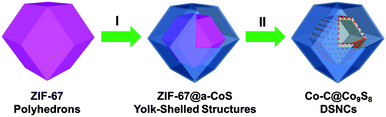
Metal–organic-framework-engaged formation of Co nanoparticle-embedded carbon@Co9S8 double-shelled nanocages for efficient oxygen reduction†
Han
Hu
a,
Lei
Han
a,
Mengzhou
Yu
b,
Zhiyu
Wang
*b and
Xiong Wen (David)
Lou
*a
aSchool of Chemical and Biomedical Engineering, Nanyang Technological University, 62 Nanyang Drive, Singapore, 637459, Singapore. E-mail: xwlou@ntu.edu.sg; davidlou88@gmail.com; Web: http://www.ntu.edu.sg/home/xwlou/
bCarbon Research Laboratory, School of Chemical Engineering, State Key Lab of Fine Chemicals, Dalian University of Technology, Dalian, Liaoning 116024, China. E-mail: zywang@dlut.edu.cn
First published on 17th November 2015
Abstract
Hollow nanostructures with a complex interior and superb structural tenability offer great advantages for constructing advanced catalysts. Herein, we report the designed synthesis of novel Co nanoparticle-embedded carbon@Co9S8 double-shelled nanocages (Co-C@Co9S8 DSNCs) by a metal–organic-framework-engaged strategy. Uniform zeolitic imidazolate framework (ZIF-67)@amorphous CoS yolk–shelled structures are first fabricated and then converted to Co-C@Co9S8 DSNCs by thermal annealing in N2 flow. The Co-C nanocages inside Co9S8 shells function as the active centers for the oxygen reduction reaction (ORR). The Co9S8 shells prevent the Co-C active centers from aggregation while acting as nanoreactors. As a result, the Co-C@Co9S8 DSNCs exhibit excellent performance for the ORR in terms of low over-potential, high current density, excellent stability and methanol tolerance capability.
The ever-growing consumption of fossil fuels and increasing global pollution, coupled with the depletion of natural resources, have boosted great interest in seeking renewable and sustainable energy sources. Fuel cells, including proton-exchange membrane fuel cells and direct methanol fuel cells, are a very appealing device for energy conversion targeted at large-scale energy applications such as electric vehicles (EVs).1–3 The development of highly active and durable catalysts for sluggish oxygen reduction reaction (ORR) is of great significance for commercial applications of fuel cells. To date, Pt and its alloys have shown the highest ORR activity in alkaline media.4,5 However, their practical applications have been hindered by a series of difficulties including the prohibitive cost, scarcity in supply, poor durability and undesirable crossover effect.2,5 Recently, hybrid nanostructures composed of non-precious metals embedded in a carbon matrix have emerged as promising low-cost substitutes for Pt-based catalysts for the ORR.1,6–8 In such catalysts, the interaction of the metal species with carbon efficiently reduces the local work function for oxygen adsorption on the carbon surface by virtue of promoted electron transfer from metal atoms to carbon.7 The presence of a carbon matrix in turn prevents the aggregation and leaching of the metal component during the reaction. As a result, this type of catalyst exhibits significantly enhanced electrocatalytic activity towards the ORR, which is even comparable to Pt-based catalysts.
Another efficient approach towards highly active catalysts is to confine catalytic materials within a permeable but robust shell, between which nanoscale free voids are formed to induce a localized high instantaneous concentration for driving fast heterogeneous catalysis.9,10 Hollow nanoparticles with complex interiors and superb structural tenability, e.g., the so-called yolk–shelled structures or multishelled nanocages, reasonably offer great advantages because of their potential in better controlling the local chemical microenvironment and multiple interface reactions for enhanced catalytic activity.11 So far, the catalyst particles of various materials such as noble metals and metal oxides have been successfully introduced into porous shells of different compositions by a straightforward layer-by-layer templating strategy.9,12–14 However, the confinement of catalytic centers with complex architecture and compositions into nanoscale hollow shells has remained a significant challenge due to the difficulty in delicate control over chemical reactions and material compatibility inside the confined nanospace.
Metal–organic frameworks (MOFs), formed by the assembly of metal ions/clusters with electron-donating organic ligands, are very appealing precursors for constructing nanostructured metal (oxide)/carbon and their nanocomposites by taking advantage of the unique thermal behavior and chemical reactivity.15–21 For example, porous carbon or carbon/metal polyhedrons have been synthesized by directly annealing zeolitic imidazolate frameworks (ZIFs), a sub-family of MOFs.22–24 Yamauchi et al. also reported the synthesis of N-doped carbon@graphitic carbon core–shelled structures by annealing their ZIF-8@ZIF-67 counterparts.25 Recently, we have developed a series of unique nanostructures ranging from Fe-based nanoboxes and MoCx nanooctahedrons to complex Co3O4/NiCo2O4 double-shelled nanocages and NiSx nanoframes from various MOFs.17,26–28 Herein, we report a novel strategy for the synthesis of Co nanoparticle-embedded carbon@Co9S8 double-shelled nanocages (Co-C@Co9S8 DSNCs) by using a Co-based ZIF (ZIF-67) as the precursor. This method involves the initial formation of ZIF-67@amorphous-CoS (ZIF-67@a-CoS) yolk–shelled structures and subsequent thermal annealing at high temperature in N2 flow. In Co-C@Co9S8 DSNCs, the inner Co-C hollow shells act as the active centers to efficiently catalyze the ORR, while the outer permeable Co9S8 shells help to build the nanoreactors around the Co-C catalysts and prevent them from deactivation. Their synergy endows Co-C@Co9S8 DSNCs with high catalytic activity towards ORR in terms of low over-potential, high current density, excellent durability and methanol tolerance capability.
The synthesis procedure of Co-C@Co9S8 DSNCs is schematically depicted in Fig. 1. Well-defined ZIF-67 polyhedrons are first synthesized by a precipitation method in the presence of a cobalt salt and 2-methylimidazole in methanol at room temperature. The as-formed ZIF-67 particles are homogenously dispersed in an ethanol solution of thioacetamide (TAA), followed by refluxing at elevated temperature. During this process, an amorphous CoS shell is formed around the scaffold of each ZIF-67 particle by the reaction between ZIF-67 and sulfide ions released by the decomposition of TAA.29 By precisely tuning the reaction time, a variety of hollow nanostructures such as amorphous CoS nanocages (a-CoS NCs) and ZIF-67@a-CoS yolk–shelled structures could be fabricated. Afterwards, thermal annealing is applied in inert gas to convert the amorphous CoS to Co9S8 shells. Interestingly, the ZIF-67 cores inside the CoS shells could be simultaneously converted to Co nanoparticle-embedded carbon nanocages (Co-C NCs) after annealing, whereas only solid Co-C particles with concave surfaces are made from pristine ZIF-67 polyhedrons under identical annealing conditions.23 As a result, Co-C@Co9S8 DSNCs with robust shells and complex interiors can be eventually obtained.
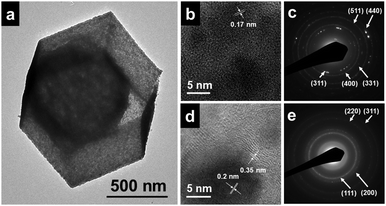
The structure of the products formed at different steps is examined by powder X-ray diffraction (XRD), field-emission scanning electron microscopy (FESEM), and transmission electron microscopy (TEM). The XRD pattern of ZIF-67 polyhedrons (Fig. S1a, see ESI†) shows typical diffraction peaks from (011), (002), (112) and (222) planes, being consistent with previous reports.29 FESEM and TEM analyses confirm the formation of a polyhedral morphology with high uniformity, smooth surface and average size of about 800 nm (Fig. S1b–d, see ESI†). After a precisely controlled reaction with TAA, XRD analysis indicates the residue of ZIF-67 cores, while no signals from CoS shells are detected due to their amorphous nature (Fig. S2a, see ESI†). FESEM characterization shows that the sample well inherits the polyhedral morphology and uniform dimensions of ZIF-67 particles but with a rough surface (Fig. 2a and b). The formation of yolk–shelled structures can be examined directly by FESEM for cracked cages (Fig. S2b, see ESI†). TEM studies further identify the presence of ZIF-67 cores inside a-CoS shells with a thickness of about 30–50 nm, between which a gap of about 100 nm is formed due to faster ZIF-67 dissolution to a-CoS deposition (Fig. 2c, Fig. S2c and d, see ESI†). Despite being very thin and amorphous, a-CoS shells possess good structural stability and are found to be robust enough to withstand thermal annealing at 600 °C. After annealing, they are converted to a Co9S8 phase (JCPDS No. 02-1459), as characterized by the distinct peaks at 29.7, 31.1, 39.5 and 51.9° from (311), (222), (331) and (440) planes in the XRD pattern, respectively (Fig. S3a, see ESI†). The signals from the ZIF-67 phase entirely disappear as a result of complete conversion to Co nanoparticles-embedded carbon. A panoramic FESEM image reveals that this sample consists entirely of uniform polyhedral particles without aggregation as shown in Fig. 2d (also Fig. S3b, see ESI†). The FESEM image (Fig. S3c, see ESI†) of a cracked particle clearly visualizes the formation of the nanocages inside Co9S8 shells. The walls of outer Co9S8 and inner Co-C shells are as thin as about 10–20 nm and 30–60 nm, respectively (Fig. 2e and f and Fig. S3d, see ESI†). The formation of Co-C hollow cages not only generates extra active sites on the catalyst surface, but also significantly shortens the diffusion pathway of the electrolyte, thereby being favourable for electrocatalytic reactions.30 To the best of our knowledge, the formation of hollow nanostructures by annealing ZIF-67 in inert gas has not been reported before.23
Fig. 3a shows that the outer shell is constructed from interconnected nanoparticles with high porosity. To investigate the structures of the outer shell and the inner shell in detail, the Co-C@Co9S8 DSNCs are broken by strong sonication to separate the inner and outer shells (Fig. S4, see ESI†). High-resolution TEM (HRTEM) characterization reveals that the outer shells are composed of Co9S8 nanoparticles with a small size of about 5 nm (Fig. 3b). The lattice fringes with a d-spacing of 0.17 nm are measured, corresponding to (440) planes of Co9S8. Selected-area electron diffraction (SAED) analysis gives a pattern of several concentric circles, implying the polycrystalline nature of the Co9S8 shells (Fig. 3c). For inner shells, typical lattice fringes of 0.2 nm and 0.35 nm can be observed, corresponding to (111) planes of cubic Co and (002) planes of carbon, respectively.23,25 With the closely surrounding carbon layer, the size of the in situ formed Co nanoparticles is limited to 3–10 nm. SAED analysis further confirms their formation by the concentric circle patterns (Fig. 3e).23 The incorporation of transition metal species is known to greatly reduce the adsorption free energy of O2 on the carbon surface by promoted electron transfer from carbon to oxygen, rendering enhanced ORR activity.7
In our work, the conversion from ZIF-67 cores to Co-C nanocages inside Co9S8 shells is interesting since the annealing of pristine ZIF-67 in inert gas usually generates solid Co-C nanocomposites (Fig. S5, see ESI†). We attribute this phenomenon to the difference in the surface characteristics of the pristine ZIF-67 and ZIF-67 cores inside the Co9S8 shells. Microscopic characterization reveals that the ZIF-67 polyhedrons have a highly homogenous structure with a smooth surface (Fig. S1c and d, see ESI†), which favours the transformation of ZIF-67 to solid Co-C nanocomposites via homogenous condensation. In contrast, the ZIF-67 cores inside Co9S8 shells feature a freshly exposed surface with high roughness caused by continuous dissolution in solution. As a result, porous Co-C shells would be preferentially formed around ZIF-67 cores at the initial stage of annealing, across which the gas generated by ZIF-67 decomposition is continuously released to leave the hollow interior behind. Eventually, Co-C nanocages could be formed inside Co9S8 shells with annealing.
The electrocatalytic activity of Co-C@Co9S8 DSNCs towards the ORR is evaluated by cyclic voltammetry (CV) tests conducted in an Ar- or O2-saturated 0.1 M KOH electrolyte at a scan rate of 10 mV s−1, as shown in Fig. 4a. A substantial reduction process is shown to occur at about 0.83 V (vs. reversible hydrogen electrode, RHE) in the O2-saturated 0.1 M KOH solution, whereas a featureless voltammogram is observed within the same voltage window in the Ar-saturated electrolyte. The onset potential is determined to be about 0.96 V (vs. RHE). Both the cathodic peak potential and onset potential of Co-C@Co9S8 DSNCs are very close to those of a commercial 20 wt% Pt/C catalyst (Fig. S6 and S7, see ESI†), suggesting relatively good electrocatalytic activity of Co-C@Co9S8 DSNCs for the ORR. The enhanced electrocatalytic activity of Co-C@Co9S8 DSNCs has been further confirmed by comparing the oxygen reduction performance with some newly developed catalysts where the activity of Co-C@Co9S8 DSNCs is superior to most of these structures (Fig. S8 and Table S1, see ESI†). To uncover the role of Co-C cores played in the ORR, CV analysis is applied for Co9S8 nanocages (Fig. S9 and S10, see ESI†) and Co-C polyhedrons (Fig. S11, see ESI†) synthesized by directly annealing ZIF-67 particles under the same conditions. The Co-C polyhedrons show similar features to Co-C@Co9S8 DSNCs with a reduction peak at 0.83 V and an onset potential of 0.96 V, whereas both values for the Co9S8 nanocages are negatively shifted. Apparently, the ORR activity of Co-C@Co9S8 DSNCs mainly originates from the inner Co-C cores.
To gain a deeper insight into the electrocatalytic activity and kinetics of Co-C@Co9S8 DSNCs towards the ORR, linear sweep voltammograms (LSVs) are recorded on a rotating disk electrode (RDE) at various rotating rates in the O2-saturated 0.1 M KOH electrolyte at a scan rate of 10 mV s−1 (Fig. 4b). The polarization curves show a sharp increase in current densities, and then the current density slowly increases when the potential is negatively shifted, which is typical for electrocatalysts with abundant mesopores (Fig. S12a, see ESI†).31,32 The diffusion-limiting current density increases with increasing rotating speed as a result of the shortened diffusion distance at high speed. Specifically, the limiting current density at a rotation speed of 1600 rpm reaches 4.5 mA cm−2 at 0.5 V, which is superior to reported ZIF-7 derived carbon, Co3S4/graphene and Co/CoO–graphene composites under the same conditions (Table S2, see ESI†).33–36 Among the three catalysts developed in this work, the Co-C@Co9S8 DSNCs show the highest limiting current density in a wide potential range (Fig. 4b, Fig. S10b and S11b, see ESI†). The Brunauer–Emmett–Teller (BET) surface area of these catalysts is measured (Fig. S12, see ESI†), where Co-C@Co9S8 DSNCs, Co-C polyhedrons and Co9S8 nanocages exhibit a BET surface area of 106.2, 275.9, and 29.2 m2 g−1, respectively. The Co-C@Co9S8 DSNCs have a smaller BET surface area but higher limiting current density than Co-C polyhedrons, further confirming the greatly enhanced activity for the ORR. The high activity of the Co-C@Co9S8 DSNCs could be ascribed to the formation of double-shelled hollow nanostructures, which play multiple roles: (i) they act as nanoreactors to confine the electrolyte with a higher instantaneous concentration for faster electrochemical reactions; (ii) they allow high exposure of active sites and fast accessibility to the reactant across large electrode–electrolyte interfaces; (iii) the presence of outer Co9S8 shells prevents Co-C catalysts from aggregation and leaching upon reaction. For Co-C polyhedrons with less active sites exposed, the current density is lower than that of Co-C@Co9S8 DSNCs under identical conditions, indicating the significance of hollow nanostructures in enhancing the electrocatalytic properties.
Fig. 4c shows the corresponding Koutecky–Levich (K–L) plots where good linearity over a wide potential range from 0.1 to 0.6 V can be observed, which is indicative of first-order reaction kinetics for the ORR with respect to the dissolved oxygen concentration.37 The electron transfer number (n) is calculated to be around 3.8, which confirms a four-electron-transfer route with OH− production at Co-C@Co9S8 DSNCs electrodes in alkaline medium.33,38,39 Besides the catalytic activity, the durability represents another crucial parameter for practical application of ORR catalysts. Therefore, chronoamperometric measurements at 0.5 V in O2-saturated 0.1 M KOH are performed to evaluate the stability of Co-C@Co9S8 DSNCs, as shown in Fig. 4d. The current–time (i–t) chronoamperometric response for the Co-C@Co9S8 DSNC electrode exhibits a very slow attenuation with high current retention of over 96% after 5 h, whereas the electrodes containing Co-C polyhedrons or Co9S8 nanocages show fast current loss of 10–20% under similar conditions. Strikingly, the durability of Co-C@Co9S8 DSNCs is also superior to the Pt/C electrode (Fig. 4d). The effect of methanol crossover of Co-C@Co9S8 DSNCs is then evaluated by CV measurement. A well-defined cathodic peak can be clearly observed in O2-saturated KOH solution containing methanol (KOH, 0.1 M; methanol, 3.0 M) without any activity deterioration (Fig. S13a, see ESI†), indicative of the remarkable methanol tolerance. In contrast, the cathodic peak of Pt/C for oxygen reduction is totally absent in the same methanol-containing electrolyte, while the peaks associated with methanol oxidation appear (Fig. S13b, see ESI†). Hence, the Co-C@Co9S8 DSNCs can deliver much higher catalytic selectivity for oxygen reduction against fuel oxidation than Pt/C for practical applications. Furthermore, the ORR performance of Co-C@Co9S8 DSNCs in acidic medium is also examined to show their versatility in electrocatalysis (Fig. S14, see ESI†). The CV tests conducted in O2-saturated 0.1 M HClO4 solution reveal the presence of a cathodic peak at 0.68 V (vs. RHE). The electron transfer number is determined to be around 3.9 by K–L tests over a potential range of 0.1–0.4 V. The durability of Co-C@Co9S8 DSNCs in acidic medium is evaluated at 0.4 V by chronoamperometric measurement, showing stable current retention of over 90% after 5 h, thanks to the protection of Co nanoparticles by the carbon matrix. The greatly enhanced electrochemical stability of Co-C@Co9S8 DSNCs is related to their unique double-shelled structure, which is more structurally robust than conventional hollow shells. The above results strongly evidence the great potential for the enhancement of ORR performance by engineering hollow nanostructured catalysts with rationally designed complex interiors and compositions.
Conclusions
In summary, Co-C@Co9S8 double-shelled nanocages (DSNCs) have been synthesized by a metal–organic-framework-engaged strategy. Electrochemical tests show that the inner Co nanoparticles-embedded carbon nanocages play the role of a catalytic center with extra active sites. The outer permeable Co9S8 shells allow fast accessibility of reactants to the active sites in solution while preventing them from aggregation and leaching out. As nanoreactors, these double-shelled Co-C@Co9S8 nanocages guarantee the confinement of reactants with a higher instantaneous concentration in the complex interior, leading to a higher driving force for the ORR. Working together, these structural merits allow Co-C@Co9S8 DSNCs to exhibit remarkable electrocatalytic activity, durability and methanol tolerance capability towards the ORR.Notes and references
- G. Wu, K. L. More, C. M. Johnston and P. Zelenay, Science, 2011, 332, 443 CrossRef CAS PubMed.
- L. M. Dai, Y. H. Xue, L. T. Qu, H. J. Choi and J. B. Baek, Chem. Rev., 2015, 115, 4823 CrossRef CAS PubMed.
- Y. Jiao, Y. Zheng, M. Jaroniec and S. Z. Qiao, Chem. Soc. Rev., 2015, 44, 2060 RSC.
- B. Y. Xia, H. B. Wu, X. Wang and X. W. Lou, Angew. Chem., Int. Ed., 2013, 52, 12337 CrossRef CAS PubMed.
- Y. Nie, L. Li and Z. D. Wei, Chem. Soc. Rev., 2015, 44, 2168 RSC.
- M. Lefevre, E. Proietti, F. Jaouen and J. P. Dodelet, Science, 2009, 324, 71 CrossRef CAS PubMed.
- D. H. Deng, L. Yu, X. Q. Chen, G. X. Wang, L. Jin, X. L. Pan, J. Deng, G. Q. Sun and X. H. Bao, Angew. Chem., Int. Ed., 2013, 52, 371 CrossRef CAS PubMed.
- S. H. Liu, Y. F. Dong, C. T. Zhao, Z. B. Zhao, C. Yu, Z. Y. Wang and J. S. Qiu, Nano Energy, 2015, 12, 578 CrossRef CAS.
- J. Liu, S. Z. Qiao, J. S. Chen, X. W. Lou, X. R. Xing and G. Q. Lu, Chem. Commun., 2011, 47, 12578 RSC.
- P. M. Arnal, M. Comotti and F. Schuth, Angew. Chem., Int. Ed., 2006, 45, 8224 CrossRef CAS PubMed.
- Z. Y. Wang, D. Y. Luan, C. M. Li, F. B. Su, S. Madhavi, F. Y. C. Boey and X. W. Lou, J. Am. Chem. Soc., 2010, 132, 16271 CrossRef CAS PubMed.
- L. Kuai, S. Z. Wang and B. Y. Geng, Chem. Commun., 2011, 47, 6093 RSC.
- C. Galeano, C. Baldizzone, H. Bongard, B. Spliethoff, C. Weidenthaler, J. C. Meier, K. J. J. Mayrhofer and F. Schuth, Adv. Funct. Mater., 2014, 24, 220 CrossRef CAS.
- C. J. Zhong and M. M. Maye, Adv. Mater., 2001, 13, 1507 CrossRef CAS.
- L. Zhang, H. B. Wu, S. Madhavi, H. H. Hng and X. W. Lou, J. Am. Chem. Soc., 2012, 134, 17388 CrossRef CAS PubMed.
- R. B. Wu, D. P. Wang, X. H. Rui, B. Liu, K. Zhou, A. W. K. Law, Q. Y. Yan, J. Wei and Z. Chen, Adv. Mater., 2015, 27, 3038 CrossRef CAS PubMed.
- L. Zhang, H. B. Wu and X. W. Lou, J. Am. Chem. Soc., 2013, 135, 10664 CrossRef CAS PubMed.
- N. L. Torad, M. Hu, S. Ishihara, H. Sukegawa, A. A. Belik, M. Imura, K. Ariga, Y. Sakka and Y. Yamauchi, Small, 2014, 10, 2096 CrossRef CAS PubMed.
- J. K. Sun and Q. Xu, Energy Environ. Sci., 2014, 7, 2071 CAS.
- B. Liu, H. Shioyama, T. Akita and Q. Xu, J. Am. Chem. Soc., 2008, 130, 5390 CrossRef CAS PubMed.
- T. Y. Ma, S. Dai, M. Jaroniec and S. Z. Qiao, J. Am. Chem. Soc., 2014, 136, 13925 CrossRef CAS PubMed.
- J. Shao, Z. Wan, H. Liu, H. Zheng, T. Gao, M. Shen, Q. Qu and H. Zheng, J. Mater. Chem. A, 2014, 2, 12194 CAS.
- W. Xia, J. H. Zhu, W. H. Guo, L. An, D. G. Xia and R. Q. Zou, J. Mater. Chem. A, 2014, 2, 11606 CAS.
- W. Chaikittisilp, M. Hu, H. J. Wang, H. S. Huang, T. Fujita, K. C. W. Wu, L. C. Chen, Y. Yamauchi and K. Ariga, Chem. Commun., 2012, 48, 7259 RSC.
- J. Tang, R. R. Salunkhe, J. Liu, N. L. Torad, M. Imura, S. Furukawa and Y. Yamauchi, J. Am. Chem. Soc., 2015, 137, 1572 CrossRef CAS PubMed.
- H. B. Wu, B. Y. Xia, L. Yu, X. Y. Yu and X. W. Lou, Nat. Commun., 2015, 6, 6512 CrossRef CAS PubMed.
- H. Hu, B. Y. Guan, B. Y. Xia and X. W. Lou, J. Am. Chem. Soc., 2015, 137, 5590 CrossRef CAS PubMed.
- X. Y. Yu, L. Yu, H. B. Wu and X. W. Lou, Angew. Chem., Int. Ed., 2015, 54, 5331 CrossRef CAS PubMed.
- Z. Jiang, W. J. Lu, Z. P. Li, K. H. Ho, X. Li, X. L. Jiao and D. R. Chen, J. Mater. Chem. A, 2014, 2, 8603 CAS.
- X. W. Lou, L. A. Archer and Z. C. Yang, Adv. Mater., 2008, 20, 3987 CrossRef CAS.
- Y. Zheng, Y. Jiao, J. Chen, J. Liu, J. Liang, A. Du, W. M. Zhang, Z. H. Zhu, S. C. Smith, M. Jaroniec, G. Q. Lu and S. Z. Qiao, J. Am. Chem. Soc., 2011, 133, 20116 CrossRef CAS PubMed.
- J. Liang, Y. Jiao, M. Jaroniec and S. Z. Qiao, Angew. Chem., Int. Ed., 2012, 51, 11496 CrossRef CAS PubMed.
- S. J. Guo, S. Zhang, L. H. Wu and S. H. Sun, Angew. Chem., Int. Ed., 2012, 51, 11770 CrossRef CAS PubMed.
- S. J. Guo, S. Zhang and S. H. Sun, Angew. Chem., Int. Ed., 2013, 52, 8526 CrossRef CAS PubMed.
- N. Mahmood, C. Z. Zhang, J. Jiang, F. Liu and Y. L. Hou, Chem. – Eur. J., 2013, 19, 5183 CrossRef CAS PubMed.
- P. Zhang, F. Sun, Z. H. Xiang, Z. G. Shen, J. Yun and D. P. Cao, Energy Environ. Sci., 2014, 7, 442 CAS.
- Y. Hou, T. Huang, Z. Wen, S. Mao, S. Cui and J. Chen, Adv. Energy Mater., 2014, 4, 1400337 Search PubMed.
- Y. Y. Liang, Y. G. Li, H. L. Wang, J. G. Zhou, J. Wang, T. Regier and H. J. Dai, Nat. Mater., 2011, 10, 780 CrossRef CAS PubMed.
- K. P. Gong, F. Du, Z. H. Xia, M. Durstock and L. M. Dai, Science, 2009, 323, 760 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, additional FESEM, TEM, HRTEM, SAED patterns, N2 adsorption–desorption isotherms, XRD patterns, and electrochemical characterization of Pt/C, Co-C@Co9S8 DSNCs, Co-C polyhedrons and Co9S8 nanocages. See DOI: 10.1039/c5ee02903a |
| This journal is © The Royal Society of Chemistry 2016 |