access of the template molecules to the recognition cavities.22,23 So far, many efforts have been devoted to improving the water-compatibility of MIPs and two main strategies have been developed. One is the post-modification of MIPs by surface grafting of hydrophilic polymer layers or by chemical modification of the MIP particles.22,24 The other is the use of hydrophilic monomers, such as 2-hydroxyethyl methacrylate,25 β-cyclodextrins,26,27 1-(α-methyl acrylate)-3-methylimidazolium bromide28 and 2-acrylamido-2-methylpropanesulfonic acid (AMPS),3,29 in the synthesis process of MIPs. This approach is simple and can effectively improve the surface hydrophilicity of MIPs. For instance, we demonstrated that water-compatible MIPs could be prepared using water-soluble AMPS as a monomer. The obtained MIPs exhibited excellent selectivity toward BPA in aqueous phase;3 whereas, the binding capacity of majority of the reported water-compatible MIPs in aqueous phase is still low,2,3,16,19 resulting from the weakening of hydrogen bonds between the template molecules and MIPs due to water molecules. To improve the binding capacities of MIPs in aqueous phase, an efficient method is to design MIPs by combining bi- or multiple functional monomer systems with different monomer–template interactions, since it will enhance the adsorption selectivity and stability toward template molecules. For example, Cai et al. synthesized Pb2+ ion imprinted polymers (IIPs) using MAA and 4-VP as bi-functional monomers for the selective solid-phase extraction of Pb2+ in water samples.30 The two functional monomers provide an excellent synergistic effect and the resultant IIPs display fast adsorption kinetics and high binding capacity for Pb2+. Zeng et al. prepared rutin imprinted polymers with acrylamide and 2-vinylpyridine as bi-functional monomers and ethylene glycol dimethacrylate and divinylbenzene as bi-crosslinkers, in which the prepared MIPs exhibited high adsorption and selective ability.31 BPA molecules can not only form hydrogen bonds with various functional groups such as –OH, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –NH, but also generate π–π stacking interactions with organic molecules having C
O and –NH, but also generate π–π stacking interactions with organic molecules having C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds or benzene rings, which are not interfered by water molecules. Therefore, the design and preparation of MIPs with higher binding capacity toward BPA in aqueous phase by virtue of two kinds of monomer–template interactions are expected.
C double bonds or benzene rings, which are not interfered by water molecules. Therefore, the design and preparation of MIPs with higher binding capacity toward BPA in aqueous phase by virtue of two kinds of monomer–template interactions are expected.
Surface molecular imprinting, in which the imprinting sites are located at or near the surface of supports, has attracted much attention because it can enhance mass transfer and binding kinetics, and hence generates higher binding capacity. So far, many materials have been studied as supports to synthesize surface MIPs, such as silica,16,19 Fe3O4 nanoparticles,18 graphene oxide32 and carbon nanotubes.33 Among them, graphene oxide (GO) is regarded as a promising matrix for preparing surface MIPs because of its little swelling, excellent stability under acidic and alkali conditions and high theoretical surface areas (2630 m2 g−1).14,32 Luo et al. described a one-step approach to synthesize a surface protein-imprinted nanomaterial employing reduced graphene oxide (RGO) nanosheets as substrates. The prepared MIPs exhibited high binding capacity, fast adsorption kinetics and good selectivity toward template protein.34 Recently, our group developed MIPs/GO for deep desulfurization using GO nanosheets as supports, which showed excellent adsorption capacity, high selectivity and fast binding kinetics for dibenzothiophene.32 However, due to the small size and excellent dispersibility of GO nanosheets and GO nanosheet–MIP nanomaterials in common solvents, it is very difficult to separate these nanomaterials from the solvents, making the preparation process of GO nanosheet–MIPs time-consuming. A simple method to solve this problem is to construct three-dimensional porous architectures using GO nanosheets as building blocks. The porous GO monolithic materials could be easily separated from water and exhibit high surface area and accessible pore volume, making them excellent scaffolds for the molecular imprinting process.
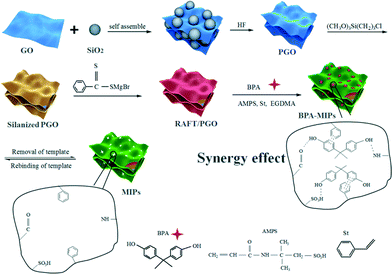
Herein, we report the synthesis of water-compatible surface MIPs for adsorbing BPA from aqueous phase by surface reversible addition–fragmentation chain transfer (RAFT) polymerization using AMPS and St as bi-monomers and porous graphene oxide (PGO) as the support. The ratio of AMPS to St is optimized to obtain MIPs with high adsorption capacity and selectivity toward BPA in aqueous solution. The morphologies and structures of the obtained MIPs are characterized by TEM, FTIR and TG. The adsorption kinetic, isotherm and thermodynamic studies of MIPs toward BPA in aqueous solution are also carried out to understand the adsorption mechanism. The MIPs were further evaluated for removal of BPA from spiked river and tap water simulating contaminated environmental water.
2. Experimental
2.1 Synthesis of RAFT/PGO
PGO was prepared by using the method of Zhao's group (details in the ESI†).35 The obtained PGO was then modified with γ-chloropropyl trimethoxysilane, a coupling agent which supplies chlorine for reacting with the RAFT agent. One milliliter of deionized water and 1.5 mL of γ-chloropropyl trimethoxysilane were added to 50 mL of ethanol containing 0.2 g of PGO and the mixture was stirred at 65 °C for 6 h. After the reaction, the silanized PGO was obtained by filtration, washing with ethanol, and drying at 50 °C overnight.The RAFT agent was immobilized onto silanized PGO as follows: 2 mL of bromobenzene and 0.24 g of magnesium were added to 25 mL of tetrahydrofuran under stirring at 35 °C for 2 h. Then, 2 mL of carbon disulfide was slowly added over 0.5 h, and the reaction system was maintained for 2 h. Next, 0.2 g of silanized PGO was added to the resulting mixture, and the reaction was kept at 35 °C for 48 h. The RAFT/PGO was obtained by filtration with ethanol until the filtered liquid became colorless and subsequent drying overnight.
2.2 Synthesis of MIPs
MIPs on PGO with different ratios of AMPS to St were prepared. St was refined with NaOH solution before polymerization. The total amount of functional monomers was fixed at 4 mmol. Taking MIPs prepared with AMPS as an example, 0.228 g of BPA (1 mmol), 0.832 g (4 mmol) of AMPS, 0.08 g of RAFT/PGO and 1.88 mL of EGDMA (10 mmol) were dispersed in 25 mL of DMF at room temperature for 1 h. Then, 0.0325 g of 2,2′-azobis(isobutyronitrile) was added to the mixed solution. The suspension was deoxygenized with nitrogen for 1 h and then sealed. The polymerization was performed at 50 °C for 24 h under nitrogen. The product was centrifuged and washed with ethanol three times to remove unreacted reagents. Then, the template molecules were removed by washing the product with 30 mL of mixture solution (ethanol and acetic acid, v/v = 9/1) under ultrasound five times. Finally, the product (denoted as AMPS/MIPs) was washed with ethanol to remove the remaining acetic acid and dried. MIPs with bi-functional monomers were prepared in a similar way and the detailed doses of each monomer are listed in Table S1 in the ESI.† The MIPs prepared with St were denoted as St/MIPs. For comparison, surface non-imprinted polymers were also prepared without adding template molecules.2.3 Adsorption experiments
Adsorption of BPA was carried out in a stirred batch system. In brief, BPA was dissolved in ethanol as a stock solution (2000 mg L−1) because of its low solubility in water and further diluted with a large amount of water to the required concentration before use. All adsorption experiments were performed in sealed 100 mL glass conical flasks that contained 20 mg of adsorbent and 40 mL of BPA solution in appropriate concentration.The adsorption kinetic study at different temperatures was carried out with an initial BPA concentration of 50 mg L−1. The concentration of BPA was detected by using a UV-vis spectrophotometer (HITACHI, U-3900) at different time intervals ranging from 2 minutes to 2 h. The adsorption isotherm experiments were similar to the adsorption kinetic study. The initial BPA concentration ranged from 2 to 50 mg L−1.
The competitive adsorption test was performed in a mixture solution of BPA, tetrabromobisphenol A (TBBPA) and 4-tert-butylphenol (BP) with each having an initial concentration of 50 mg L−1. After binding equilibrium, the suspension was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. The concentrations of BPA, TBBPA and BP were determined by using a high-performance liquid chromatography (HPLC, Waters) system with an Akasil-C18 column (Agela Technologies Inc., 4.6 × 150 mm) and a UV absorbance detector operated at 276 nm for BPA and BP and 292 nm for TBBPA. The mobile phase was 0.5 mL min−1 of 60% acetonitrile, 39% deionized water and 1% acetic acid.
000 rpm for 10 min. The concentrations of BPA, TBBPA and BP were determined by using a high-performance liquid chromatography (HPLC, Waters) system with an Akasil-C18 column (Agela Technologies Inc., 4.6 × 150 mm) and a UV absorbance detector operated at 276 nm for BPA and BP and 292 nm for TBBPA. The mobile phase was 0.5 mL min−1 of 60% acetonitrile, 39% deionized water and 1% acetic acid.
The effect of pH on the adsorption capacity of MIPs toward BPA was studied with an initial BPA concentration of 50 mg L−1 in a pH range of 1.0 to 11.0 at 293 K. The solution pH was adjusted with 1 M HCl or NaOH solution. The effect of ionic strength on the adsorption of BPA was studied by adding NaCl to 50 mg L−1 BPA solutions with a concentration ranging from 0.02 to 0.5 M at 293 K and pH 6.0.
The desorption and reutilization of the MIPs were investigated. The adsorbent MIPs saturated with BPA were washed with a mixture solution (ethanol and acetic acid, V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V = 9
V = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and then the regenerated MIPs were reused for the next adsorption. The adsorption–desorption cycles were repeated five times under the same conditions.
1), and then the regenerated MIPs were reused for the next adsorption. The adsorption–desorption cycles were repeated five times under the same conditions.
2.4 Application of the MIPs to real samples
River water samples were collected from Fen River in Taiyuan, China. Tap water samples were from the Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan, China. All the water samples were filtered through a 0.22 μm filter before use. The adsorption experiments were carried out in spiked water samples with BPA (50 mg L−1) at 293 K. After binding equilibrium, the suspension was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. The concentration of BPA in the supernatant was detected by HPLC.
000 rpm for 10 min. The concentration of BPA in the supernatant was detected by HPLC.
3. Results and discussion
3.1 Effect of molar ratio of AMPS and St on adsorption capacities of MIPs
Here, water-soluble AMPS and aromatic St were chosen as bi-functional monomers to introduce double monomer–template interactions into water-compatible BPA MIPs. AMPS has been demonstrated to be an efficient monomer to improve the surface hydrophilicity of MIPs.3 However, the hydrogen bonds between AMPS and BPA are partially disturbed by water molecules, so it is necessary to introduce other monomer–template interactions into MIPs which are not disturbed by water molecules, for example, π–π stacking interactions. St, an aromatic monomer, can easily form stable π–π stacking interactions with BPA molecules. In this bi-functional monomer-imprinted system, AMPS enhances the water-compatible properties of the imprinted polymers and St lessens the interference by water molecules. The synergetic effect of AMPS and St is expected to greatly improve the adsorption ability of MIPs toward BPA in aqueous phase.The overall preparation of MIPs on PGO is depicted in Scheme 1, which involves a four-step process: (1) PGO is prepared by the hard-template method; (2) the silane coupling agent is grafted onto PGO, and silanized PGO is obtained; (3) the RAFT agent is grafted onto the surface of silanized PGO; (4) MIP coatings are introduced onto the surface of PGO via surface RAFT polymerization using AMPS and St as bi-functional monomers and BPA as the template molecule. After imprinting, the template molecules are removed by repetitive washing in ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetic acid (90
acetic acid (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, v/v), and then recognition cavities complementary to BPA in shape, size and chemical functionality are formed, which could selectively rebind BPA molecules from a mixture of BPA and the analogs.
10, v/v), and then recognition cavities complementary to BPA in shape, size and chemical functionality are formed, which could selectively rebind BPA molecules from a mixture of BPA and the analogs.
To obtain more effective MIPs, the ratio of AMPS to St was optimized. The adsorption capacities of MIPs prepared with different molar ratios of AMPS to St are shown in Fig. S1 in the ESI.† It can be seen that the adsorption capacity of MIPs is greatly enhanced by the synergy of AMPS and St. Furthermore, the ratio of AMPS to St has an obvious influence on the adsorption capacity of MIPs. With the decrease in the molar ratio of AMPS to St from 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, the adsorption capacity of MIPs initially increases and subsequently decreases. The adsorption capacities of MIPs prepared with AMPS
4, the adsorption capacity of MIPs initially increases and subsequently decreases. The adsorption capacities of MIPs prepared with AMPS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) St ratios of 4
St ratios of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3
1, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 2.5
2, 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5, 2
2.5, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 1
3 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 were 58.5, 69.0, 85.7, 69.0 and 67.5 mg g−1, respectively, much higher than those of AMPS/MIPs (50.7 mg g−1) and St/MIPs (9.11 mg g−1). These results demonstrate that the combination of AMPS and St as bi-functional monomers is a feasible route to greatly improve the adsorption capacity of MIPs toward BPA in aqueous solution. The MIPs synthesized with bi-functional monomers are water-compatible because of the rich hydrophilic functional groups in AMPS, which is beneficial for target molecules to access the recognition cavities. Moreover, the π–π stacking interaction between St and BPA cannot be easily disturbed by water molecules. As a result, the adsorption ability of MIPs synthesized with bi-functional monomers was enhanced. The MIPs with the AMPS-to-St ratio of 2.5
4 were 58.5, 69.0, 85.7, 69.0 and 67.5 mg g−1, respectively, much higher than those of AMPS/MIPs (50.7 mg g−1) and St/MIPs (9.11 mg g−1). These results demonstrate that the combination of AMPS and St as bi-functional monomers is a feasible route to greatly improve the adsorption capacity of MIPs toward BPA in aqueous solution. The MIPs synthesized with bi-functional monomers are water-compatible because of the rich hydrophilic functional groups in AMPS, which is beneficial for target molecules to access the recognition cavities. Moreover, the π–π stacking interaction between St and BPA cannot be easily disturbed by water molecules. As a result, the adsorption ability of MIPs synthesized with bi-functional monomers was enhanced. The MIPs with the AMPS-to-St ratio of 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 show the highest adsorption capacity. For convenience, the corresponding MIPs were denoted as AMPS–St/MIPs and their properties and adsorption performance were studied in detail.
2.5 show the highest adsorption capacity. For convenience, the corresponding MIPs were denoted as AMPS–St/MIPs and their properties and adsorption performance were studied in detail.
3.2 Characterization of MIPs
FTIR analysis was carried out to characterize the structural details of PGO and functionalized PGO at different steps. In the FTIR spectrum of PGO (Fig. 1), the wide and strong band at 3425 cm−1 indicates the existence of –OH, while the band at 1634 cm−1 corresponds to the aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups.32,36 The band at around 1042 cm−1 can be assigned to C–O groups.36 For silanized PGO, the expected band corresponding to Si–O is not observed probably because the band is weak and overlaps with the C–O groups of PGO. Compared with PGO, RAFT/PGO shows a weak band around 1165 cm−1, which can be attributed to C
C groups.32,36 The band at around 1042 cm−1 can be assigned to C–O groups.36 For silanized PGO, the expected band corresponding to Si–O is not observed probably because the band is weak and overlaps with the C–O groups of PGO. Compared with PGO, RAFT/PGO shows a weak band around 1165 cm−1, which can be attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S groups.37 For St/MIPs, AMPS/MIPs and AMPS–St/MIPs, they all show a new weak band at 2318 cm−1 which can be assigned to –CH2–. In addition, two new bands at 1729 cm−1 and 1114 cm−1 are observed in the spectra of AMPS/MIPs and AMPS–St/MIPs, which can be assigned to C
S groups.37 For St/MIPs, AMPS/MIPs and AMPS–St/MIPs, they all show a new weak band at 2318 cm−1 which can be assigned to –CH2–. In addition, two new bands at 1729 cm−1 and 1114 cm−1 are observed in the spectra of AMPS/MIPs and AMPS–St/MIPs, which can be assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –SO3H.38 Meanwhile, the expected bands ascribed to C
O and –SO3H.38 Meanwhile, the expected bands ascribed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C of St in St/MIPs and AMPS–St/MIPs are overlapped with the characteristic bands of aromatic C
C of St in St/MIPs and AMPS–St/MIPs are overlapped with the characteristic bands of aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups of PGO. All these results indicate that the imprinted polymers are grafted onto the surface of PGO. For AMPS–St/MIPs without washing (denoted as AMPS–St/BPA–MIPs), a new band around 2800–3000 cm−1 is observed, which is in agreement with the band from the FTIR spectrum of BPA. Interestingly, the stretching frequency of the O–H group exhibits a shift from 3425 to 3371 cm−1, which can be due to hydrogen bonds between hydroxyl groups contained in both BPA and AMPS–St/MIPs. After washing with ethanol and acetic acid five times, no characteristic bands of BPA can be observed in the FTIR spectrum of AMPS–St/MIPs, indicating that the template BPA molecules are removed from AMPS–St/MIPs. The grafting of MIPs on the surface of PGO was also confirmed by thermogravimetric analysis (Fig. S2 in the ESI†). The PGO and functionalized PGO at different steps show different thermal stabilities, decomposition temperatures and weight losses.
C groups of PGO. All these results indicate that the imprinted polymers are grafted onto the surface of PGO. For AMPS–St/MIPs without washing (denoted as AMPS–St/BPA–MIPs), a new band around 2800–3000 cm−1 is observed, which is in agreement with the band from the FTIR spectrum of BPA. Interestingly, the stretching frequency of the O–H group exhibits a shift from 3425 to 3371 cm−1, which can be due to hydrogen bonds between hydroxyl groups contained in both BPA and AMPS–St/MIPs. After washing with ethanol and acetic acid five times, no characteristic bands of BPA can be observed in the FTIR spectrum of AMPS–St/MIPs, indicating that the template BPA molecules are removed from AMPS–St/MIPs. The grafting of MIPs on the surface of PGO was also confirmed by thermogravimetric analysis (Fig. S2 in the ESI†). The PGO and functionalized PGO at different steps show different thermal stabilities, decomposition temperatures and weight losses.
 | ||
| Fig. 1 FTIR spectra of PGO, silanized PGO, RAFT/PGO, St/MIPs, AMPS/MIPs, AMPS–St/MIPs, AMPS–St/BPA–MIPs and BPA. | ||
The dispersion of MIPs in water plays an important role in the adsorption performance of MIPs toward BPA in aqueous phase. Fig. 2 shows the dispersion stability of PGO, AMPS/MIPs, St/MIPs and AMPS–St/MIPs in water. PGO can well disperse in water even after settling for 4 h because there are many hydrophilic functional groups such as –OH and –COOH on the surface of PGO. AMPS/MIPs, St/MIPs and AMPS–St/MIPs show different dispersion stabilities in water. AMPS/MIPs show excellent dispersion in aqueous phase because of the incorporation of hydrophilic functional monomer AMPS, which is consistent with our previous work.3 In contrast, St/MIPs float on water even immediately after stirring, indicating that St/MIPs could not disperse in aqueous phase. This phenomenon can be explained by the hydrophobic properties of St.39 Interestingly, AMPS–St/MIPs could disperse homogeneously in water after stirring, though slow sedimentation is observed after settling for 4 h. These results further verified the incorporation of both AMPS and St into MIPs.
 | ||
| Fig. 2 Photograph of the dispersion of PGO and functionalized PGO in pure water (1 mg mL−1) (a) after stirring for 10 min and (b) after settling for 4 h. | ||
PGO was selected as the substrate for MIPs because of its porous structure, high surface area and easy separation from water. The morphology and structure of PGO and functionalized PGO at different steps were examined by TEM, as shown in Fig. 3. The porous structure throughout the entire PGO can be clearly observed (Fig. 3a), which is in agreement with the literature.35 The obtained PGO exhibits a surface area of 94.5 m2 g−1. Fig. 3b–e show the TEM images of the silanized PGO, AMPS/MIPs, AMPS–St/MIPs and St/MIPs, respectively. These images clearly show that the porous structure of the PGO substrate is preserved after grafting the silane coupling agent and MIPs. Though it is hard to measure the definitive thickness of the MIP layers on the GO nanosheet surfaces, it can be speculated that the MIPs/GO sheets are very thin with nanoscale thickness according to the TEM images, which is expected to enhance mass transfer and adsorption kinetics. In addition, Si and Cl signals can be seen in the EDS spectrum of silanized PGO (Fig. 3f), which suggests that the silane coupling agent (γ-chloropropyl trimethoxysilane) is grafted on the surface of PGO. After imprinting, S is observed in the EDS spectra of AMPS–St/MIPs and AMPS/MIPs (Fig. 3g and h, respectively), which originates from the AMPS monomer, while the peak of N overlaps with that of C. These results further confirm that the MIPs are coated onto PGO.
 | ||
| Fig. 3 TEM images of (a) PGO, (b) silanized PGO, (c) AMPS/MIPs, (d) AMPS–St/MIPs and (e) St/MIPs; EDS spectra of (f) silanized PGO, (g) AMPS–St/MIPs and (h) AMPS/MIPs. | ||
3.3 Adsorption kinetic studies of AMPS–St/MIPs
To evaluate the effectiveness of AMPS–St/MIPs for adsorption of BPA from aqueous phase, the adsorption capacity and equilibrium time of PGO, AMPS–St/MIPs and AMPS–St/NIPs were investigated at 293 K with an initial BPA concentration of 50 mg L−1. Fig. 4a displays the adsorption kinetic curves of PGO, AMPS–St/MIPs and AMPS–St/NIPs toward BPA. It can be seen that the equilibrium times of PGO, AMPS–St/MIPs and AMPS–St/NIPs are about 20, 60 and 60 minutes, respectively, which are faster than our previous results for MIPs/carbon microspheres (about 120 min),3 indicative of fast mass transfer on these PGO-based materials because of the porous structure and nanoscale thickness of the pore walls (MIPs/GO sheets). Moreover, as expected, the adsorption capacity of AMPS–St/MIPs (85.7 mg g−1) is much higher than those of AMPS–St/NIPs (40.9 mg g−1) and PGO (66.3 mg g−1), demonstrating the formation of specific recognition sites on the surface of PGO, which benefits BPA to bind with the recognition sites.To further elucidate the mechanism of the adsorption process of BPA onto AMPS–St/MIPs, adsorption kinetic tests at three different temperatures (293 K, 298 K and 303 K) were carried out and two conventional kinetic models (pseudo-first-order3,40–42 and pseudo-second-order43,44) were applied to analyze the experimental data (Fig. 4b). A detailed description of the kinetic models is provided in Table S2 in the ESI.† As shown in Fig. 4b and Table S2,† the sorption kinetics of BPA on AMPS–St/MIPs can be better fitted by a pseudo-second-order model.
3.4 Adsorption isotherm and thermodynamic studies of AMPS–St/MIPs
Adsorption isotherm models are efficient avenues in revealing the interaction between the adsorbent and adsorbate when the adsorption process reaches equilibrium. Fig. 5 shows the adsorption isotherms of BPA on AMPS–St/MIPs at three different temperatures. It can be observed that the adsorption capacity of AMPS–St/MIPs increases with increasing equilibrium concentration of BPA. This could be explained by the enhanced driving force of the concentration gradient because the increase in BPA concentration could accelerate the diffusion of BPA molecules onto AMPS–St/MIPs.3 Two commonly used models, the Langmuir34,45,46 and Freundlich models,47,48 were adopted to describe the adsorption isotherms of BPA on AMPS–St/MIPs (Fig. 5). A more detailed description and the corresponding parameters of the Langmuir and Freundlich models are presented in Table S3 in the ESI.† It can be seen that the Freundlich model is more suitable for describing the adsorption isotherm of BPA on AMPS–St/MIPs. The values of the Freundlich constant n are all greater than 1 at the three temperatures, representing favorable adsorption conditions,45 and the values of the Freundlich constant KF indicate that the adsorption capacity decreases with increasing temperature from 293 K to 303 K, which agrees with experimental data.In recent years, adsorption of BPA from aqueous solution with molecular imprinting technology has attracted much interest. Table 1 compares the adsorption capacity of AMPS–St/MIPs toward BPA against other surface MIPs previously reported in the literature.2,3,15–19 Apparently, AMPS–St/MIPs have the highest adsorption capacity toward BPA in aqueous solution. This can be attributed to the high surface area of PGO, excellent dispersion of AMPS–St/MIPs in aqueous solution and strong interaction between BPA and AMPS–St/MIPs which is not disturbed by water molecules. The results confirm that the bi-functional monomer molecular imprinting strategy can be effectively applied to adsorption of BPA from aqueous solution.
| Support | Functional monomer | Temperature | Solution | Adsorption capacity (mg g−1) | Ref. |
|---|---|---|---|---|---|
| MCM-48 | 4-VP | Room temperature | Toluene | 152.76 | 15 |
| Fe3O4 | 4-VP | Room temperature | Acetonitrile | 16.28 | 18 |
| Fe2O3 | Methacrylic acid | 293 K | Ethanol | 3.94 | 17 |
| SiO2 | Diethylenetriamine pentaacetic acid | 298 K | Aqueous | 6.89 | 19 |
| Silica | Methacrylic acid | Room temperature | Aqueous | 2.1 | 16 |
| CMSs | AMPS | 293 K | Aqueous | 5.38 | 3 |
| Hollow porous PS particle | 4-VP | 293 K | Aqueous with buffer | 5.03 | 2 |
| PGO | AMPS and St | 293 K | Aqueous | 85.7 | This work |
The standard free-energy change (ΔG°), the standard enthalpy change (ΔH°) and the standard entropy change (ΔS°) can be calculated from the temperature-dependent adsorption isotherms to provide in-depth information about internal energy changes concerning adsorption. The thermodynamic parameters at three different temperatures were calculated and are listed in Table S4 in the ESI.† All the negative values of ΔG° at various temperatures imply that the adsorption of BPA onto AMPS–St/MIPs is a spontaneous process, and the values of ΔG° become more negative with decreasing temperature, suggesting that a lower temperature is beneficial for adsorption of BPA on AMPS–St/MIPs. The negative value of ΔH° indicates that the adsorption process is exothermic, which is supported by the decline in the adsorption capacity of BPA with increasing temperature from 293 K to 303 K. Moreover, the negative value of ΔS° reflects a decrease in randomness at the solid–liquid interface during the adsorption process.
3.5 Effect of solution pH and ionic strength on adsorption properties
It is well known that pH is an important parameter that can affect the adsorption properties of MIPs in aqueous phase because it determines the charge of both MIPs and template molecules and therefore governs the MIP–template electrostatic interactions. The effect of solution pH on the adsorption capacity of AMPS–St/MIPs was investigated over pH values ranging from 1.0 to 11.0, and the results are shown in Fig. S3a in the ESI.† The adsorption capacity of AMPS–St/MIPs toward BPA is highly dependent on pH. With an increasing solution pH from 1 to 7, the adsorption capacity of AMPS–St/MIPs toward BPA gradually increases from 35.5 mg g−1 to 85.7 mg g−1. Afterwards, a drop in adsorption capacity is observed when pH > 7.0. This phenomenon can be explained by the net charge of BPA and AMPS–St/MIPs at different pH values. The state of BPA and various functional groups (such as –NH and –S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) in AMPS–St/MIPs is significantly influenced by pH.3 BPA is found to be in its molecular form at pH values below 8 and is negatively charged at high pH (>8).4 AMPS dissociates completely over the entire pH range because of its strongly ionizable sulfonate group,49 while St undergoes no deprotonation or protonation. Thus, the decrease in the adsorption capacity of AMPS–St/MIPs in alkaline solution might be due to the repulsive electrostatic interaction between the negatively charged surface of AMPS–St/MIPs and the bisphenolate anion. Under acidic conditions, the amino groups of AMPS are easier to protonate and remain in an ionic state, resulting in the partial breakage of hydrogen bonds and hence a decline in adsorption capacity.
O) in AMPS–St/MIPs is significantly influenced by pH.3 BPA is found to be in its molecular form at pH values below 8 and is negatively charged at high pH (>8).4 AMPS dissociates completely over the entire pH range because of its strongly ionizable sulfonate group,49 while St undergoes no deprotonation or protonation. Thus, the decrease in the adsorption capacity of AMPS–St/MIPs in alkaline solution might be due to the repulsive electrostatic interaction between the negatively charged surface of AMPS–St/MIPs and the bisphenolate anion. Under acidic conditions, the amino groups of AMPS are easier to protonate and remain in an ionic state, resulting in the partial breakage of hydrogen bonds and hence a decline in adsorption capacity.
Generally, besides organic pollutants, there are also high concentrations of salts in wastewater, which may affect the adsorption ability of pollutants. Therefore, the effect of ionic strength on the adsorption capacity of AMPS–St/MIPs toward BPA was also examined using NaCl as a model salt at pH = 6.0. As shown in Fig. S3b in the ESI,† when the NaCl concentration is below 0.1 mol L−1, the adsorption capacity increases rapidly. This phenomenon can be explained by the negative charge of AMPS–St/MIPs and the molecular form of BPA at pH 6.0. The ions from NaCl, which locate between the surface of AMPS–St/MIPs and BPA, enhance the MIP–template interactions via the screening effect.4 However, when the concentration of NaCl is above 0.1 mol L−1, the adsorption capacity decreases, indicating that NaCl also competes with BPA for the adsorption sites on AMPS–St/MIPs.
3.6 Adsorption specificity
To evaluate the binding specificity of AMPS–St/MIPs toward BPA in aqueous phase, competitive adsorption experiments were carried out using BP and TBBPA as reference compounds. Fig. 6a shows the adsorption capacities of the obtained MIPs, NIPs and PGO for these molecules in mixed solution. It is obvious that all MIPs exhibit excellent adsorption selectivity toward BPA. The adsorption capacities of all MIPs toward BPA are much higher than those toward BP and TBBPA, confirming the formation of specific BPA recognition cavities in MIPs. In contrast, NIPs and PGO have not shown specific adsorption ability toward BPA. The high adsorption capacities of NIPs and PGO toward TBBPA can be explained by the rich functional groups in the TBBPA molecule. The selectivity coefficient (k) is shown in Table 2. The selectivity coefficient of the adsorbent suggests the difference of the two substances (e.g. BPA and BP) adsorbed by one adsorbent, k = qe(BPA)/qe(BP). The k values of all MIPs are much higher than those of AMPS–St/NIPs for both BP and TBBPA. In particular, the MIPs prepared with the AMPS-to-St molar ratio of 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 exhibit the highest k values (7.73 for BP and 13.61 for TBBPA). These results confirm that AMPS–St/MIPs have excellent recognition ability and high selectivity for BPA against its analogues in aqueous phase.
2.5 exhibit the highest k values (7.73 for BP and 13.61 for TBBPA). These results confirm that AMPS–St/MIPs have excellent recognition ability and high selectivity for BPA against its analogues in aqueous phase.
 | ||
| Fig. 6 (a) Selective adsorption of different MIPs, AMPS–St/NIPs and PGO (20 mg of each sample in 40 mL of 50 mg L−1 mixed solution for 1.5 h at 293 K). (b) Regeneration cycles for AMPS–St/MIPs. | ||
| k | AMPS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) St molar ratio St molar ratio |
AMPS–St/NIPs | |||||
|---|---|---|---|---|---|---|---|
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
||
q
e(BPA)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe(BP) qe(BP) |
4.40 | 6.41 | 7.18 | 7.73 | 6.01 | 5.13 | 1.67 |
q
e(BPA)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe(TBBPA) qe(TBBPA) |
3.98 | 7.12 | 11.70 | 13.61 | 10.43 | 6.55 | 0.18 |
3.7 Regeneration
The regeneration of adsorbents is important in practical applications. The regenerated AMPS–St/MIPs were used to adsorb BPA in subsequent cycles. The adsorption capacity of AMPS–St/MIPs in five consecutive adsorption–regeneration cycles is shown in Fig. 6b. It can be seen that AMPS–St/MIPs exhibit excellent stability. In the fifth adsorption–desorption cycle, the adsorption capacity of AMPS/MIPs is 78.43 mg g−1 and the adsorption efficiency loss is only 7.7% compared with the initial adsorption.3.8 Real sample analysis
The feasibility of applying AMPS–St/MIPs to removal of BPA from real water samples was demonstrated by recovering BPA from 50 mg L−1 BPA-spiked river water (Fen River in Taiyuan, China) and tap water (Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan, China). The adsorption capacities of AMPS–St/MIPs toward BPA from river and tap water were 60.6 and 68.9 mg g−1, respectively. Though these values are slightly lower than those in deionized water, they are in good agreement with the data in Fig. S3a in the ESI.† The weak alkalinity of the river (pH = 7.8) and tap water (pH = 7.5) leads to a small drop in the adsorption capacity of AMPS–St/MIPs. In addition, the presence of pollutants which could also bind to MIPs is another reason for the decrease of adsorption capacity in river water. These results demonstrate that the prepared MIPs were promising adsorbents for the selective removal of BPA from environmental water.Conclusions
In conclusion, water-compatible BPA MIPs have been prepared via synergy of two functional monomers using porous GO as a substrate for the selective adsorption of BPA from aqueous solution. FTIR, TG, TEM and dispersion analysis results confirm that MIP layers were successfully grafted on both sides of the GO nanosheets, which enhanced the accessibility of target molecules to the recognition cavities because of the porous structure of MIPs/PGO and nanoscale thickness of the MIPs/GO sheets. The two functional monomers, AMPS and St, form hydrogen bonds and π–π stacking interactions with the template BPA, respectively. Furthermore, the water-soluble AMPS enhances the water-compatibility of the synthesized AMPS–St/MIPs, which reduces the nonspecific adsorption of AMPS–St/MIPs. The adsorption capacity of MIPs is greatly enhanced by virtue of the synergy of AMPS and St and the high surface area of PGO. The MIPs prepared with an AMPS-to-St molar ratio of 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 exhibit the highest adsorption capacity (up to 85.7 mg g−1 at 293 K) and excellent stability toward BPA in aqueous media. The kinetics and isotherm data can be well fitted with the pseudo-second-order kinetic model and the Freundlich isotherm, respectively. Competitive adsorption studies show that the synthesized MIPs display excellent selectivity toward BPA versus other competitive molecules such as tetrabromobisphenol A and 4-tert-butylphenol. AMPS–St/MIPs show excellent adsorption affinity even in complex river water and good recoverability with a slight loss of the initial adsorption capacity of 7.7%. This remarkable improvement in the adsorption performance of MIPs in aqueous phase demonstrates a facile and promising approach to design water-compatible MIPs with high adsorption capacity and selectivity in aqueous media using bi-functional monomers.
2.5 exhibit the highest adsorption capacity (up to 85.7 mg g−1 at 293 K) and excellent stability toward BPA in aqueous media. The kinetics and isotherm data can be well fitted with the pseudo-second-order kinetic model and the Freundlich isotherm, respectively. Competitive adsorption studies show that the synthesized MIPs display excellent selectivity toward BPA versus other competitive molecules such as tetrabromobisphenol A and 4-tert-butylphenol. AMPS–St/MIPs show excellent adsorption affinity even in complex river water and good recoverability with a slight loss of the initial adsorption capacity of 7.7%. This remarkable improvement in the adsorption performance of MIPs in aqueous phase demonstrates a facile and promising approach to design water-compatible MIPs with high adsorption capacity and selectivity in aqueous media using bi-functional monomers.
Acknowledgements
The authors acknowledge financial support from the Program for Changjiang Scholar and Innovative Research Team in University (IRT0972), the National Natural Science Foundation of China (20971094, 21176169, 51152001), the Ph.D. Programs Foundation of Ministry of Education of China (20101402110007), the International S&T Cooperation Program of Shanxi Province (2010081017), the Research Project supported by the Shanxi Scholarship Council of China (2012–038), the Hundred Talent Program of the Chinese Academy of Sciences, and the Hundred Talent Program of Shanxi Province.Notes and references
- C. A. Staples, P. B. Dome, G. M. Klecka, S. T. Oblock and L. R. Harris, Chemosphere, 1998, 36(10), 2149–2173 CrossRef CAS.
- R. Dong, J. Li, H. Xiong, W. Lu, H. Peng and L. Chen, Talanta, 2014, 130, 182–191 CrossRef CAS.
- F. Duan, C. Chen, L. Chen, Y. Sun, Y. Wang, Y. Yang, X. Liu and Y. Qin, Ind. Eng. Chem. Res., 2014, 53(37), 14291–14300 CrossRef CAS.
- J. Xu, L. Wang and Y. Zhu, Langmuir, 2012, 28(22), 8418–8425 CrossRef CAS.
- G. O. Noonan, L. K. Ackerman and T. H. Begley, J. Agric. Food Chem., 2011, 59(13), 7178–7185 CrossRef CAS.
- Y. Zhang, C. Causserand, P. Aimar and J.-P. Cravedi, Water Res., 2006, 40(20), 3793–3799 CrossRef CAS.
- D. Rana, R. M. Narbaitz, A.-M. Garand-Sheridan, A. Westgate, T. Matsuura, S. Tabe and S. Y. Jasim, J. Mater. Chem. A, 2014, 2(26), 10059–10072 CAS.
- T. Kamegawa, Y. Ishiguro, H. Seto and H. Yamashita, J. Mater. Chem. A, 2015, 3(5), 2323–2330 CAS.
- C. Guo, M. Ge, L. Liu, G. Gao, Y. Feng and Y. Wang, Environ. Sci. Technol., 2009, 44(1), 419–425 CrossRef.
- Y.-H. Kim, B. Lee, K.-H. Choo and S.-J. Choi, Microporous Mesoporous Mater., 2011, 138(1–3), 184–190 CrossRef CAS.
- M. Clara, B. Strenn, E. Saracevic and N. Kreuzinger, Chemosphere, 2004, 56(9), 843–851 CrossRef CAS.
- G. Liu, J. Ma, X. Li and Q. Qin, J. Hazard. Mater., 2009, 164(2), 1275–1280 CrossRef CAS.
- L. Chen, S. Xu and J. Li, Chem. Soc. Rev., 2011, 40(5), 2922–2942 RSC.
- X. Shen, L. Zhu, N. Wang, L. Ye and H. Tang, Chem. Commun., 2012, 48(6), 788–798 RSC.
- X.-B. Zhang, J. Li, B. You, G.-P. Yong, H.-W. Tong and S.-M. Liu, RSC Adv., 2012, 2(26), 9778–9780 RSC.
- N. Griffete, H. Frederich, A. S. Maître, S. Ravaine, M. M. Chehimi and C. Mangeney, Langmuir, 2011, 28(1), 1005–1012 CrossRef.
- N. Griffete, H. Li, A. Lamouri, C. Redeuilh, K. Chen, C.-Z. Dong, S. Nowak, S. Ammar and C. Mangeney, J. Mater. Chem., 2012, 22(5), 1807–1811 RSC.
- J. Liu, W. Wang, Y. Xie, Y. Huang, Y. Liu, X. Liu, R. Zhao, G. Liu and Y. Chen, J. Mater. Chem., 2011, 21(25), 9232–9238 RSC.
- Y. Ren, W. Ma, J. Ma, Q. Wen, J. Wang and F. Zhao, J. Colloid Interface Sci., 2012, 367(1), 355–361 CrossRef CAS.
- G. Pan, Y. Zhang, Y. Ma, C. Li and H. Zhang, Angew. Chem., Int. Ed., 2011, 50(49), 11731–11734 CrossRef CAS.
- H. Zhang, Polymer, 2014, 55(3), 699–714 CrossRef CAS.
- G. Pan, Y. Ma, Y. Zhang, X. Guo, C. Li and H. Zhang, Soft Matter, 2011, 7(18), 8428–8439 RSC.
- X. Shen, C. Xu and L. Ye, Ind. Eng. Chem. Res., 2013, 52(39), 13890–13899 CrossRef CAS.
- P. Manesiotis, C. Borrelli, C. S. Aureliano, C. Svensson and B. Sellergren, J. Mater. Chem., 2009, 19(34), 6185–6193 RSC.
- Y. Liu, K. Hoshina and J. Haginaka, Talanta, 2010, 80(5), 1713–1718 CrossRef CAS.
- P. Martin, I. D. Wilson and G. R. Jones, J. Chromatogr. A, 2000, 889(1–2), 143–147 CrossRef CAS.
- G. Z. Kyzas, D. N. Lazaridis and N. K. Lazaridis, Chem. Eng. J., 2009, 149(1–3), 263–272 CrossRef CAS.
- X. Luo, Y. Zhan, Y. Huang, L. Yang, X. Tu and S. Luo, J. Hazard. Mater., 2011, 187(1–3), 274–282 CrossRef CAS.
- Y.-S. Chang, T.-H. Ko, T.-J. Hsu and M.-J. Syu, Anal. Chem., 2009, 81(6), 2098–2105 CrossRef CAS.
- X. Cai, J. Li, Z. Zhang, F. Yang, R. Dong and L. Chen, ACS Appl. Mater. Interfaces, 2013, 6(1), 305–313 Search PubMed.
- H. Zeng, Y. Wang, X. Liu, J. Kong and C. Nie, Talanta, 2012, 93, 172–181 CrossRef CAS.
- F. Duan, C. Chen, G. Wang, Y. Yang, X. Liu and Y. Qin, RSC Adv., 2014, 4(3), 1469–1475 RSC.
- H. Dai, D. Xiao, H. He, H. Li, D. Yuan and C. Zhang, Microchim. Acta, 2014, 1–16 Search PubMed.
- J. Luo, S. Jiang and X. Liu, J. Phys. Chem. C, 2013, 117(36), 18448–18456 CAS.
- X. Huang, K. Qian, J. Yang, J. Zhang, L. Li, C. Yu and D. Zhao, Adv. Mater., 2012, 24(32), 4419–4423 CrossRef CAS.
- F. Niu, J.-M. Liu, L.-M. Tao, W. Wang and W.-G. Song, J. Mater. Chem. A, 2013, 1(20), 6130–6133 CAS.
- Y. He, Y. Huang, Y. Jin, X. Liu, G. Liu and R. Zhao, ACS Appl. Mater. Interfaces, 2014, 6(12), 9634–9642 CAS.
- S. Zhong, X. Cui, H. Cai, T. Fu, K. Shao and H. Na, J. Power Sources, 2007, 168(1), 154–161 CrossRef CAS.
- A. Faghihnejad and H. Zeng, Soft Matter, 2012, 8(9), 2746–2759 RSC.
- M. Li, H. Wang, S. Wu, F. Li and P. Zhi, RSC Adv., 2012, 2(3), 900–907 RSC.
- S. Deng, R. Wang, H. Xu, X. Jiang and J. Yin, J. Mater. Chem., 2012, 22(19), 10055–10061 RSC.
- X. Zhou, J. Wei, K. Liu, N. Liu and B. Zhou, Langmuir, 2014, 30(46), 13861–13868 CrossRef CAS.
- J.-Q. Jiang, C.-X. Yang and X.-P. Yan, ACS Appl. Mater. Interfaces, 2013, 5(19), 9837–9842 CAS.
- D. Kong, F. Zhang, K. Wang, Z. Ren and W. Zhang, Ind. Eng. Chem. Res., 2014, 53(11), 4434–4441 CrossRef CAS.
- H. Deng, L. Gao, S. Zhang and J. Yuan, Ind. Eng. Chem. Res., 2012, 51(43), 14018–14025 CrossRef CAS.
- J. Zhou, C. Tang, B. Cheng, J. Yu and M. Jaroniec, ACS Appl. Mater. Interfaces, 2012, 4(4), 2174–2179 CAS.
- X. Wang, J. Pan, W. Guan, J. Dai, X. Zou, Y. Yan, C. Li and W. Hu, J. Chem. Eng. Data, 2011, 56(6), 2793–2801 CrossRef CAS.
- X. He, K. B. Male, P. N. Nesterenko, D. Brabazon, B. Paull and J. H. Luong, ACS Appl. Mater. Interfaces, 2013, 5(17), 8796–8804 CAS.
- T. Anirudhan and S. Sandeep, Polym. Chem., 2011, 2(9), 2052–2061 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details of PGO preparation, adsorption kinetics of BPA on different MIPs with different molar ratios of AMPS to St, TG curves of PGO and functionalized PGO, photograph of the dispersion of PGO and MIPs and the parameters of model simulation. See DOI: 10.1039/c5en00198f |
| This journal is © The Royal Society of Chemistry 2016 |