Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Riparian shading controls instream spring phytoplankton and benthic algal growth
S. J.
Halliday
 a,
R. A.
Skeffington
*a,
A. J.
Wade
a,
M. J.
Bowes
b,
D. S.
Read
b,
H. P.
Jarvie
b and
M.
Loewenthal
c
a,
R. A.
Skeffington
*a,
A. J.
Wade
a,
M. J.
Bowes
b,
D. S.
Read
b,
H. P.
Jarvie
b and
M.
Loewenthal
c
aDepartment of Geography and Environmental Sciences, University of Reading, Reading, RG6 6AB, UK. E-mail: r.a.skeffington@reading.ac.uk
bCentre for Ecology and Hydrology, Wallingford, Oxon, OX10 8BB, UK
cEnvironment Agency, National Water Quality Instrumentation Service, Henley Road, Reading, Berkshire RG4 9RA, UK
First published on 10th May 2016
Abstract
Dissolved oxygen (DO) concentrations showed a striking pattern in a multi-year study of the River Enborne, a small river in SE England. In each of three years (2010–2012), maximum DO concentrations were attained in mid-April, preceded by a period of steadily increasing diurnal amplitudes, followed by a steady reduction in both amplitude and concentration. Flow events during the reduction period reduce DO to low concentrations until the following spring. Evidence is presented that this pattern is mainly due to benthic algal growth which is eventually suppressed by the growth of the riparian tree canopy. Nitrate and silicate concentrations are too high to inhibit the growth of either benthic algae or phytoplankton, but phosphate concentrations might have started to reduce growth if the tree canopy development had been delayed. This interpretation is supported by evidence from weekly flow cytometry measurements and analysis of the diurnal, seasonal and annual patterns of nutrient concentrations. As the tree canopy develops, the river switches from an autotrophic to a heterotrophic state. The results support the use of riparian shading to help control algal growth, and highlight the risks of reducing riparian shade.
Environmental impactThis paper provides insight into the processes controlling algal growth in streams. Excess growth of algae in rivers is a world-wide problem, and clearly manifests itself in some of the rivers in SE England, which have high nutrient inputs due to dense human populations and intensive agriculture. This study of the River Enborne uses high-frequency chemical monitoring data and innovative flow cytometry methods to evaluate the processes controlling algal growth and to demonstrate the importance of riparian shading in this system. Riparian shading should be considered as an effective, and cost-effective, management tool. |
1. Introduction
It is generally accepted that nuisance algae and a shift in plant community composition can be a consequence of nutrient enrichment (eutrophication) by nitrogen (N) and phosphorus (P) compounds, which is in turn due to increasing human effluent inputs and runoff from intensive agriculture (e.g.ref. 1 and 2). Attempts to manage these problems have therefore concentrated on reducing nutrient inputs, especially P, since P is assumed to be the primary limiting nutrient (e.g.ref. 1, 3 and 4). Large expenditures have been incurred in reducing nutrient inputs from both point and diffuse sources, but results, especially for running waters, have been mixed at best (e.g.ref. 4 and 5). A number of papers have suggested recently that promoting riparian shading would be a more effective, and certainly more cost-effective, management tool for the control of nuisance algae in rivers.6–8 Riparian shading is expected to work by reducing photosynthetic rates and water temperatures, and though modelling studies tend to show this would be highly effective in reducing algal growth especially under scenarios of increased water temperature resulting from climate change,6,8 observational evidence of its effectiveness is more limited. This paper explores the controls on algal growth in a small river in SE England, the River Enborne, where riparian shading, by deciduous trees, is heavy but seasonal. Using high-frequency hydrochemical data coupled with weekly grab sampling of a wider range of chemicals and the river's phytoplankton community, we can test the hypothesis that riparian shading controls algal growth for at least part of the year.Burrell et al.9 discuss in depth the effects of riparian shading on stream ecosystems in agricultural landscapes, which include enhancing litter inputs and reducing excess nutrients and sediment as well as reducing water temperatures and photosynthetic rates. In their study of 21 streams in New Zealand,9 shading reduced both gross primary productivity (GPP) and ecosystem respiration, but had a stronger effect on GPP. In their case, however, macrophytes rather than algae were the main driver of stream GPP. Shading also affects periphyton growth and productivity – for instance Bowes et al.7 showed in an experimental study on the River Thames that shading could reduce the periphyton accrual rate by 50%. It is not however self-evident that shading will always reduce primary productivity even in temperate zone streams. Nutrients may be limiting factors, or interact with light intensity; photosynthetic organisms may adapt to lower light intensities; these effects may vary seasonally. Hill et al.10 showed in an experimental study that stream periphyton from shaded sites were twice as efficient at photosynthesis in low light intensities than those from open sites (though not enough to compensate for the lower irradiance in this case). Interactive effects of light and nutrients on algae depend on nutrient concentrations and how close they are to limiting values (e.g.ref. 7, 11 and 12). For phytoplankton, Reynolds13 suggested that growth-limiting concentrations are normally much lower than those found in streams in agricultural areas and densely-populated countries like the UK: ca. 4 μg P l−1 and ca. 15–30 μg N l−1. For periphyton where the nutrients have to diffuse through biofilms, the suggested limiting concentrations are higher: from 25 μg P l−1 to 80 μg P l−1,7,11 hence nutrient limitation or co-limitation may be a possibility. Limiting factors may vary seasonally: for instance Rosemond et al.14 showed for a stream in Tennessee, USA, that light, nutrients and grazing snails co-limited periphyton biomass through most of the year, but their relative importance varied seasonally. For instance, nutrients were more limiting in summer when light intensities were higher. Algal growth is thus determined by a complex set of interacting factors which vary in space and time.
Increasing riparian vegetation is unlikely to be a universal panacea for improving water quality. Dense riparian vegetation has been shown to reduce salmonid populations, for instance in Ireland,15,16 acting through a reduction in primary production. Along some reaches of the River Kennet, which is adjacent to the River Enborne, riparian tree canopies have been removed to allow light to reach the river banks and bed, with the assertion that the river was over-shaded and would benefit from more macrophyte growth.17 Such conflicting views on stream management highlight the need for more data to evaluate the effects of riparian shading on stream ecosystems. This paper uses existing monitoring data to test the hypothesis that riparian shading controls phytoplankton growth and river metabolism on the River Enborne.
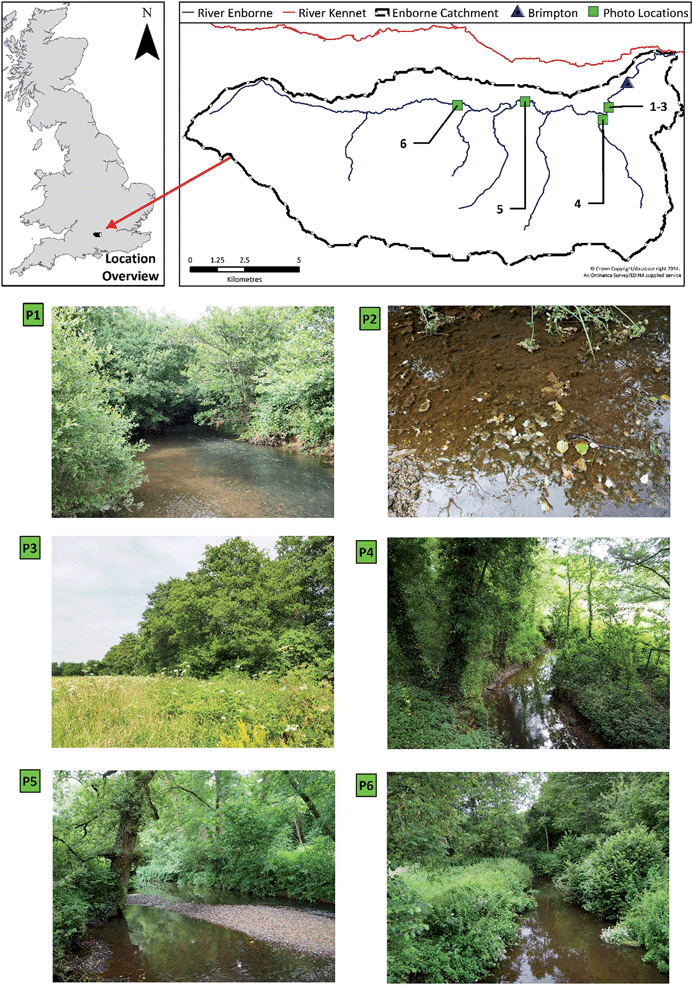
2. Study area
The River Enborne drains a 148 km2 rural catchment situated in southeast England (Fig. 1). The catchment has been described extensively in previous publications,18,19 consequently only a brief overview of the system is provided here. The river is located in the Thames basin and is a tributary of the River Kennet (Fig. 1). The catchment monitoring point for this study was located at the flow gauging station at Brimpton, 2 km upstream of the confluence with the Kennet (SU567647). Although the catchment geology is dominated by Cretaceous chalk in the headwaters, tertiary clays dominate in the lower reaches, and thus the river's baseflow index, 0.53, is lower than for other rivers in this area. The dominant catchment land use is agricultural, with 39% of the catchment designated as “Arable and Horticulture” land.20Despite the rural nature of the catchment, the population is ca. 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 260 people, and there are six sewage treatment works discharging to the river network: Washwater (Population Equivalent (PE) – 7000); Kingsclere (PE 2500); Greenham Common (PE 1700); Ashford Hill (PE 100); Wolverton Townsend (PE 50); and Bishop's Green (PE 10). In addition, there is a high density of registered septic tank systems (STS) throughout the catchment (163), with the estimated number of unregistered systems approximately 2600.18 It has been previously shown that despite the agricultural nature of the catchment, these effluent discharges exert significant control on the hydrochemical dynamics of the river.18
260 people, and there are six sewage treatment works discharging to the river network: Washwater (Population Equivalent (PE) – 7000); Kingsclere (PE 2500); Greenham Common (PE 1700); Ashford Hill (PE 100); Wolverton Townsend (PE 50); and Bishop's Green (PE 10). In addition, there is a high density of registered septic tank systems (STS) throughout the catchment (163), with the estimated number of unregistered systems approximately 2600.18 It has been previously shown that despite the agricultural nature of the catchment, these effluent discharges exert significant control on the hydrochemical dynamics of the river.18
3. Methods
3.1 Data
3.2 Data analysis
| yt = Tt + et, et ∼ N(0,σ2) | (1) |
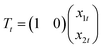 | (2) |
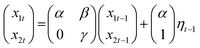 | (3) |
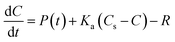 | (4) |
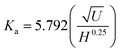 | (5) |
4. Results and discussion
4.1 Annual and diurnal dissolved oxygen patterns
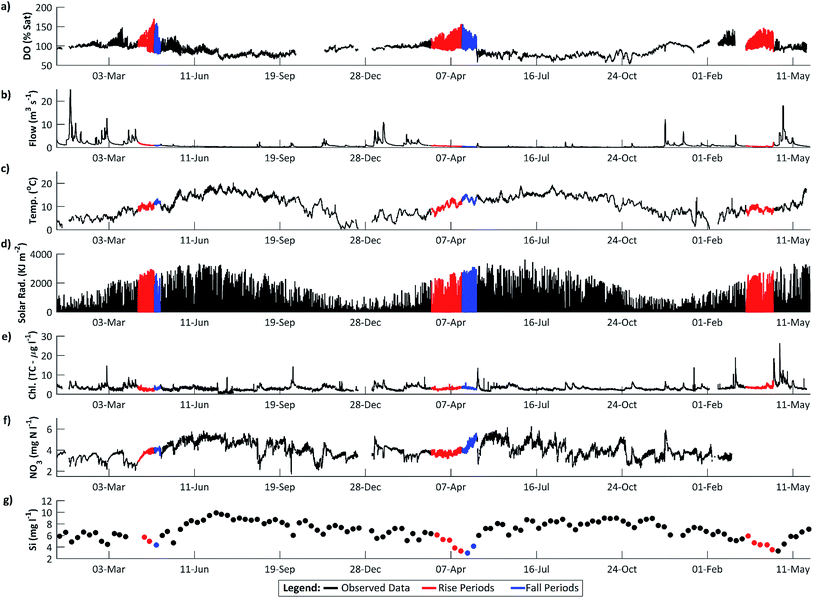
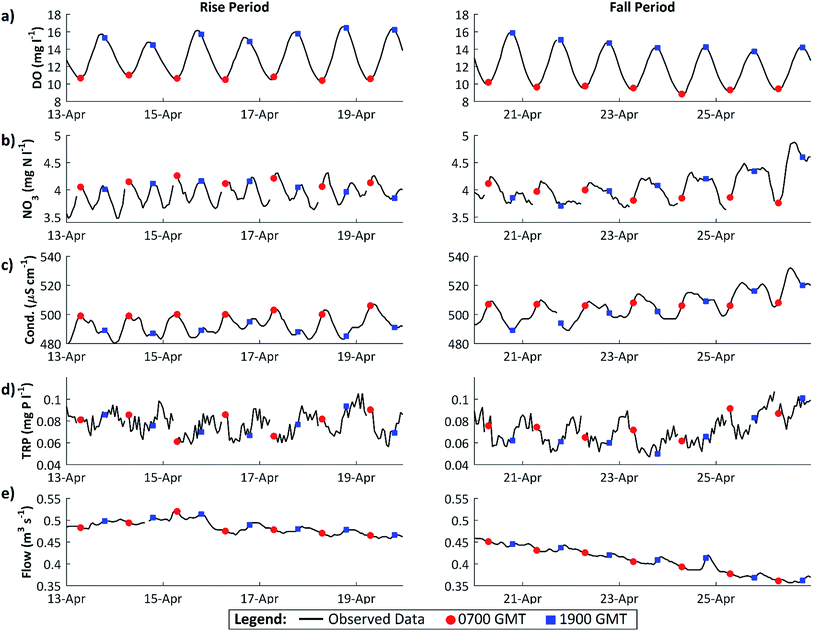
Dissolved oxygen (DO) concentrations during the study period followed a striking repetitive pattern (Fig. 2a). In each of the three years, maximum DO concentrations were attained in mid-April, 16.6 mg l−1 (157%) on 18 April 2011, preceded by a period of steadily increasing diurnal amplitudes, with the diurnal range increasing from 1.6 to 6.2 mg l−1 (highlighted in red on Fig. 2a). This was followed by a period of declining DO concentration and diurnal amplitude, with the diurnal range decreasing from 6.0 to 3.0 mg l−1 (Fig. 2a, highlighted in blue). These periods we term the ‘DO rise’ and ‘DO fall’ periods respectively. The DO fall period was terminated abruptly by a flow event in 2011 and 2012, whereas in 2010 a steady decline continued, but in all cases overall DO concentrations and amplitudes declined and remained lower than in mid-April for the remainder of the year. Dates for the two periods are given in Table 1.The diurnal variation in DO is due to the changing balance between photosynthesis and respiration during the 24 h period, and is commonly observed in the Enborne and other local rivers (e.g.ref. 39 and 40). During the DO rise period, the river is net autotrophic, with average daily photosynthesis exceeding respiration (Table 2). Respiration is low probably because river temperatures are still low (Fig. 3c). Maximum DO concentrations are reached after 12 noon at the point where photosynthesis has declined so it equals respiration ± gas exchange (eqn (4)): in the DO rise and fall periods this does not occur until about an hour before sunset, 6–7 hours after solar noon (Fig. 4). Increases in the diurnal amplitude of DO and the maximum oxygen saturation percentage imply increased photosynthetic rates relative to the volume of water flowing. This increase could be due to a number of factors, including increasing temperatures; reducing flow volume; increasing solar radiation and increasing biomass of photosynthetic organisms. The values of these factors are also shown in Fig. 2, and in more detail for the relevant period of 2011 in Fig. 3.
| Determinanda | 2010 | 2011 | ||
|---|---|---|---|---|
| Rise period | Summer | Rise period | Summer | |
| a DO – dissolved oxygen; NO3 – nitrate; TRP – total reactive phosphorus; Si – silicon; Temp – temperature; P/R – estimated of photosynthesis/respiration ratio. | ||||
| DO (mg l−1) | 12.6 | 8.01 | 12.3 | 7.42 |
| DO (% Sat.) | 112 | 80.9 | 109 | 73.5 |
| Flow (m3 s−1) | 1.25 | 0.33 | 0.62 | 0.23 |
| NO3 (mg N l−1) | 3.66 | 4.79 | 3.74 | 4.34 |
| TRP (μg P l−1) | 80.7 | 284 | 92.0 | 217 |
| Si (mg Si l−1) | 5.34 | 8.85 | 4.74 | 7.77 |
| Water Temp. (°C) | 9.88 | 15.9 | 10.1 | 15.0 |
| Solar Rad. (kJ m−2 d−1) | 789 | 740 | 526 | 691 |
| P/R | 4.20 | 0.27 | 3.22 | 0.17 |
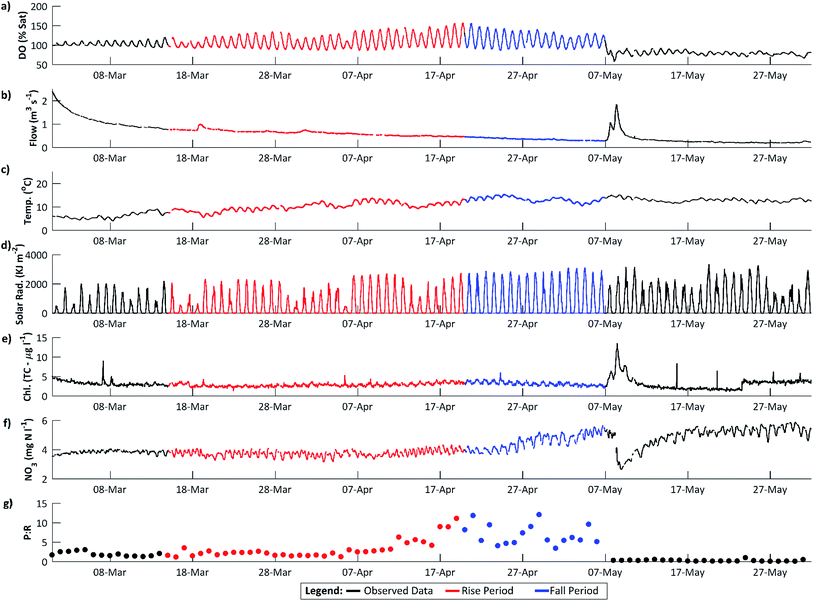 | ||
| Fig. 3 The DO rise and fall periods in 2011 at higher resolution. (a–f) as for Fig. 2 and (g), calculated daily mean photosynthesis to respiration ratio (eqn (4)) in mg O2 per l per day. | ||
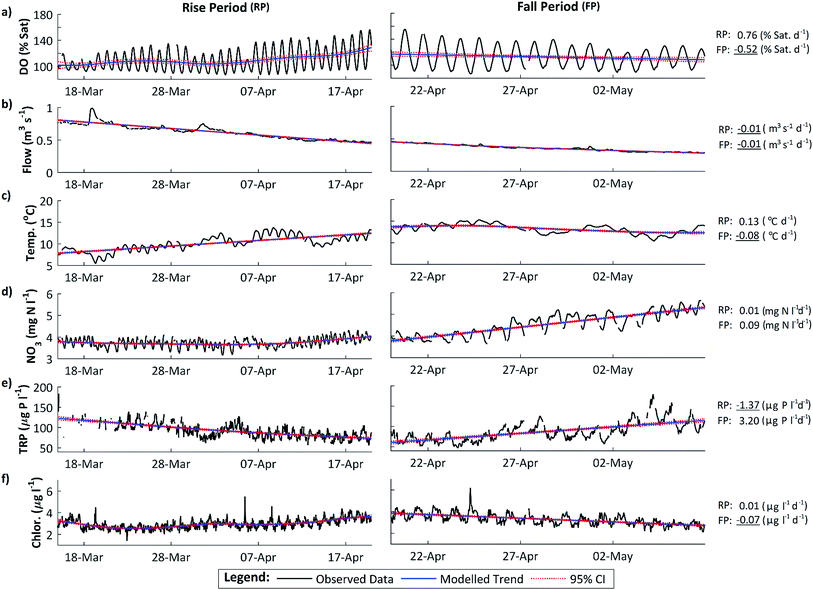
The maximum DO and thus rate of photosynthesis appears to be related to solar radiation as days with lower radiation, such as 5 April 2011, exhibit lower maximum DO. There is a significant positive trend in solar radiation during the DO rise period (Fig. 5), but in 2011 this trend continued (and solar radiation is thus higher) during the DO fall period. Similarly, flow declined slowly during the DO rise period (which would increase DO amplitude and maximum concentrations due to reduced dilution) but this flow decline continued during the DO fall period (see Section 4.3). Stream temperature increased during the DO rise period, and decreased slightly during the fall period, which is consistent with the DO pattern, except that mean temperatures are still considerably higher during the DO fall period than in the DO rise period (Fig. 2c, 3c and 5). Some change in conditions is thus needed to account for the switch between the DO rise and DO fall periods. We hypothesize that this switch is the development of the riparian tree canopy, and that the DO dynamics demonstrate that in the River Enborne riparian shading controls algal growth through most of the year. We examine the evidence for this hypothesis in more detail below.
4.2 Benthic algae as a source of DO
Instream total chlorophyll concentrations are a measure of the photosynthetic capacity of phytoplankton, and these increase slightly during the DO rise period and decrease slightly in the fall period (Fig. 5). These changes cannot however explain the changes in DO concentration for several reasons. The percent change in chlorophyll (ca. 10% in the rise period) is small compared to the change in DO amplitude (ca. 165%). Similarly the change in chlorophyll concentration in the DO fall period (Fig. 3) is −25% compared to a change in DO amplitude of −47%. Chlorophyll concentrations in the water column are low, with a maximum total chlorophyll and chlorophyll-a concentration of only 5.5 and 4.7 μg l−1 respectively in the 2011 period. Although the high-frequency data show small, on average 1.4 μg l−1, diurnal fluctuations in total chlorophyll concentration, these fluctuations are highly variable and are insufficient to account for the large changes in DO dynamics. Furthermore, if all photosynthetic activity was due to phytoplankton, the specific activity relative to chlorophyll concentration would be about 180 mg O2 per (mg chlorophyll) per h in the DO rise period. This can be compared with a maximal rate of 20 mg O2 per (mg chlorophyll) per h found in summer in a eutrophic temperate lake (Loch Leven41). It seems unlikely therefore that the DO pattern is primarily driven by phytoplankton.Alternative sources of photosynthetic oxygen other than phytoplankton are macrophytes or benthic algae. Williams et al.,42 in a study of the adjacent River Kennet in late summer, also found much more DO than could be attributed to phytoplankton, and suggested that photosynthesis by macrophytes was the major source. Palmer-Felgate et al.43 added periphyton to the possible sources in this river. Macrophytes are however uncommon in the River Enborne (e.g.Fig. 1) and the main macrophyte growth period would in any case be expected later in the year in late May–June. In nine years of summer surveys (2006–15) upstream of Brimpton Gauging station, the Environment Agency (pers. comm.) recorded an average macrophyte cover of only 4.6% (mostly Cladonia spp. and Sparganium erectum). It thus probable that the DO dynamics observed in early spring are primarily due to the growth of benthic algae.
Benthic algae were not measured directly in this study. The Environment Agency have undertaken biannual monitoring of benthic algae at selected sites along the river. This data showed a diatom flora which is characteristic of some nutrient enrichment, such as Amphora pediculus and Achnanthidium minutissimum, with very few planktonic species entrained in the biofilm (3.5% on average). There was also evidence that filamentous algae were present in the algal assemblages, with diatom species such as Rhoicosphenia abbreviata identified, a common epiphyte of filamentous algae, in particular Cladophora glomerata.44
The FCM data reveal a marked peak in large diatoms on 18 April (Fig. 6a), coinciding with the observed maximum in DO dynamics. A spring diatom peak is a characteristic of western European rivers (e.g.ref. 5 and 45) and the importance of benthic diatoms is further supported by the fact that the annual minimum in dissolved silicon concentration, which is required by diatoms to make their frustules, occurs at the same time as the transition from the DO rise to the DO fall patterns (Fig. 2). The spring peak in FCM diatom cell abundance may also reflect the self-detachment of mature epilithic biofilms under the sustained low-flow conditions.24,25 There is a subsidiary diatom peak on 9 May in the aftermath of a small flow event, suggesting that some of the diatoms observed are benthic diatoms abraded from the substrate. Though other organisms may make a contribution, it seems likely that the photosynthetic organisms driving the spring dissolved oxygen cycling are benthic algae.
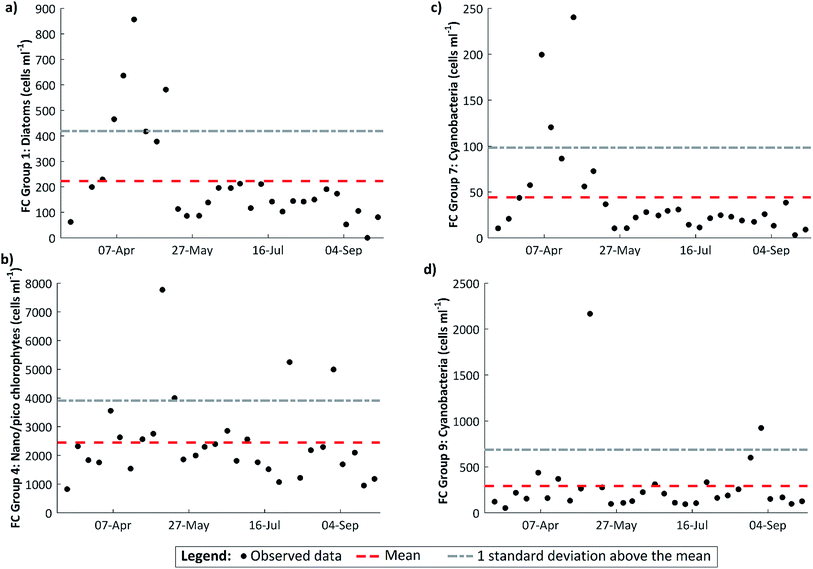 | ||
| Fig. 6 Weekly cell abundance for various algal groups derived from flow cytometry measurements (FC). The mean value and standard deviation for the whole period are also shown. (a) FC Group 1 – large diatoms 12–20 μm, with high levels of chlorophyll (CHL) and phycocyanin (PC) but low phycoerythrin (PE) levels; (b) FC Group 4 – 2–12 μm with low CHL levels; (c) FC Group 7 – 5–20 μm, with very high levels of PC; (d) FC Group 9 – 5–12 μm with high levels of PC, but very low levels of both PE and CHL.23 | ||
4.3 Control of algal dynamics by flow
During the DO rise period in 2011, a significant negative correlation was observed between flow and DO (Spearman's rank correlation ρ = −0.29, p < 0.001). As flows decline, DO concentrations in the river would be expected to increase as photosynthetic O2 dissolves into a lower volume of water. However, the rapid DO concentration increases observed in spring, with diurnal DO ranges increasing from 1.63 to 6.21 mg l−1 between the 16 March and 18 April 2011, are too large to be explained by a roughly 38% decrease in river volume over the same period. A more probable explanation is that the stable low flow conditions in the river are facilitating algal growth, and the increased DO concentrations are caused by increasing daytime photosynthesis rates (Fig. 3).The links between DO dynamics and flow is supported in the 2010 data (Fig. 7). The amplitude of the diurnal DO fluctuations and the DO trend start to increase in early spring, around the 3 March, as flows decline following a high flow period which started in mid-February. This is accompanied by an increase in daily photosynthesis rates (ρ = −0.91, p < 0.001). However, this pattern ceases on the 19–20 March following a high flow event (Fig. 7). Photosynthetic rates then start to increase again, but a much larger flow event on 25 March reduces them for the remainder of the spring season. This can be interpreted as high flow scouring the streambed and removing a proportion of the benthic diatom algal growth which had begun to develop. While flows remain high the re-establishment of algal growth is inhibited. Consequently the DO diurnal fluctuations are reduced to approximately 1.38 mg l−1. The system continued like this until flow reaches the pre-event level. Only once the stable low flow conditions have been re-established, around the 6 April, do the dissolved oxygen concentrations and the amplitude of the diurnal dynamics start to increase again, indicating that benthic algal growth has re-commenced.
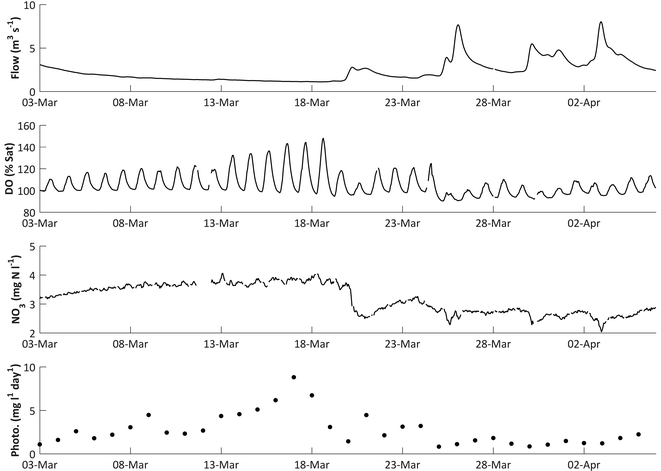 | ||
| Fig. 7 The influence of flow on dissolved oxygen, nitrate and calculated daily mean photosynthesis in March 2010. | ||
Although DO concentrations peaked in mid-April in 2011, flows continued to decline until the 6 May, with significant declining trends observed in both periods (Fig. 2). During the DO fall period, there was no significant correlation between DO and flow. The period ended when a high flow event occurred between the 7 and 9 May, peaking at 1.84 m3 s−1 on the 8 May at 0900 GMT. Flows do not return to pre-event levels until the 14 May. Although the peak on 7–9 May appears small, in terms of preceding catchment conditions this is a significant event. In the 2 months before the flow event, flows were constantly below 1 m3 s−1, with flows <0.5 m3 s−1 from 12 April. Consequently, the event causes a significant reduction in both the DO concentration and the amplitude of the diurnal DO cycling.
This event washed out the instream processing signal and appeared to flush the system, with peaks in a number of the algal groups at this time (Fig. 6). An explanation is the wash out of benthic algae from the stream substrates, in particular filamentous algae which are released into the water column with a modest increases in flow. This event may have also washed out mature biofilms which had begun to self-detach under the preceding low flow conditions. The data thus suggest that stable low flow conditions are required for the establishment and development of benthic algal growth. However, low flow conditions alone are insufficient to maintain algal growth in this system. During the DO fall period, other factors are at work.
4.4 Solar radiation and riparian shading
During the DO rise period in 2011, a strong positive correlation was observed between the daily solar radiation (SR) and water temperature (WT) ranges and the daily DO range (Spearman's Rank, SR, ρ = 0.75; WT, ρ = 0.54, p < 0.001). This indicates that as the diurnal range in solar radiation and water temperature increased, so too did the instream DO range. However, during the DO fall period these relationships reverse or weaken, with the diurnal DO range exhibiting a strong negative correlation with solar radiation (ρ = −0.73, p < 0.001), and no significant relationship with water temperature. Although the daily range in solar radiation continues to increase, with annual maximums in solar radiation not observed until early/mid-summer (Table 3), the diurnal DO amplitude is now declining. This suggests that at this time the instream DO dynamics have become decoupled from the solar radiation signal. In addition, during the DO fall period, the solar radiation and streamwater temperature dynamics have also become decoupled from each other with no significant correlation identified, despite a strong positive correlation in the DO rise period (ρ = 0.84, p < 0.001). We suggest that the decoupling of the DO and radiation dynamics is caused by the development of riparian shading.| Factor | 2010 | 2011 | 2012b |
|---|---|---|---|
| a Si data is collected on a weekly basis, so the exact date of the annual concentration minimum is unknown, therefore a window covering the two lowest concentration measurements is provided. b Indicative dates based on incomplete annual dataset. | |||
| Annual DO max. | 24 April | 18 April | 16 April |
| Budburst | 11 April | 01 April | 01 April |
| First leaf | 19 April | 14 April | 14 April |
| Si min.a | 19 April | 18 April | 16 April |
| 27 April | 26 April | 23 April | |
| Solar Rad. max. | 16 June | 02 July | 20 June |
| Photo. max. | 16 April | 21 April | 15 April |
| G1 diatom max. | — | 18 April | — |
Based on the 2007 Land Cover Map,20 26% of the Enborne riparian corridor, defined as a 50 m buffer zone on either side of the river, is classified as broadleaf woodland. However, it is clear from site visits and catchment aerial imagery that the resolution of the land cover mapping does not account for the significant riparian tree growth present directly along the river banks. As part of the EA's “Keeping Rivers Cool Project” the extent of shading along the River Kennet was estimated. The project classified shading into 20 classes, with 1 indicating the least shaded and 20 the most. For the River Enborne, 64% of river was classified as ≥16 (41% ≥ 18), with <1% between 1 and 5, indicating that the river is heavily dominated by riparian shading. Riparian tree cover is dominated by the European alder Alnus glutinosa, which casts a dense shade (Fig. 1). The timing of canopy development of this tree species is thus crucial to light penetration to the river. The UK Phenology Network27 recorded the timings of budburst and of the emergence of the first leaf for 22 alder trees within 70 km of Brimpton. As shown in Table 3, the mean date of budburst was around 1 April in 2011 and 2012, with the first leaves developed around April 14. In 2010 these dates were a little later. The standard deviations on these dates were about 9 days for budburst and 11 days for first leaf. Light penetration to the stream will be greatest in early spring as external solar radiation is increasing, but before riparian shading has fully developed. The timing of the switch from the DO rise to the DO fall periods is consistent with the development of the alder canopy, with the annual maximums in DO following first leaf dates by approximately 5 days.
The importance of riparian shading in controlling algal growth dynamics in the River Enborne is shown by the fact that the maximal DO dynamics observed in spring are not observed at any other time of year (Fig. 2). This is in spite of the other possible controlling factors being more conducive to algal growth (Table 2):
• Higher nutrient concentrations;
• Higher water temperatures;
• Higher solar radiation; and
• A prolonged period of low flows.
After the tree canopy has developed and a flow event has removed the benthic algae, photosynthesis reduces to a very low level (Fig. 3), respiration increases (Table 2) and the river switches to a net heterotrophic state. The data are thus consistent with the hypothesis that algal growth and productivity in the River Enborne is controlled by light penetration through the riparian tree canopy when this is present. In the next section, we consider whether nutrients also have a role in algal dynamics.
4.5 Nutrient dynamics
Previous work on P and N dynamics on the Enborne19,39 has demonstrated that sewage effluent discharges exert a significant influence on the instream nutrient dynamics. Annual nutrient maximums are observed in the summer during the low flow periods, linked to the river's reduced capacity to dilute point source discharges. Groundwater becomes a particularly important contributor to NO3 concentrations during the summer months. The dominance of effluent discharges is also evidenced through the appearance of two-peak diurnal nutrient cycles, linked to the diurnal pattern in sewage effluent discharges. The hypothesis that these contributions avert nutrient limitation of algal growth rates is examined in more detail in this section.During the entire study period, the minimum NO3 concentration observed was 1.7 mg N l−1, and the mean, 4.0 mg N l−1,39 demonstrating that NO3 concentrations are unlikely to limit algal growth given a limiting concentration for phytoplanktonic algae of c.0.03 mg NO3–N l−1.13 P limitation cannot be ruled out so easily. Although the mean soluble reactive phosphorus (SRP) concentration of 130 μg P l−1 is well above proposed growth-limiting concentrations for benthic algae of 25 μg P l−1 (ref. 11) or 80 μg P l−1,7 the minimum value observed in the weekly grab samples was 24 μg P l−1, and 37% were <80 μg P l−1, mostly in the spring growth periods. Only TRP was measured in the high-frequency data: here some samples were below the detection limit of ca. 10 μg P l−1. Using the above SRP thresholds as a rough guide, only 0.1% of the 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 276 high frequency samples had TRP of <25 μg P l−1, whereas 19% had TRP <80 μg P l−1 (Fig. 2). However, the lowest TRP concentrations occurred in winter when higher flows were diluting the inputs: at this time algal growth is likely to be temperature or light-limited. During the DO rise periods, the minimum concentrations observed in 2010 and 2011 respectively were: NO3, 2.86 and 3.16 mg N l−1; TRP, 30 and 56 μg P l−1. A degree of P limitation of algal growth rates in the DO rise period is thus a possibility, though there was always P available. The supply of Si is also relevant given the evidence that diatoms dominate photosynthesis in early spring. Mean Si concentrations in the DO rise periods in 2010 and 2011 respectively were 4.34 and 2.96 mg l−1. These are well above the putative limiting concentration of 0.5 mg l−1.13
276 high frequency samples had TRP of <25 μg P l−1, whereas 19% had TRP <80 μg P l−1 (Fig. 2). However, the lowest TRP concentrations occurred in winter when higher flows were diluting the inputs: at this time algal growth is likely to be temperature or light-limited. During the DO rise periods, the minimum concentrations observed in 2010 and 2011 respectively were: NO3, 2.86 and 3.16 mg N l−1; TRP, 30 and 56 μg P l−1. A degree of P limitation of algal growth rates in the DO rise period is thus a possibility, though there was always P available. The supply of Si is also relevant given the evidence that diatoms dominate photosynthesis in early spring. Mean Si concentrations in the DO rise periods in 2010 and 2011 respectively were 4.34 and 2.96 mg l−1. These are well above the putative limiting concentration of 0.5 mg l−1.13
Between 15 March and 4 April, a slight declining trend in NO3 concentrations can be observed but daily mean NO3 concentrations remained almost constant, ranging from 3.67 to 3.77 mg N l−1. This was then followed by a slight increasing trend between 5 March and 20 March, with daily mean NO3 concentrations increasing from 3.78 to 3.99 mg N l−1. However, during the DO fall period this increasing trend becomes much steeper, with daily mean NO3 concentrations increasing from 3.95 to 5.30 mg N l−1. These trends indicate that instream uptake of N during the DO rise period is sufficient to maintain the N concentrations roughly constant. The marked increasing trend in the DO fall period shows reduced instream uptake.
The weekly hydrochemical data also demonstrate marked changes in Si dynamics between the DO rise and DO fall periods. For example, in 2011 Si concentrations decreased from 6.33 to 2.96 mg l−1 between 14 March and 26 April (equating to a daily uptake rate of approximately 0.08 mg l−1). There was then a marked increase in concentration, with Si concentrations returning to 6.01 mg l−1 by 9 May. This highlights that instream uptake of Si, largely by the benthic diatoms, is controlling the observed trends in Si concentration.
| Day | 1st min. | 1st peak | 2nd min. | 2nd peak | ||||
|---|---|---|---|---|---|---|---|---|
| Hour | NO3 | Hour | NO3 | Hour | NO3 | Hour | NO3 | |
| 13 | 0100 | 3.47 | 0700 | 4.05 | 1300 | 3.63 | 1800 | 4.08 |
| 14 | 0100 | 3.47 | 0700 | 4.15 | 1400 | 3.66 | 1900 | 4.12 |
| 15 | 0100 | 3.67 | 0700 | 4.26 | 1300 | 3.76 | 2000 | 4.17 |
| 16 | 0200 | 3.65 | 0700 | 4.12 | 1400 | 3.83 | 2000 | 4.23 |
| 17 | 0200 | 3.69 | 0800 | 4.30 | 1500 | 3.80 | 2100 | 4.09 |
| 18 | 0300 | 3.63 | 1000 | 4.31 | 1500 | 3.72 | 2200 | 4.13 |
| 19 | 0200 | 3.87 | 0900 | 4.26 | 1700 | 3.83 | 2200 | 4.01 |
In the DO fall period, immediately following the algal growth maximum, the diurnal pattern in nutrient dynamics changes. For NO3, at this time, minimum daily concentrations were observed between 0300 and 0700 GMT and maximum concentrations occurred between 1000 and 1400 GMT. However, during this period the marked daytime drop in concentration, observed in the DO rise period, is less prominent with an average daytime decrease of only 0.26 mg N l−1. In addition, as the 7 day period progresses the instream NO3 concentrations tend to remain high until 2100–2300 GMT, rather than exhibit a significant daytime decline. These changes in NO3 dynamics are dramatic, relative to the change in DO, and likely result from a range of contributory factors: the increasing importance of groundwater contributions and effluent discharges to the river flow as flows continue to decline;18 reduced NO3 uptake as algal growth begins to decline; possible release of NO3 from dying algae; and the increased importance of daytime nitrification to the overall NO3 signal, supported by the conductivity dynamics with peak conductivity at 1400 GMT along with peak NO3 concentrations18 (Fig. 4). TRP diurnal dynamics remain noisy in the DO fall period, with no discernible pattern in concentration minimums.
The presence and absence of the regular daytime drop in NO3 concentration between the DO rise and fall periods suggests that the mechanism driving this daytime NO3 removal has become less important. This is further evidence that instream N uptake driven by daytime benthic algal photosynthesis is producing this daytime N concentration drop. The occurrence of more regular daytime P concentrations minimums in the DO rise period also suggests that instream P uptake is taking place through bioaccumulation. However, there is no evidence in Fig. 4 that N or P concentrations were reduced to concentrations which would limit algal growth. Possibly, if algal growth had continued unabated for several more days, this might have reduced P concentrations to limiting levels. Instead, shading by the developing tree canopy probably curbed algal growth before P concentrations were depleted to limiting levels.
4.6 Overall discussion
The evidence discussed above suggests strongly that the development of a riparian canopy controls algal growth throughout the growing season. It is not however completely conclusive, as important components of the stream ecosystem were not measured in this monitoring study. Grazing by zooplankton and invertebrates affects algal biomass and is likely to change seasonally. Budburst correlates with increasing temperature and light duration and intensity, which can all affect algal growth directly. Direct measurement of benthic biomass and composition together with measurements of light penetration to the stream would be required to resolve these questions conclusively. Nevertheless, control by the riparian tree canopy remains the most likely explanation.5. Conclusions
High frequency hydrochemical data together with weekly flow cytometry data and catchment information, have revealed new understanding on the control of instream algal dynamics on the River Enborne.The phytoplankton biomass is not large enough to explain the observed seasonal and diurnal patterns in dissolved oxygen and nutrients. Instead, benthic algae seem to be the key primary producers.
Stable low flow conditions are important for the development of benthic algal growth. Moderate-sized flow events can reduce this considerably.
In early spring a diatom bloom starts to develop, principally of benthic diatoms. This starts to deplete P concentrations towards possibly limiting concentrations. P limitation is unlikely at other times, and N or Si limitation at any time.
Algal growth exerts a strong influence on observed nutrient concentrations, causing observable trends and diurnal patterns in spite of the high nutrient inputs.
The most probable explanation of these observations is that shading by riparian trees controls algal growth through most of the growing season.
Acknowledgements
We thank the Natural Environment Research Council (NERC) for funding the analysis of this data under the Turf2Surf Macronutrients Project (NE/J011967/1); the Engineering and Physical Sciences Research Council for funding the LIMPIDS project (EP/G019967/1); NERC for financially supporting the CEH Thames Initiative monitoring; Linda Armstrong, Sarah Harman and Heather Wickham (CEH) for carrying out the laboratory analysis; Colin Roberts (CEH) for the weekly river sampling; the Woodland trust and UKPN for providing budburst data. River monitoring data can be obtained from the NERC Environmental Information Data Centre (DOI: 10/f272wn). The budburst and first leaf data are freely accessible from the Woodland Trust and can be accessed via an email request from http://naturescalendar.org.uk.Notes and references
- V. H. Smith, G. D. Tilman and J. C. Nekola, Environ. Pollut., 1999, 100, 179–196 CrossRef CAS PubMed.
- S. R. Carpenter, E. H. Stanley and M. J. Vander Zanden, Annu. Rev. Environ. Resour., 2011, 36, 75–99 CrossRef.
- V. H. Smith and D. W. Schindler, Trends Ecol. Evol., 2009, 24, 201–207 CrossRef PubMed.
- H. P. Jarvie, A. N. Sharpley, P. J. Withers, J. T. Scott, B. E. Haggard and C. Neal, J. Environ. Qual., 2013, 42, 295–304 CrossRef CAS PubMed.
- M. J. Bowes, J. T. Smith, C. Neal, D. V. Leach, P. M. Scarlett, H. D. Wickham, S. A. Harman, L. K. Armstrong, J. Davy-Bowker, M. Haft and C. E. Davies, Sci. Total Environ., 2011, 409, 3418–3430 CrossRef CAS PubMed.
- A. Ghermandi, V. Vandenberghe, L. Benedetti, W. Bauwens and P. A. Vanrolleghem, Ecol. Eng., 2009, 35, 92–104 CrossRef.
- M. J. Bowes, N. L. Ings, S. J. McCall, A. Warwick, C. Barrett, H. D. Wickham, S. A. Harman, L. K. Armstrong, P. M. Scarlett, C. Roberts, K. Lehmann and A. C. Singer, Sci. Total Environ., 2012, 434, 201–212 CrossRef CAS PubMed.
- M. G. Hutchins, A. C. Johnson, A. Deflandre-Vlandas, S. Comber, P. Posen and D. Boorman, Sci. Total Environ., 2010, 408, 5065–5077 CrossRef CAS PubMed.
- T. K. Burrell, J. M. O'Brien, S. E. Graham, K. S. Simon, J. S. Harding and A. R. McIntosh, Freshwat. Sci., 2014, 33, 73–84 CrossRef.
- W. R. Hill, M. G. Ryon and E. M. Schilling, Ecology, 1995, 1297–1309 CrossRef.
- W. R. Hill, S. E. Fanta and B. J. Roberts, Limnol. Oceanogr., 2009, 54, 368–380 CrossRef CAS.
- T. D. Mosisch, S. E. Bunn and P. M. Davies, Freshwater Biol., 2001, 46, 1269–1278 CrossRef.
- C. S. Reynolds, Ecology of Phytoplankton, Cambridge University Press, Cambridge, 2006 Search PubMed.
- A. D. Rosemond, P. J. Mulholland and S. H. Brawley, Can. J. Fish. Aquat. Sci., 2000, 57, 66–75 CrossRef.
- D. P. McCormick and S. S. C. Harrison, Fish. Manag. Ecol., 2011, 18, 444–455 CrossRef.
- M. F. O'Grady, Aquacult. Res., 1993, 24, 563–573 CrossRef.
- ARK, Chalkstream habitat and water meadow restoration project, Coppers Meadow, River Kennet, Action for the River Kennet, Accessed online, http://www.riverkennet.org/uploads/files/documents/Projects/Cooper's%20Meadow%20Final%20Report%20June%202009.pdf, 21/09/14, 2009.
- S. Halliday, R. Skeffington, M. Bowes, E. Gozzard, J. Newman, M. Loewenthal, E. Palmer-Felgate, H. Jarvie and A. Wade, Water, 2014, 6, 150–180 CrossRef.
- M. Bowes, H. Jarvie, S. Halliday, R. Skeffington, A. Wade, M. Loewenthal, E. Gozzard, J. Newman and E. Palmer-Felgate, Sci. Total Environ., 2015, 511, 608–620 CrossRef CAS PubMed.
- D. Morton, C. Rowland, C. Wood, L. Meek, C. Marston, G. Smith, R. Wadsworth and I. C. Simpson, Final Report for LCM2007-the New UK Land Cover Map. Countryside Survey Technical Report No. 11/07, NERC/Centre for Ecology & Hydrology, 2011 Search PubMed.
- A. J. Wade, E. J. Palmer-Felgate, S. J. Halliday, R. A. Skeffington, M. Loewenthal, H. P. Jarvie, M. J. Bowes, G. M. Greenway, S. J. Haswell, I. M. Bell, E. Joly, A. Fallatah, C. Neal, R. J. Williams, E. Gozzard and J. R. Newman, Hydrol. Earth Syst. Sci., 2012, 16, 4323–4342 CrossRef.
- M. J. Bowes, E. Gozzard, A. C. Johnson, P. M. Scarlett, C. Roberts, D. S. Read, L. K. Armstrong, S. A. Harman and H. D. Wickham, Sci. Total Environ., 2012, 426, 45–55 CrossRef CAS PubMed.
- D. S. Read, M. J. Bowes, L. K. Newbold and A. S. Whiteley, Environ. Sci.: Processes Impacts, 2014, 16, 594–603 CAS.
- S. Boulêtreau, F. Garabétian, S. Sauvage and J. M. Sánchez-Pérez, Freshwater Biol., 2006, 51, 901–912 CrossRef.
- C. Mendoza-Lera, L. L. Federlein, M. Knie and M. Mutz, Water Resour. Res., 2016, 52, 108–118 CrossRef.
- UK Met Office, Global Radiation Observations, Part of the Met Office Integrated Data Archive System (MIDAS). NCAS British Atmospheric Data Centre, 2016, 02/02/16, http://catalogue.ceda.ac.uk/uuid/b4c028814a666a651f52f2b37a97c7c7.
- Woodland Trust, http://www.naturescalendar.org.uk/, Online 2015.
- P. C. Young, C. J. Taylor, W. Tych and D. J. Pedregal, The CAPTAIN Toolbox, http://captaintoolbox.co.uk/, Online 2016 Search PubMed.
- C. J. Taylor, D. J. Pedregal, P. C. Young and W. Tych, Environ. Model. Software, 2007, 22, 797–814 CrossRef.
- T. Vogt, P. Schneider, L. Hahn-Woernle and O. A. Cirpka, J. Hydrol., 2010, 380, 154–164 CrossRef.
- S. J. Halliday, R. A. Skeffington, A. J. Wade, C. Neal, B. Reynolds, D. Norris and J. W. Kirchner, Biogeosciences, 2013, 10, 8013–8038 CrossRef.
- S. J. Halliday, A. J. Wade, R. A. Skeffington, C. Neal, B. Reynolds, P. Rowland, M. Neal and D. Norris, Sci. Total Environ., 2012, 434, 186–200 CrossRef CAS PubMed.
- J. Keery, A. Binley, N. Crook and J. W. N. Smith, J. Hydrol., 2007, 336, 1–16 CrossRef.
- N. A. Chappell and W. Tych, Hydrol. Processes, 2012, 26, 100–116 CrossRef.
- H. Wang, M. Hondzo, C. Xu, V. Poole and A. Spacie, Ecol. Modell., 2003, 160, 145–161 CrossRef CAS.
- J. C. Correa-González, M. d. C. Chávez-Parga, J. A. Cortés and R. M. Pérez-Munguía, Ecol. Modell., 2014, 273, 220–227 CrossRef.
- H. Haider, W. Ali and S. Haydar, Hydrol. Processes, 2013, 27, 3949–3963 CrossRef CAS.
- R. Jha, C. S. P. Ojha and K. K. S. Bhatia, Hydrol. Processes, 2001, 15, 1047–1060 CrossRef.
- S. J. Halliday, R. A. Skeffington, M. J. Bowes, E. Gozzard, J. R. Newman, M. Loewenthal, E. J. Palmer-Felgate, H. P. Jarvie and A. J. Wade, Water, 2014, 6, 150–180 CrossRef.
- S. J. Halliday, R. A. Skeffington, A. J. Wade, M. J. Bowes, E. Gozzard, J. R. Newman, M. Loewenthal, E. J. Palmer-Felgate and H. P. Jarvie, Hydrol. Processes, 2015, 29, 3388–3407 CrossRef CAS.
- M. E. Bindloss, Proc. R. Soc. Edinburgh, Sect. B: Biol. Sci., 1974, 74, 157–181 CrossRef.
- R. J. Williams, C. White, M. L. Harrow and C. Neal, Sci. Total Environ., 2000, 251, 497–510 CrossRef PubMed.
- E. J. Palmer-Felgate, H. P. Jarvie, R. J. Williams, R. J. Mortimer, M. Loewenthal and C. Neal, J. Hydrol., 2008, 351, 87–97 CrossRef CAS.
- M. G. Kelly, Water Res., 1998, 32, 236–242 CrossRef CAS.
- J. Garnier, G. Billen and M. Coste, Limnol. Oceanogr., 1995, 40, 750–765 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |