Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Light and oxygen induced degradation limits the operational stability of methylammonium lead triiodide perovskite solar cells†
Daniel
Bryant
ab,
Nicholas
Aristidou
a,
Sebastian
Pont
a,
Irene
Sanchez-Molina
a,
Thana
Chotchunangatchaval
a,
Scot
Wheeler
a,
James R.
Durrant
ab and
Saif A.
Haque
*a
aDepartment of Chemistry and Centre for Plastic Electronics, Imperial College London, South Kensington Campus, SW7 2AZ, UK. E-mail: s.a.haque@imperial.ac.uk
bSPECIFIC, College of Engineering, Swansea University, Baglan Bay Innovation and Knowledge Centre, Central Avenue, Baglan, SA12 7AX, UK
First published on 13th April 2016
Abstract
Here, we demonstrate that light and oxygen-induced degradation is the main reason for the low operational stability of methylammonium lead triiodide (MeNH3PbI3) perovskite solar cells exposed to ambient conditions. When exposed to both light and dry air, unencapsulated MeNH3PbI3 solar cells rapidly degrade on timescales of minutes to a few hours. This rapid degradation is also observed under electrically bias driven current flow in the dark in the presence of O2. In contrast, significantly slower degradation is observed when the MeNH3PbI3 devices are exposed to moisture alone (e.g. 85% relative humidity in N2). We show that this light and oxygen induced degradation can be slowed down by the use of interlayers that are able to remove electrons from the perovskite film before they can react with oxygen to form O2−. These observations demonstrate that the operational stability of electronic and optoelectronic devices that exploit the electron transporting properties of MeNH3PbI3 will be critically dependent upon the use of suitable barrier layers and device configurations to mitigate the oxygen sensitivity of this remarkable material.
Broader contextHybrid organic–inorganic metal halide perovskite solar cells (PSCs) are a potentially low cost and efficient third generation photovoltaic technology. Recent advances in the performance of lab based devices have seen certified efficiencies in excess of 20%. This, in combination with the solution processible nature and low cost of the precursor materials, has seen worldwide scientific and industrial interest in perovskite solar cells grow rapidly in the past 4 years. However, the instability of the perovskite photoactive layer still represents a major barrier that must be overcome before the successful commercialisation of PSC. Until now much of the published literature on PSC stability has been concerned with the inherent moisture sensitivity demonstrated by the perovskite layer. We demonstrate herein that an additional degradation mechanism induced by the combination of light and oxygen in combination, and show that this can be the primary degradation pathway under device operation. We show this degradation pathway can be partially suppressed under conditions of rapid electron extraction from the perovskite layer. We go on to demonstrate how this oxygen induced degradation pathway is not limited to only photovoltaic devices, but is also relevant to all electric devices that employ an organic–inorganic perovskite layer exposed to oxygen under operation. This is an important finding since it affects not only considerations of barrier layer and encapsulation design for such hybrid perovskite electronic devices but also suggests a key materials design challenge in developing hybrid perovskite active layers with reduced oxygen sensitivity. |
Methylammonium lead trihalide perovskites (e.g. MeNH3PbI3) are currently generating extensive interest with respect to their use in a range of devices such as light emitting diodes, solar cells, transistors and lasers.1–7 The successful exploitation of these technologies ultimately depends on the ability to achieve both high device performance and operational stability. A device configuration of particular interest is the MeNH3PbI3 perovskite solar cell. Over the last few years, remarkable progress has been made and solar light to electrical power conversion efficiencies of such devices have quickly risen from approximately 10% in 2012 to over 20% at present.4,8 As such, it is increasingly recognised that stability is a key challenge for this technology.9 Promising operational stability has been reported for MeNH3PbI3 solar cells encapsulated under an inert atmosphere using glass as a barrier layer.10–12 Whilst it is common place to encapsulate similar optoelectronic devices (such as OPVs and OLEDs), sensitivity to environmental exposure, and specifically to water and/or oxygen ingress, is likely to be key concern for many practical technology applications where flexibility is needed and glass barrier layers are not viable. Some progress has been made in this regard, with promising shelf life (dark) stability reported for unencapsulated MeNH3PbI3 employing a metal-oxide top contact,13 and under operation for devices employing enhanced electron extraction.10,13 However, the underlying origin(s) of these improvements in device stability have not been elucidated, and in particular in the literature to date relatively little attention has been placed on determination of the relative importance of different factors limiting material and device stability. While several external agents (e.g. temperature, phase behaviour, pressure, ultraviolet light, moisture and crystallinity) have been reported to influence the stability of perovskite solar cells,14–19 it has largely been assumed that moisture-induced degradation is the dominant issue affecting MeNH3PbI3 device stability under ambient conditions. As such, most stability studies have focussed on the role of moisture in the deterioration of MeNH3PbI3 solar cell performance.17,20,21 In contrast, we have recently demonstrated that exposure of MeNH3PbI3 films to both light and molecular oxygen can cause rapid degradation.22,23 Specifically, we have shown that this reaction is initiated by the deprotonation of the methylammonium cation of the perovskite by a photogenerated reactive oxygen species (superoxide, O2−) where the O2− is generated by the reaction of photo-generated electrons in the perovskite and molecular oxygen.22 These observations raise two important questions, namely does oxygen induced degradation affect the stability of fully-functioning perovskite solar cells and secondly whether the degradation can also be induced by the reaction of electrically injected electrons in the perovskite and oxygen. The ability of oxygen induced degradation to be facilitated by electrical bias would be expected to have a profound impact on the stability of a wide range of devices such as light emitting diodes and transistors as well as solar cells. Herein we address these two issues. Specifically, in this paper we focus on the stability of MeNH3PbI3 based solar cell devices under different operating (e.g. light and dark) and environmental exposure conditions and conclude, remarkably, that oxygen induced degradation, rather than moisture induced degradation, is the dominant process that limits the operational stability of optoelectronic devices containing MeNH3PbI3 under ambient conditions. Furthermore, we show that this drop in device performance can be slowed down by the integration of electron acceptor layers within device architecture. Such layers are shown to enhance electron extraction from the perovskite absorber before they can react with oxygen, thus reducing the yield of superoxide O2− and improving device stability.
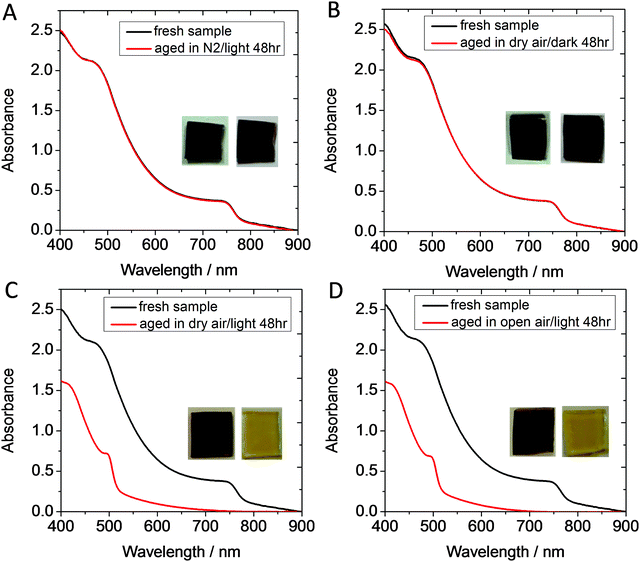
When exposed to both light and oxygen, MeNH3PbI3 photoactive layers rapidly degrade, as illustrated in Fig. 1. Specifically, Fig. 1 presents the absorption characteristics of MeNH3PbI3 films prepared on non-conducting glass before (blue trace) and after (red trace) ageing for 48 hours in controlled environments (where (A) is exposed to light and under a N2 atmosphere, (B) is in the dark under clean dry air, (C) is in light under clean dry air and (D) is in light under ambient air (RH ≈ 48%)). The as-prepared MeNH3PbI3 films all exhibit the reported absorption onset at approximately 780 nm.4 It is apparent that degradation of the MeNH3PbI3 films takes place in dry air with light (Fig. 1C) and ambient air with light (Fig. 1D). However, no degradation is observed in control samples aged in N2 with light (Fig. 1A) and dry air in the dark (Fig. 1B). Moreover, it can be seen in Fig. 1C and D, that exposure to light and oxygen for just 48 hours results in a dramatic blue shift in the absorption onset from 780 nm to 520 nm and a profound change in colour of the sample from dark brown to yellow; both these observations being consistent with the breakdown perovskite crystal and the subsequent presence of PbI2 in the final degraded films.22 The data presented herein are consistent with our previous work and further confirm the presence of a degradation pathway that is light activated, independent of moisture and requires molecular oxygen.22,23
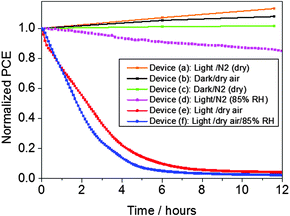
Our observation of a profound sensitivity of MeNH3PbI3 films to light and oxygen raises a key question, namely whether and to what extent this degradation pathway contributes to the low stability of MeNH3PbI3 based solar cells. To investigate how light and oxygen affect the performance of complete photovoltaic devices, stability studies were undertaken. The relevant experimental details of device fabrication are given in the ESI† section. Briefly, solar cells of type: ITO/compact-TiO2/meso-TiO2/MeNH3PbI3/spiro-OMeTAD/Au were fabricated in a dry, oxygen-free glove-box. These devices, without further encapsulation, were then placed in an environmental chamber for stability measurements. Ageing illumination was provided by a 1 sun equivalent white LED array equipped with a UV blocking filter. Power conversion efficiencies (PCE's) of the solar cells were determined from photocurrent–voltage measurements at regular time intervals (0.02 V s−1 scan speed, using same LED light source) over the course of 12 hours with the solar cells being subjected to different environmental conditions. Fig. 2 shows the PCE versus time profile with the solar cell devices aged in controlled conditions (where device (a) is exposed to light and N2, (b) exposed to dry air in dark, (c) exposed to N2 in the dark (d) exposed to light and N2 with 85% relative humidity, (e) exposed to dry air in light and (f) exposed to dry air and 85% relative humidity in the light). Raw data (device parameters obtained from current–voltage scans under these different environmental conditions as a function of time) are presented in Fig. S1 (ESI†). It is evident from the data presented in Fig. 2 that the performance of control devices a, b and c remains relatively constant, even increasing marginally in performance over 12 hours. In contrast, the device exposed to light and dry air (device e) shows a dramatic and large drop in PCE with ageing time with, for example, this device exhibiting a 50% drop in PCE from its original value within 2 hours. This observation clearly demonstrates that the performance and stability of MeNH3PbI3 based solar cells is critically affected when exposed to both light and oxygen. In contrast, the PCE of device (d) exposed to light, 85% relative humidity (RH) in N2 remains relatively constant, with this device showing a comparatively small 10% drop in PCE over the 12 hour aging period. Furthermore, it can be seen from the data presented in Fig. 2 that the rate of decrease in PCE is similar in devices exposed to light and dry air (device e) and light, dry air and 85% RH (device f). We therefore conclude that light and oxygen induced degradation, and not moisture induced degradation, is the dominant factor that determines the stability of MeNH3PbI3 based solar cells under ambient operating conditions.
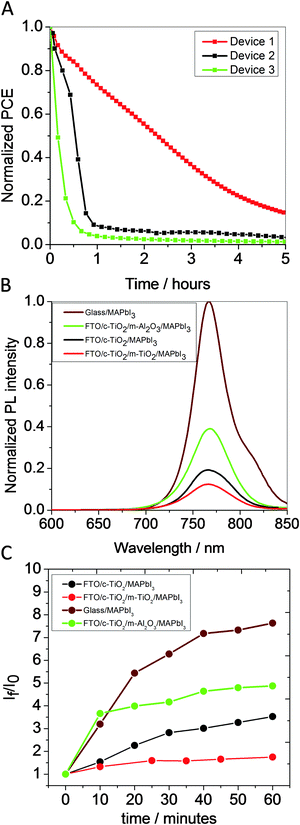
Next, we consider how the impact of light and oxygen induced degradation on device stability can be minimized in MeNH3PbI3 solar cells. We have recently demonstrated that the yield of O2− generation can be decreased by the use of suitable electron acceptor layers within the MeNH3PbI3 film.22 In this context, the electron acceptor layer functions to extract photogenerated electrons from the MeNH3PbI3 layer before they react with oxygen thereby competing with the formation of O2−.22 As such, it is reasonable to expect that perovskite solar cell structures comprising layers that are able to efficiently extract electrons would exhibit better stability. To test this hypothesis, the relative stability of three types of MeNH3PbI3 perovskite solar cell structures comprising different electron extractor layers were investigated. In this study, device (1) employed a compact-TiO2/mesoporous-TiO2/MeNH3PbI3 film; device (2) employed a compact-TiO2/MeNH3PbI3 film; and device (3) employed a compact-TiO2/mesoporous Al2O3/MeNH3PbI3 film. All three devices (1, 2 and 3) employed doped spiro-OMeTAD and gold as the hole-transporting material and top metal contact respectively.24Fig. 3A shows the PCE versus time profile for devices 1, 2 and 3 aged in dry air and light. Full current–voltage data are presented in Fig. S2 (ESI†). As can be seen by a comparison of the PCE versus time profiles for devices 1, 2 and 3 (Fig. 3A) the time taken for the performance of these devices to drop by 50% is 2.2, 0.6 and 0.17 hours respectively. Relatively better stability of device 1 was attributed to the superior electron extraction capability of the compact-TiO2/mesoporous-TiO2 electrode as compared to the compact-TiO2 and compact-TiO2/mesoporous-Al2O3 electrodes.
MeNH3PbI3 photoluminescence (PL) quenching experiments and O2− yield were employed to investigate the origin of the trend in device stabilities shown in Fig. 3A. Fig. 3B presents the steady-state PL data for compact-TiO2/mesoporous-TiO2/MeNH3PbI3, compact-TiO2/MeNH3PbI3, compact-TiO2/mesoporous-Al2O3/MeNH3PbI3 and glass/MeNH3PbI3 films. It is clear that, all three electrode systems show reduced emission intensity relative the glass/MeNH3PbI3 sample. By evaluation of the integrated areas of the respective spectra in Fig. 3B, we find that yield of MeNH3PbI3 PL quenching is 85%, 78% and 56% for the compact-TiO2/mesoporous-TiO2, compact-TiO2, compact-TiO2/mesoporous-Al2O3 films respectively, indicative of a trend in the efficiency of electron extraction between these three device architectures, in agreement with literature data.25 We note that the same trend in PL quenching efficiency was obtained from time-resolved PL measurement, as illustrated in Fig. S3 (ESI†). We next consider the correlation between the yields of MeNH3PbI3 PL quenching and O2− generation in the three films. The presence of superoxide was detected, as previously, by using a fluorescent molecular probe, hydroethidine.22Fig. 3C shows the rate of increase in probe emission ([IF(t)]/[IF(to)] versus ageing time) and therefore O2− generation yield. As expected, a good correlation can be seen between the MeNH3PbI3 PL quenching efficiency and O2− generation yield. Interestingly, it is apparent that the compact-TiO2/mesoporous-Al2O3/MeNH3PbI3 film exhibits a lower PL quenching efficiency and a higher O2− yield as compared to the compact-TiO2/MeNH3PbI3 film. This is most likely due to: (i) the presence of the mesoporous-Al2O3 film reducing the effective electron extraction area of the compact TiO2 layer and (ii) reduced crystal size of MeNH3PbI3 in compact-TiO2/mesoporous-Al2O3/MeNH3PbI3 relative to that in the ‘planar-type’ compact-TiO2/MeNH3PbI3 film. It is reasonable to suppose that both these factors would lead to poorer electron extraction and therefore an increased probability of electron transfer to oxygen. Taken together, the data presented in Fig. 3 indicates that better electron extraction leads to better device stability. This improved stability derives from a reduction in yield of O2− generation as a consequence of better electron extraction from the MeNH3PbI3 film.
Further evidence for the importance of electron extraction for the stability of MeNH3PbI3 solar cells was obtained from device performance measurements as a function of externally applied bias. Fig. 4 shows the PCE versus time profile for solar cells biased at short-circuit (Vbias = 0 V) and open-circuit (Vbias = Voc = 0.9 V). As expected, more rapid degradation is observed when the device is biased at open-circuit rather than short-circuit, consistent with better electron extraction under short circuit conditions. This enhanced electron extraction was confirmed by enhanced MAPI3 PL quenching at short-circuit compared to open-circuit (Fig. 4A (inset)). These observations further highlight the importance to device stability of removing electrons from the photoactive layer before they can react with O2 to form O2−.
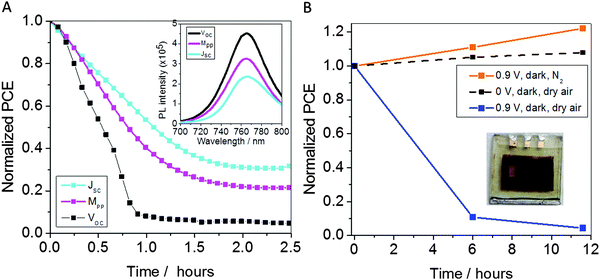 | ||
| Fig. 4 (A) Shows the relative performance of FTO/c-TiO2/MeNH3PbI3/spiro-OMeTAD/Au solar cells as function of aging time. Measurements were performed with the solar cells being exposed to light and oxygen. Data was collected with the solar cells were biased at open circuit (Vbias = 0.9 V), short circuit (Vbias = 0 V) and maximum power point (Vbias = 0.66 V). (A) (inset) Shows the corresponding MAPI3 PL data measured under these applied biases, employing 485 nm excitation. (B) Shows the relative performance of such solar cells aged in the dark in the presence of either dry air or nitrogen under electrical bias (Vbias = 0.9 V, current density 20 mA cm−2). For reference the equivalent data for a cell held in dark and dry air conditions with no external bias is included (black dashed line, taken from Fig. 2). From (B) (inset) shows the degraded device pixel (1) after ageing at Vbias 0.9 V; decolouration of the pixel from dark brown to yellow indicates degradation of the MeNH3PbI3. For the data presented in (B) the aging was performed in the dark but the PCE measurements were performed under 1 sun equivalent illumination using a white LED array. | ||
We now consider the wider relevance of our observations to other electronic and optoelectronic devices that exploit the semiconducting properties of MeNH3PbI3. Thus far, we have shown that the degradation of MeNH3PbI3 solar cells can be triggered by the reaction of photogenerated electrons and oxygen. The next question that arises relates to whether degradation can also be induced by the reaction of electrically injected electrons and oxygen. In order to address this, we investigated the degradation of the MeNH3PbI3 solar cells in the dark (no illumination) under forward bias (Vbias = 0.9 V, J = 20 mA cm−2) in a N2 or dry air environment. Device degradation in the dark was monitored by brief measurements of device photovoltaic PCE under light irradiation (Fig. 4B), as well as by monitoring MeNH3PbI3 decoloration using a camera (Fig. 4B inset). Remarkably, we find that the MeNH3PbI3 devices degrade even in the dark and operating in the forward bias regime when oxygen is present (Fig. 4B, blue trace and inset LH pixel). However, such degradation was not observed when the device was in a nitrogen atmosphere or in the absence of forward bias. Our observation of device degradation under forward bias in the dark suggests that in the presence of oxygen, MeNH3PbI3 is fundamentally unstable when transporting electrons. This finding indicates that oxygen induced degradation of MeNH3PbI3 reported in this paper is likely to be important in limiting the operational stability of not only MeNH3PbI3 based solar cells, but also that of other electronic and optoelectronic devices employing MeNH3PbI3 as an electron transport material, such as LED's and transistors.
Herein, we have shown that MeNH3PbI3 based films and solar cells degrade remarkably quickly, on timescales of minutes to a few hours when exposed to both light and oxygen. In organic solar cells, the presence of a metal top contact has been observed to substantially improve device stability under light/oxygen exposure, attributed to slow lateral oxygen diffusion kinetics through the organic layer.26 For MeNH3PbI3 devices studied herein, in contrast, the presence of a metal top contact does not appear to produce the same effect (i.e. enhance stability). This observation is indicative of relatively rapid oxygen diffusion kinetics within the device structure. Such rapid diffusion kinetics would be consistent with the observation that even much larger species (e.g. I−, Pb2+ and CH3NH3+) are able to migrate within the MeNH3PbI3 perovskite films.27 Further studies addressing oxygen diffusion in MeNH3PbI3 films, and its impact upon device stability are currently underway and will be reported in our future work.
In summary, this work elucidates a major cause of the relatively low stability of MeNH3PbI3 solar cells when operating in ambient conditions and provides a pathway to address the problem. Specifically, we have demonstrated that light and oxygen induced degradation can be the dominant factor that determines the operational stability of MeNH3PbI3 solar cells in ambient conditions. We show that, under these technologically important conditions, light and oxygen induced degradation occurs faster than the more widely studied moisture induced degradation. Moreover, we have shown that this degradation can be slowed down by the integration of electron extraction layers within the device architecture. Specifically, we have demonstrated that better electron extraction leads to a reduction in the yield of O2− and an improvement in device stability. Finally, we have shown that MeNH3PbI3 devices also degrade in the dark under forward bias when exposed to oxygen. The present findings suggest that oxygen induced degradation can influence the operational stability of not only solar cells but also other devices that utilize MeNH3PbI3 as an electron transporting material. Ultimately, in their current state, the long term stability of optoelectronic and electronic devices containing organic–inorganic lead halide perovskite semiconductors will be reliant on successful encapsulation and effective barrier layers.
Acknowledgements
S. A. H. acknowledges financial support from the Engineering and Physical Sciences Research Council (EPSRC) through (EP/M023532/1) and (EP/K010298/1) projects and the European Community's Seventh Framework Programme (Nanomatcell, grant agreement number 308997). J. R. D. and D. B. acknowledge financial support from the EPSRC (EP/IO19278/1 & EP/M023532/1) and the Welsh Assembly government funded Sêr Solar project. Thanks to Diego Alonso Alvarez and Ned Ekins-Dawkes for their assistance with the TRPL measurements and Li Xiaoe for her assistance with device manufacture.References
- Z.-K. Tan, R. S. Moghaddam, M. L. Lai, P. Docampo, R. Higler, F. Deschler, M. Price, A. Sadhanala, L. M. Pazos, D. Credgington, F. Hanusch, T. Bein, H. J. Snaith and R. H. Friend, Nat. Nanotechnol., 2014, 9, 687 CrossRef CAS PubMed.
- G. Li, Z.-K. Tan, D. Di, M. L. Lai, L. Jiang, J. H.-W. Lim, R. H. Friend and N. C. Greenham, Nano Lett., 2015, 15, 2640 CrossRef CAS PubMed.
- M. M. Lee, J. Teuscher, T. Miyasaka, T. N. Murakami and H. J. Snaith, Science, 2012, 338, 643 CrossRef CAS PubMed.
- H.-S. Kim, C.-R. Lee, J.-H. Im, K.-B. Lee, T. Moehl, A. Marchioro, S.-J. Moon, R. Humphry-Baker, J.-H. Yum, J. E. Moser, M. Grätzel and N.-G. Park, Sci. Rep., 2012, 2, 591 Search PubMed.
- G. Xing, N. Mathews, S. S. Lim, N. Yantara, X. Liu, D. Sabba, M. Grätzel, S. Mhaisalkar and T. C. Sum, Nat. Mater., 2014, 13, 476 CrossRef CAS PubMed.
- F. Deschler, M. Price, S. Pathak, L. E. Klintberg, D.-D. Jarausch, R. Higler, S. Hüttner, T. Leijtens, S. D. Stranks, H. J. Snaith, M. Atatüre, R. T. Phillips and R. H. Friend, J. Phys. Chem. Lett., 2014, 5, 1421 CrossRef CAS PubMed.
- X. Y. Chin, D. Cortecchia, J. Yin, A. Bruno and C. Soci, Nat. Commun., 2015, 6, 7383 CrossRef CAS PubMed.
- N. J. Jeon, J. H. Noh, W. S. Yang, Y. C. Kim, S. Ryu, J. Seo and S. I. Seok, Nature, 2015, 517, 476 CrossRef CAS PubMed.
- Leijtens, G. E. Eperon, N. K. Noel, S. N. Habisreutinger, A. Petrozza and H. J. Snaith, Adv. Eng. Mater., 2015, 5, 1500963 Search PubMed.
- W. Chen, Y. Wu, Y. Yue, J. Liu, W. Zhang, X. Yang, H. Chen, E. Bi, I. Ashraful, M. Grätzel and L. Han, Science, 2015, 350, 944 CrossRef CAS PubMed.
- X. Li, M. Tschumi, H. Han, S. S. Babkair, R. A. Alzubaydi, A. A. Ansari, S. S. Habib, M. K. Nazeeruddin, S. M. Zakeeruddin and M. Grätzel, Eng. Tech., 2015, 3, 551 CAS.
- J. Burschka, N. Pellet, S.-J. Moon, R. Humphry-Baker, P. Gao, M. K. Nazeeruddin and M. Gratzel, Nature, 2013, 499, 316 CrossRef CAS PubMed.
- J. You, L. Meng, T.-B. Song, T.-F. Guo, Y. Yang, W.-H. Chang, Z. Hong, H. Chen, H. Zhou, Q. Chen, Y. Liu, N. De Marco and Y. Yang, Nat. Nanotechnol., 2016, 11, 75–81 CrossRef CAS PubMed.
- B. Conings, J. Drijkoningen, N. Gauquelin, A. Babayigit, J. D'Haen, L. D'Olieslaeger, A. Ethirajan, J. Verbeeck, J. Manca, E. Mosconi, F. D. Angelis and H.-G. Boyen, Adv. Energy Mater., 2015, 5, 1500477 Search PubMed.
- C. C. Stoumpos, C. D. Malliakas and M. G. Kanatzidis, Inorg. Chem., 2013, 52, 9019 CrossRef CAS PubMed.
- T. Leijtens, G. E. Eperon, S. Pathak, A. Abate, M. M. Lee and H. J. Snaith, Nat. Commun., 2013, 4, 2885 Search PubMed.
- A. M. A. Leguy, Y. Hu, M. Campoy-Quiles, M. I. Alonso, O. J. Weber, P. Azarhoosh, M. van Schilfgaarde, M. T. Weller, T. Bein, J. Nelson, P. Docampo and P. R. F. Barnes, Chem. Mater., 2015, 27, 3397 CrossRef CAS.
- F. K. Aldibaja, L. Badia, E. Mas-Marza, R. S. Sanchez, E. M. Barea and I. Mora-Sero, J. Mater. Chem. A, 2015, 3, 9194 CAS.
- A. Dualeh, P. Gao, S. I. Seok, M. K. Nazeeruddin and M. Grätzel, Chem. Mater., 2014, 26, 6160 CrossRef CAS.
- J. A. Christians, P. A. Miranda Herrera and P. V. Kamat, J. Am. Chem. Soc., 2015, 137, 1530 CrossRef CAS PubMed.
- J. Yang, B. D. Siempelkamp, D. Liu and T. L. Kelly, ACS Nano, 2015, 9, 1955 CrossRef CAS PubMed.
- N. Aristidou, I. Sanchez-Molina, T. Chotchuangchutchaval, M. Brown, L. Martinez, T. Rath and S. A. Haque, Angew. Chem., Int. Ed., 2015, 54, 8208 CrossRef CAS PubMed.
- F. T. F. O'Mahony, Y. H. Lee, C. Jellett, S. Dmitrov, D. T. J. Bryant, J. R. Durrant, B. C. O'Regan, M. Graetzel, M. K. Nazeeruddin and S. A. Haque, J. Mater. Chem. A, 2015, 3, 7219 Search PubMed.
- J. Troughton, D. Bryant, K. Wojciechowski, M. J. Carnie, H. Snaith, D. A. Worsley and T. M. Watson, J. Mater. Chem. A, 2015, 3, 9141 CAS.
- A. Listorti, E. J. Juarez-Perez, C. Frontera, V. Roiati, L. Garcia-Andrade, S. Colella, A. Rizzo, P. Ortiz and I. Mora-Sero, J. Phys. Chem. Lett., 2015, 6, 1628 CrossRef CAS PubMed.
- S. Shoaee and J. R. Durrant, J. Mater. Chem. C, 2015, 3, 10079 RSC.
- C. Eames, J. M. Frost, P. R. F. Barnes, B. C. O'Regan, A. Walsh and M. S. Islam, Nat. Commun., 2015, 6, 7497 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ee00409a |
| This journal is © The Royal Society of Chemistry 2016 |