Open Access Article
Open Access ArticleHigh-performance ternary blend all-polymer solar cells with complementary absorption bands from visible to near-infrared wavelengths†
Hiroaki
Benten
*,
Takaya
Nishida
,
Daisuke
Mori
,
Huajun
Xu
,
Hideo
Ohkita
and
Shinzaburo
Ito
*
Department of Polymer Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo, Kyoto 615-8510, Japan. E-mail: benten@photo.polym.kyoto-u.ac.jp; sito@photo.polym.kyoto-u.ac.jp
First published on 9th December 2015
Abstract
We developed high-performance ternary blend all-polymer solar cells with complementary absorption bands from visible to near-infrared wavelengths. A power conversion efficiency of 6.7% was obtained with an external quantum efficiency over 60% both in the visible and near-infrared regions. Our results demonstrate that the ternary blend all-polymer systems open a new avenue for accelerating improvement in the efficiency of non-fullerene thin-film polymer solar cells.
Broader contextContinued expansion of energy consumption worldwide necessitates future global energy that is based on limitless resources and that generates less greenhouse gases than fossil-fuel-based energy sources. The use of photovoltaic energy is a promising approach that can solve the energy problems we will face in the foreseeable future. Recently, organic photovoltaics (OPVs) have gained increasing attention as an inexpensive source of renewable energy owing to their unique advantages including high throughout and large-area production with low-cost printing processes. Among the OPVs, all-polymer solar cells based on a blend of two types of conjugated polymers acting as an electron donor (hole transport) and an electron acceptor (electron transport) have numerous potential advantages over conventional fullerene-based solar cells, though the polymer/polymer blend systems still lag behind their polymer/fullerene counterparts in power conversion efficiencies (PCEs). Herein, we report high-performance ternary blend all-polymer solar cells in which a wide-bandgap visible polymer is introduced as a third polymer into a low-bandgap near-infrared donor/acceptor binary polymer blend. Owing to the complementary absorption bands of the ternary blend, the PCE is improved up to 6.7% with an external quantum efficiency over 60% both in the visible and near-infrared regions. Our results demonstrate that the ternary blend all-polymer solar cells open a new avenue for accelerating improvement in the efficiency of non-fullerene thin-film OPVs. |
Organic photovoltaics have gained increasing attention as an inexpensive source of renewable energy owing to their unique advantages including high throughout and large-area production with low-cost printing processes.1 The power conversion efficiencies (PCEs) of organic thin-film solar cells have made significant progress over the past decade, and approached 10% in single-junction cells.2 The most widely studied solar cells consist of a bulk-heterojunction (BHJ) structure in which a conjugated polymer is mixed with a low-molecular-weight fullerene derivative.3 In the systems, the conjugated polymer acts as an electron donor and the fullerene derivative acts as an electron acceptor. On the other hand, polymer/polymer blend BHJ solar cells that utilize conjugated polymers as both an electron donor and an electron acceptor have recently attracted much attention because they have numerous potential advantages over conventional polymer/fullerene BHJ solar cells. In particular, the flexible molecular design of not only the donor but also the acceptor material affords a large scope for tuning the optical, electronic, morphological, and mechanical properties of their blended films, which should give an optimal device performance superior to that of polymer/fullerene blend BHJ solar cells. While the PCE of polymer/polymer BHJ solar cells was limited to around 2% until 2012,4 it has steeply increased owing to the development of low-bandgap polymer acceptors, which show both high electron mobility (μe) and high electron affinity similar to those of fullerenes.5 Very recently, PCE values approaching 6–7%6–8 and 7.7%9 have been reported.
One of the promising strategies for achieving efficient performance of the polymer/polymer BHJ solar cells is to use donor and acceptor polymers that exhibit efficient light-absorption capabilities at near-infrared (NIR) wavelengths where the photon flux of the solar spectrum is at its highest levels. This is important for obtaining large short-circuit current density (JSC) values even for the thin films optimal for charge carrier collection. Recently, we developed polymer/polymer BHJ solar cells based on a pair of low-bandgap donors and acceptors, poly[4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-b;4,5-b′]dithiophene-2,6-diyl-alt-(4-(2-ethylhexyl)-3-fluorothieno[3,4-b]thiophene-)-2-carboxylate-2-6-diyl] (PBDTTT-EF-T) and poly{[N,N′-bis(2-octyldodecyl)-naphthalene-1,4,5,8-bis(dicarboximide)-2,6-diyl]-alt-5,5′-(2,2′-bithiophene)} [P(NDI2OD-T2); Polyera ActivInk™ N2200], respectively (Fig. 1a).7 The PBDTTT-EF-T/N2200 active layer having a thickness of as low as 100 nm can absorb over 90% of the incident sunlight at a wavelength of around 700 nm, resulting in an average JSC of 13 mA cm−2 and a PCE of 5.5%.7 Further, the generation and collection efficiencies of free charge carriers are each estimated to be 80% for the PBDTTT-EF-T/N2200 blend systems,7 which is as high as those of efficient polymer/fullerene BHJ solar cells.3 On the other hand, as shown in Fig. 1b, the combination of low-bandgap donor and acceptor polymers inevitably results in an weak light absorptivity in the visible region, owing to the intrinsic narrow absorption bandwidth of organic semiconductors. It should be noted that the polymer/polymer BHJ solar cells with the highest level of PCEs reported in the recent literature8–10 also face a comparable challenge: the external quantum efficiency (EQE) in the visible region is relatively low although the EQE in the NIR region shows excellent values higher than 60%. As a consequence, the PCE still continues to be lower than state-of-the-art polymer/fullerene solar cells.2 Therefore, further improvement in the PCE requires new design strategies that can complement the weak absorption in the visible range, while taking full advantage of the excellent photovoltaic conversion characteristics of these highly efficient low-bandgap donor/acceptor polymer blends.
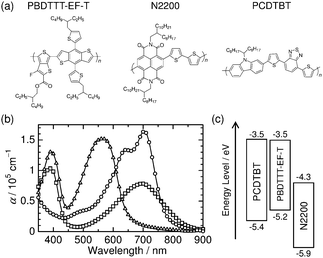 | ||
| Fig. 1 (a) Chemical structures of PBDTTT-EF-T, N2200, and PCDTBT. (b) Absorption coefficients α of PBDTTT-EF-T (circles), N2200 (squares), and PCDTBT (triangles) measured in neat film spin-coated from chlorobenzene. (c) Energy level diagram for neat films of each polymer. The HOMO levels were determined by photoelectron yield spectroscopy, whereas the LUMO levels were estimated by adding the optical energy gap calculated from the 0–0 transition to the HOMO energy (ESI,† Fig. S1). | ||
The ternary blend polymer solar cell, which is fabricated by blending a second polymer donor or a dye molecule into a binary blend of a polymer donor and a [6,6]-phenyl-C61-butyric acid methyl ester (PCBM) acceptor, is emerging as a fascinating alternative for broadening the absorption bandwidth of the photoactive layer.11,12 Owing to the advantage of overcoming the absorption limitation, the ternary blends based on wide- and low-bandgap donor polymers and PCBM have been investigated to achieve a PCE higher than that of the original binary polymer/PCBM solar cells.13,14 However, this concept has not been widely applied to enhance the efficiency of polymer/polymer BHJs,15 because such ternary blend systems require not only complementary absorption bands but also sufficient free carrier generation and effective charge transport properties of both constituent binary blends. Unlike the case with polymer/PCBM systems, there are few such ideal binary polymer/polymer blends that work well individually at different wavelength ranges, while they share the common polymer acceptor.
In this study, we have designed ternary blend all-polymer solar cells in which a wide-bandgap polymer, poly[N-9′-heptadecanyl-2,7-carbazole-alt-5,5-(4′,7′-di-2-thienyl-2′,1′,3′-benzothiadiazole)] (PCDTBT), is introduced as a second donor into the highly efficient low-bandgap PBDTTT-EF-T/N2200 blend. We found that PCDTBT can contribute to efficient photocurrent generation in the ternary blends even though photovoltaic performance is poor for PCDTBT/N2200 binary blends. As a result, we have developed high-performance ternary blend all-polymer solar cells with much better efficiency than the PBDTTT-EF-T/N2200 binary blend, with an enhanced EQE of 65–70% even at visible wavelengths, and with a PCE of 6.65%. The values are the highest reported for ternary blend all-polymer BHJ solar cells. Furthermore, these results provide the first example of enhanced performance in ternary blend all-polymer systems based on highly efficient polymer/polymer BHJ solar cells.
As shown in Fig. 1b, the ternary blends are capable of providing broad and strong absorption covering the range of wavelengths from the visible to NIR regions because PCDTBT has an absorption band in the visible region that is complementary to the other two polymers, PBDTTT-EF-T and N2200. The PBDTTT-EF-T/N2200 binary BHJ solar cells were fabricated according to the method described in the previous report and used as a reference for the ternary blends.7 The PBDTTT-EF-T/N2200 active layer was spin-coated from a chlorobenzene solution onto poly(3,4-ethylenedioxythiophene):poly(4-styrenesulfonate)-coated (PEDOT:PSS-coated) indium–tin-oxide (ITO) substrates. The weight ratio of PBDTTT-EF-T to N2200 was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the film thickness of the PBDTTT-EF-T/N2200 blend was optimized at ∼100 nm.7 In the case of the PBDTTT-EF-T/N2200/PCDTBT ternary BHJ solar cells, PCDTBT was added to the PBDTTT-EF-T/N2200 binary blend, such that the weight fraction of PCDTBT in the ternary blends ranged from 0 to 30 wt%. Here, the PBDTTT-EF-T:N2200 weight ratio remained constant at 1
1 and the film thickness of the PBDTTT-EF-T/N2200 blend was optimized at ∼100 nm.7 In the case of the PBDTTT-EF-T/N2200/PCDTBT ternary BHJ solar cells, PCDTBT was added to the PBDTTT-EF-T/N2200 binary blend, such that the weight fraction of PCDTBT in the ternary blends ranged from 0 to 30 wt%. Here, the PBDTTT-EF-T:N2200 weight ratio remained constant at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
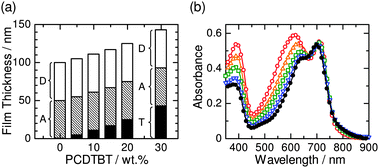
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the absorbances of PBDTTT-EF-T and N2200 were adjusted to the same value as that of the PBDTTT-EF-T/N2200 binary blend. Therefore, as shown in Fig. 2a, the thickness of the ternary blend active layer increases with an increase in the amount of PCDTBT. The absorption spectra of the PBDTTT-EF-T/N2200 binary and PBDTTT-EF-T/N2200/PCDTBT ternary blends are shown in Fig. 2b; the absorbance in the visible region at around 400 nm and 450–650 nm increased upon the addition of PCDTBT, whereas the absorbances of both PBDTTT-EF-T and N2200 at wavelength longer than 670 nm remained the same. As controls, the PCDTBT/N2200 binary and PBDTTT-EF-T/PCDTBT binary BHJ solar cells were also fabricated by spin-coating from chlorobenzene solutions onto the PEDOT:PSS films. The weight ratio of PCDTBT to N2200 and that of PBDTTT-EF-T to PCDTBT was 1
1, and the absorbances of PBDTTT-EF-T and N2200 were adjusted to the same value as that of the PBDTTT-EF-T/N2200 binary blend. Therefore, as shown in Fig. 2a, the thickness of the ternary blend active layer increases with an increase in the amount of PCDTBT. The absorption spectra of the PBDTTT-EF-T/N2200 binary and PBDTTT-EF-T/N2200/PCDTBT ternary blends are shown in Fig. 2b; the absorbance in the visible region at around 400 nm and 450–650 nm increased upon the addition of PCDTBT, whereas the absorbances of both PBDTTT-EF-T and N2200 at wavelength longer than 670 nm remained the same. As controls, the PCDTBT/N2200 binary and PBDTTT-EF-T/PCDTBT binary BHJ solar cells were also fabricated by spin-coating from chlorobenzene solutions onto the PEDOT:PSS films. The weight ratio of PCDTBT to N2200 and that of PBDTTT-EF-T to PCDTBT was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the film thickness of these binary blends was adjusted to be ∼100 nm.
1 and the film thickness of these binary blends was adjusted to be ∼100 nm.
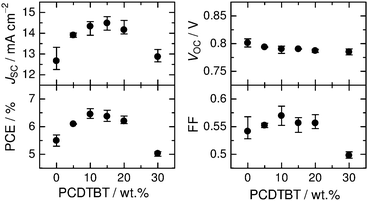
Fig. 3 shows the dependence of the device performance of the PBDTTT-EF-T/N2200/PCDTBT ternary blend solar cells on the amount of PCDTBT loading. The PBDTTT-EF-T/N2200 binary blend used as a reference device gave an average PCE of 5.49% with a JSC of 12.7 mA cm−2, an open-circuit voltage (VOC) of 0.801 V, and a fill factor (FF) of 0.542. It is clear from this figure that JSC is enhanced significantly with an increase in the amount of PCDTBT and it reaches 14.48 mA cm−2 for the ternary blend containing 15 wt% PCDTBT. The value of VOC was nearly constant at around 0.80 V and the FF values were as high as 0.54–0.57, regardless of the amount of PCDTBT in the range of 0–20 wt%. As a result, the PCE increased in the same fashion as JSC, with maximum values achieved at 10–15 wt% PCDTBT. The improvement in PCE is thus primarily due to the increase in JSC from the binary device. On the other hand, PCDTBT loadings of 30 wt% have a detrimental effect on the device performance, with decreases in both JSC and FF and consequently, PCE.
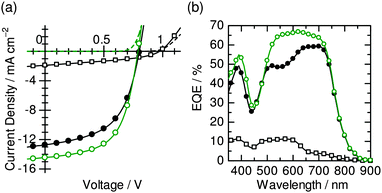
Fig. 4 shows the current density–voltage (J–V) curves and the EQE spectrum of the PBDTTT-EF-T/N2200/PCDTBT ternary (open circles) BHJ solar cell with the best PCE of 6.65% under AM 1.5G illumination with an intensity of 100 mW cm−2. The best photovoltaic performance was obtained for the ternary device with 10 wt% PCDTBT; the device parameters were a JSC of 14.4 mA cm−2, an VOC of 0.790 V, and a FF of 0.583. For comparison, J–V curves and EQE spectra for the PBDTTT-EF-T/N2200 binary (solid circles) and PCDTBT/N2200 binary (open squares) blends are also shown in the figure, and the photovoltaic parameters of these devices are summarized in Table 1. Herein, we note that the device performance of the PBDTTT-EF-T/PCDTBT binary blend was much poorer than that of the PCDTBT/N2200 binary blend, with a JSC of 0.118 mA cm−2 and a PCE of 0.042% (ESI,† Fig. S2), indicating that PBDTTT-EF-T/PCDTBT heterojunctions are unsuitable for charge generation. The origin of the enhanced JSC for the ternary blends can be deduced from the EQE spectra. For the PBDTTT-EF-T/N2200 binary device, the EQEs were limited to 50% in the visible range from 500 to 600 nm. For the ternary device, the EQEs were improved noticeably mainly in the visible wavelengths, approaching values as high as 65–70%. Improvement in the EQEs at the PCDTBT absorption wavelength demonstrates that the increase in JSC for the ternary device is caused by the additional light absorption of PCDTBT. In addition, we note that the existence of EQEs higher than 60% at the NIR wavelengths reveals that the photovoltaic conversion through direct light absorption by both PBDTTT-EF-T and N2200 host polymers remained as excellent as that of the individually optimized PBDTTT-EF-T/N2200 binary blend, which is an essential prerequisite for improving overall device efficiency.
| Devicea | J SC (mA cm−2) | V OC (V) | FF | PCE (%) | J SC (calc.) (mA cm−2) | Error (%) | |
|---|---|---|---|---|---|---|---|
| a The values are the photovoltaic parameters of solar cells with the highest PCEs. Averaged values over at least 14 devices are shown in parentheses. | |||||||
| Ternary | PBDTTT-EF-T/N2200/PCDTBT | 14.4 (14.3 ± 0.18) | 0.790 (0.790 ± 0.00) | 0.583 (0.570 ± 0.01) | 6.65 (6.45 ± 0.11) | 14.7 | 2.1 |
| Binary | PBDTTT-EF-T/N2200 | 12.4 (12.7 ± 0.24) | 0.806 (0.801 ± 0.00) | 0.568 (0.542 ± 0.01) | 5.70 (5.49 ± 0.10) | 12.8 | 3.2 |
| Binary | PCDTBT/N2200 | 2.00 (1.76 ± 0.11) | 0.970 (0.959 ± 0.06) | 0.397 (0.370 ± 0.01) | 0.77 (0.634 ± 0.06) | 1.94 | 3.0 |
As shown in Fig. 5, the photoluminescence (PL) spectrum of PCDTBT overlaps well with the absorption spectra of both PBDTTT-EF-T and N2200, giving a Förster radius (R0) of 3.5 nm for PCDTBT to PBDTTT-EF-T and 3.3 nm for PCDTBT to N2200 (ESI,† Fig. S3).16 Therefore, in addition to the enhanced light absorption by PCDTBT, in the ternary blends, resonant Förster energy transfer should occur from the wide-bandgap visible polymer PCDTBT to the low-bandgap NIR host polymers, PBDTTT-EF-T and N2200, after the light-absorption by PCDTBT.14,17 The energy transfer enables long-range transport of PCDTBT excitons directly to both PBDTTT-EF-T and N2200. To unravel the mechanism whereby our ternary blend system functioned so beautifully, we focus on the charge generation from the excited states of PBDTTT-EF-T and N2200 even when light is absorbed by PCDTBT because PCDTBT excitons should be collected to PBDTTT-EF-T and N2200 by long-range exciton transport as mentioned above. Indeed, the PL of PCDTBT was completely quenched in the ternary blends regardless of the loading amount of PCDTBT ranging from 0 to 30 wt%, suggesting that all of the PCDTBT excitons are utilized for the charge generation (ESI,† Fig. S4). Firstly, the PBDTTT-EF-T excited state is not quenched at the interface with PCDTBT, owing to the negligible energy offset between the lowest unoccupied molecular orbital (LUMO) levels of PBDTTT-EF-T and PCDTBT (Fig. 1c and ESI,† Fig. S5a, b). The non-quenching of PBDTTT-EF-T excitons at the heterojunction with PCDTBT is consistent with the result of negligible JSC values for the PBDTTT-EF-T/PCDTBT binary device (ESI,† Fig. S2). Therefore, PBDTTT-EF-T excitons, which are generated both directly by the NIR-light absorption and indirectly through the energy transfer from PCDTBT, can be converted to free charge carriers at the PBDTTT-EF-T/N2200 interface as efficiently as those in the case of the PBDTTT-EF-T/N2200 binary blends. The holes and electrons are transported through the PBDTTT-EF-T and N2200 networks, respectively, to the electrodes. Secondly, the N2200 excited state is quenched to form charges at the interfaces with PCDTBT, owing to an energy offset of 0.5 eV between the highest occupied molecular orbital (HOMO) levels of N2200 and PCDTBT (Fig. 1c). Here, the hole transfer from the N2200 excited state to PCDTBT will proceed in competition with charge generation at the other PBDTTT-EF-T/N2200 interface. The energy diagram suggests that the resulting holes on PCDTBT at the PCDTBT/N2200 interfaces can migrate towards PBDTTT-EF-T, owing to the preferred cascade alignment of the HOMO energy levels of PCDTBT and PBDTTT-EF-T (Fig. 1c). Therefore, they can be transported as holes of PBDTTT-EF-T through the high-hole-mobility PBDTTT-EF-T networks7 to the electrode rather than being energetically trapped in relatively low-hole-mobility PCDTBT domains.18 Consequently, the quenching of N2200 excitons at the PCDTBT/N2200 interface does not necessarily result in hole trapping but contributes to photocurrent generation. The efficient collection of PCDTBT excitons by long-range resonant energy transfer and the rational alignment of the HOMO–LUMO energy levels of PCDTBT that minimizes quenching losses of both PBDTTT-EF-T and N2200 excitons are keys for improving the EQEs in the visible region while retaining the excellent EQE values at the NIR wavelengths.
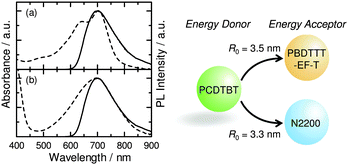 | ||
| Fig. 5 (a) The absorption spectrum of PBDTTT-EF-T neat film (broken line) and the PL spectrum of PCDTBT neat film (solid line). (b) The absorption spectrum of N2200 neat film (broken line) and the PL spectrum of PCDTBT neat film (solid line). The strong overlap between the PCDTBT PL and the absorption bands of PBDTTT-EF-T and N2200 gave a Förster radius (R0) of 3.5 nm and 3.3 nm, respectively (ESI,† Fig. S3) | ||
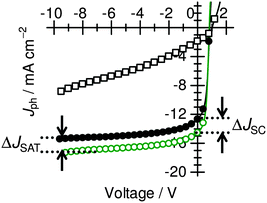
To confirm this scenario, we further examine the dependence of the photocurrent on the applied reverse (negative) voltage in the ternary blends with the best PCE. Fig. 6 shows the net photocurrent density (Jph) of the PBDTTT-EF-T/N2200/PCDTBT ternary (open circles), PBDTTT-EF-T/N2200 binary (solid circles), and PCDTBT/N2200 binary (open squares) BHJ solar cells under AM 1.5G illumination at 100 mW cm−2. Here, Jph is given by Jph = JL − JD, where JL and JD are the current densities under illumination and in the dark, respectively. It is worth noting that the PCDTBT/N2200 binary blend showed noticeable dependence of Jph on the applied reverse voltage, suggesting poor efficiency of either charge dissociation (dissociation of bound electron–hole pairs) into free carriers or charge carrier transport, or both.19 On the other hand, the PBDTTT-EF-T/N2200/PCDTBT ternary blend showed a weak dependence of Jph on the applied reverse voltage, and the Jph was saturated at reverse voltage higher than −8 V just as in the case of the PBDTTT-EF-T/N2200 binary blend,7 suggesting that all of the photogenerated free charge carriers in the devices were collected at the reverse voltage higher than −8 V.20 The increase in the reverse saturation photocurrent density Jph,sat (ΔJSAT) was 1.81 mA cm−2, which agrees well with the increase in JSC (ΔJSC) of 1.72 mA cm−2. This agreement indicates that the additional free charge carriers resulting from the PCDTBT light absorption are free from bimolecular recombination loss and hence are efficiently collected under short circuit conditions. The overall charge collection efficiencies under short-circuit conditions, which are defined as the ratio of JSC to Jph,sat, were estimated to be as high as 84% in the ternary blend and 83% in the binary blend. These results demonstrate that the charge collection in the PBDTTT-EF-T/N2200/PCDTBT ternary BHJ solar cells can be as efficient as in the PBDTTT-EF-T/N2200 binary BHJ solar cells even though poor charge dissociation and/or transport is expected from the PCDTBT/N2200 binary BHJ solar cells. In other words, the PBDTTT-EF-T/N2200/PCDTBT ternary BHJ solar cell functioned well by taking full advantage of the excellent free carrier generation and transport capabilities of the PBDTTT-EF-T/N2200 binary BHJ.
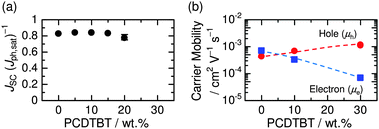
Finally, we examine the dependence of JSC/Jph,sat and charge carrier mobilities on the amount of PCDTBT loading. As shown in Fig. 7a, we found that the overall charge collection efficiency under short circuit conditions remained close to 80% even for the ternary blend containing 20 wt% PCDTBT. Fig. 7b shows μh and μe determined using the space-charge-limited current (SCLC) method with the Mott-Gurney equation.21 The μh increased from 4.3 × 10−4 cm2 V−1 s−1 in the PBDTTT-EF-T/N2200 binary blend to 1.3 × 10−3 cm2 V−1 s−1 in the ternary blend containing 30 wt% PCDTBT, which is much larger than that of PCDTBT neat film (on the order of 10−5 cm2 V−1 s−1).18 These results support our proposed mechanism that the bottleneck of charge-transport of the PCDTBT/N2200 binary device is overcome by energetically-feasible hole transfer from PCDTBT to PBDTTT-EF-T. On the other hand, the μe was found to decrease by an order of magnitude from 7.0 × 10−4 cm2 V−1 s−1 in the binary blend to 6.9 × 10−5 cm2 V−1 s−1 in the ternary blend containing 30 wt% PCDTBT, resulting in an imbalance between hole and electron mobilities. As shown in Fig. 3, the decrease in JSC and FF was observed for the ternary blend containing 30 wt% PCDTBT. Such a detrimental effect may be attributed to reduced charge collection efficiency caused by the increased film thickness of ∼150 nm and the decreased electron mobility.
As shown in Fig. 4 and 6, the second polymer donor PCDTBT forms BHJs with N2200 that exhibit limited photovoltaic performance and different VOC values from the PBDTTT-EF-T/N2200 binary blend. However, our ternary blends composed of 0–20 wt% PCDTBT improved the PCE while retaining similar values of JSC/Jph,sat, VOC, and FF to those of the PBDTTT-EF-T/N2200 host binary blend. The compositional dependence strongly suggests that PCDTBT contributed to the photocurrent generation as a visible sensitizer through efficient energy transfer for both PBDTTT-EF-T and N2200 rather than as a PCDTBT/N2200 device working in parallel with the PBDTTT-EF-T/N2200.11 In this case, PCDTBT absorbs visible light but relies on both PBDTTT-EF-T and N2200 host polymers to generate and transport free charge carriers. As a result, improvement in the PCE can be achieved by taking full advantage of the excellent photovoltaic conversion characteristics of the PBDTTT-EF-T/N2200 binary blend.
Conclusions
We have demonstrated that the PCE of low-bandgap PBDTTT-EF-T/N2200 binary BHJ solar cells can be improved by introducing wide-bandgap PCDTBT to complement the weak absorption at visible wavelengths. For the ternary blend all-polymer BHJ solar cell containing 10 wt% PCDTBT, the EQEs at visible wavelengths were successfully increased up to 65–70%, and a PCE as high as 6.65% was obtained. These values are the highest reported for ternary blend all-polymer BHJ solar cells. In the ternary blends, PCDTBT excitons can be transported directly to both PBDTTT-EF-T and N2200 through long-range resonant Förster energy transfer, contributing to the photocurrent generation as a visible sensitizer. Consequently, light absorption by PCDTBT can lead to an increase in JSC, by taking advantage of the excellent free carrier generation and transport characteristics of the PBDTTT-EF-T/N2200 binary blends. Our results demonstrate that the use of ternary blends composed of a wide-bandgap polymer along with an efficient low-bandgap donor/acceptor polymer blend is an effective strategy for achieving more efficient all-polymer BHJ solar cells. This approach should be applicable to the recently reported low-bandgap donor/acceptor polymer BHJ solar cells with the highest level of PCEs. The ternary blend all-polymer BHJs open a new avenue for accelerating improvement in the efficiency of non-fullerene thin-film polymer solar cells.Acknowledgements
This work was supported by the JSPS KAKENHI Grant Number 26288060 and the CREST program of the Japan Science and Technology Agency.Notes and references
- R. Søndergaard, M. Hösel, D. Angmo, T. T. Larsen-Olsen and F. C. Krebs, Mater. Today, 2012, 15, 36 CrossRef CAS; S. B. Darling and F. You, RSC Adv., 2013, 3, 17633 RSC.
- Z. He, C. Zhong, S. Su, M. Xu, H. Wu and Y. Cao, Nat. Photonics, 2012, 6, 591 Search PubMed; Z. He, B. Xiao, F. Liu, H. Wu, Y. Yang, S. Xiao, C. Wang, T. P. Russell and Y. Cao, Nat. Photonics, 2015, 9, 174 CrossRef CAS; Y. Liu, J. Zhao, Z. Li, C. Mu, W. Ma, H. Hu, K. Jiang, H. Lin, H. Ade and H. Yan, Nat. Commun., 2014, 5, 5293 CrossRef PubMed; M. A. Green, K. Emery, Y. Hishikawa, W. Warta and E. D. Dunlop, Prog. Photovolt.: Res. Appl., 2015 DOI:10.1002/pip.2728.
- L. Bian, E. Zhu, J. Tang, W. Tang and F. Zhang, Prog. Polym. Sci., 2012, 37, 1292 CrossRef CAS; C. J. Brabec, S. Gowrisanker, J. J. M. Halls, D. Laird, S. Jia and S. P. Williams, Adv. Mater., 2010, 22, 3839 CrossRef PubMed; G. Dennler, M. C. Scharber and C. J. Brabec, Adv. Mater., 2009, 21, 1323 CrossRef.
- A. Facchetti, Mater. Today, 2013, 16, 123 CrossRef CAS.
- X. Zhan, Z. Tan, B. Domercq, Z. An, X. Zhang, S. Barlow, Y. Li, D. Zhu, B. Kippelen and S. R. Marder, J. Am. Chem. Soc., 2007, 129, 7246 CrossRef CAS PubMed; H. Yan, Z. Chen, Y. Zheng, C. Newman, J. R. Quinn, F. Dötz, M. Kastler and A. Facchetti, Nature, 2009, 457, 679 CrossRef PubMed; E. Zhou, J. Cong, Q. Wei, K. Tajima, C. Yang and K. Hashimoto, Angew. Chem., Int. Ed., 2011, 50, 2799 CrossRef PubMed; X. Zhao and X. Zhan, Chem. Soc. Rev., 2011, 40, 3728 RSC.
- L. Ye, X. Jiao, M. Zhou, S. Zhang, H. Yao, W. Zhao, A. Xia, H. Ade and J. Hou, Adv. Mater., 2015, 27, 6046 CrossRef CAS PubMed.
- D. Mori, H. Benten, I. Okada, H. Ohkita and S. Ito, Energy Environ. Sci., 2014, 7, 2939 CAS.
- J. W. Jung, J. W. Jo, C. C. Chueh, F. Liu, W. H. Jo, T. P. Russell and A. K.-Y. Jen, Adv. Mater., 2015, 27, 3310 CrossRef CAS PubMed; Y. J. Hwang, T. Earmme, B. A. E. Courtright, F. N. Eberle and S. A. Jenekhe, J. Am. Chem. Soc., 2015, 137, 4424 CrossRef PubMed; C. Lee, H. Kang, W. Lee, T. Kim, K. H. Kim, H. Y. Woo, C. Wang and B. J. Kim, Adv. Mater., 2015, 27, 2466 CrossRef PubMed; T. Kim, J. H. Kim, T. E. Kang, C. Lee, H. Kang, M. Shin, C. Wang, B. Ma, U. Jeong, T. S. Kim and B. J. Kim, Nat. Commun., 2015, 6, 8547 CrossRef PubMed.
- Y. J. Hwang, B. A. E. Courtright, A. S. Ferreira, S. H. Tolbert and S. A. Jenekhe, Adv. Mater., 2015, 27, 4578 CrossRef CAS PubMed.
- C. Mu, P. Liu, W. Ma, K. Jiang, J. Zhao, K. Zhang, Z. Chen, Z. Wei, Y. Yi, J. Wang, S. Yang, F. Huang, A. Facchetti, H. Ade and H. Yan, Adv. Mater., 2014, 26, 7224 CrossRef CAS PubMed.
- L. Lu, M. A. Kelly, W. You and L. Yu, Nat. Photonics, 2015, 9, 491 CrossRef CAS; B. M. Savoie, S. Dunaisky, T. J. Marks and M. A. Ratner, Adv. Energy Mater., 2014, 1400891 Search PubMed; T. Ameri, P. Khoram, J. Min and C. J. Brabec, Adv. Mater., 2013, 25, 4245 CrossRef PubMed; L. Yang, L. Yan and W. You, J. Phys. Chem. Lett., 2013, 4, 1802 CrossRef PubMed; Y. C. Chen, C. Y. Hsu, R. Y. Y. Lin, K. C. Ho and J. T. Lin, ChemSusChem, 2013, 6, 20 CrossRef PubMed; P. P. Khlyabich, B. Burkhart, A. E. Rudenko and B. C. Thompson, Polymer, 2013, 54, 5267 CrossRef.
- S. Honda, H. Ohkita, H. Benten and S. Ito, Chem. Commun., 2010, 46, 6596 RSC; S. Honda, T. Nogami, H. Ohkita, H. Benten and S. Ito, ACS Appl. Mater. Interfaces, 2009, 1, 804 Search PubMed; Y. Wang, B. Zheng, Y. Tamai, H. Ohkita, H. Benten and S. Ito, J. Electrochem. Soc., 2014, 161, D3093 CrossRef CAS; H. Xu, T. Wada, H. Ohkita, H. Benten and S. Ito, Sci. Rep., 2015, 5, 9321 CrossRef PubMed; H. Xu, H. Ohkita, Y. Tamai, H. Benten and S. Ito, Adv. Mater., 2015, 27, 5868 CrossRef PubMed.
- Y. Yang, W. Chen, L. Dou, W. H. Chang, H. S. Duan, B. Bob, G. Li and Y. Yang, Nat. Photonics, 2015, 9, 190 CrossRef CAS; K. Yao, Y. X. Xu, F. Li, X. Wang and L. Zhou, Adv. Opt. Mater., 2015, 3, 321 CrossRef; T. Ameri, J. Min, N. Li, F. Machui, D. Baran, M. Forster, K. J. Schottler, D. Dolfen, U. Scherf and C. J. Brabec, Adv. Energy Mater., 2012, 2, 1198 CrossRef; L. Yang, H. Zhou, S. C. Price and W. You, J. Am. Chem. Soc., 2012, 134, 5432 CrossRef PubMed; P. P. Khlyabich, B. Burkhart and B. C. Thompson, J. Am. Chem. Soc., 2012, 134, 9074 CrossRef PubMed.
- Y. Wang, H. Ohkita, H. Benten and S. Ito, Phys. Chem. Chem. Phys., 2015, 17, 27217 RSC; V. Gupta, V. Bharti, M. Kumar, S. Chand and A. J. Heeger, Adv. Mater., 2015, 27, 4398 CrossRef CAS PubMed.
- Y. J. Hwang, B. A. E. Courtright and S. A. Jenekhe, MRS Commun., 2015, 5, 229 CrossRef CAS.
- T. Förster, Discuss. Faraday Soc., 1959, 27, 7 RSC; I. B. Berlman, Energy Transfer Parameters of Aromatic Compounds, Academic Press, New York and London, 1973, pp. 27–32 Search PubMed.
- Y. Liu, M. A. Summers, C. Edder, J. M. J Fréchet and M. D. McGehee, Adv. Mater., 2005, 17, 2960 CrossRef CAS; S. R. Scully, P. B. Armstrong, C. Edder, J. M. J. Fréchet and M. D. McGehee, Adv. Mater., 2007, 19, 2961 CrossRef.
- K. K. H. Chan, S. W. Tsang, H. K. H. Lee, F. So and S. K. So, Org. Electron., 2012, 13, 850 CrossRef CAS.
- M. M. Mandoc, W. Veurman, L. J. A. Koster, B. Boer and P. W. M. Blom, Adv. Funct. Mater., 2007, 17, 2167 CrossRef CAS.
- V. D. Mihailetchi, L. J. A. Koster, P. W. M. Blom, C. Melzer, B. Boer, J. K. J. Duren and R. A. J. Janssen, Adv. Funct. Mater., 2005, 15, 795 CrossRef CAS; V. D. Mihailetchi, H. Xie, B. Boer, L. J. A. Koster and P. W. M. Blom, Adv. Funct. Mater., 2006, 16, 699 CrossRef; P. W. M. Blom, V. D. Mihailetchi, L. J. A. Koster and D. E. Markov, Adv. Mater., 2007, 19, 1551 CrossRef.
- G. G. Malliaras, J. R. Salem, P. J. Brock and C. Scott, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 58, R13411 CrossRef CAS; Y. Shen, A. R. Hosseini, M. H. Wong and G. G. Malliaras, ChemPhysChem, 2004, 5, 16 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ee03460d |
| This journal is © The Royal Society of Chemistry 2016 |