Superior performance of borocarbonitrides, BxCyNz, as stable, low-cost metal-free electrocatalysts for the hydrogen evolution reaction†
Manjeet
Chhetri
a,
Somak
Maitra
a,
Himanshu
Chakraborty
b,
Umesh V.
Waghmare
b and
C. N. R.
Rao
*a
aNew Chemistry Unit, International Centre for Materials Science, Jawaharlal Nehru Centre for Advanced Scientific Research, P.O Jakkur, Bangalore, 560064, India. E-mail: cnrrao@jncasr.ac.in
bTheoretical Sciences Unit, Jawaharlal Nehru Centre for Advanced Scientific Research, P.O Jakkur, Bangalore 560064, India
First published on 2nd November 2015
Abstract
We report superior hydrogen evolution activity of metal-free borocarbonitride (BCN) catalysts. The highly positive onset potential (−56 mV vs. RHE) and the current density of 10 mA cm−2 at an overpotential of 70 mV exhibited by a carbon-rich BCN with the composition BC7N2 demonstrates the extraordinary electrocatalytic activity at par with Pt. Theoretical studies throw light on the cause of high activity of this composition. The high activity and good stability of BCNs surpass the characteristics of other metal-free catalysts reported in recent literature.
Broader contextThe use of hydrogen as a clean and efficient fuel has the potential of replacing scarce carbon based fuels. Using hydrogen as a fuel produces only water as the product thereby making it most suitable from environmental considerations. One of the ways to generate hydrogen is by the electrolysis of water by cathodically reducing hydrogen ions. However, hydrogen ion reduction is accompanied by a substantial overpotential unless an effective catalyst is used to reduce the gap between standard reduction potential of hydrogen ions and the overpotential. Pt or Pt group metals (PGMs) have been extensively used for the hydrogen evolution reaction (HER) owing to their low overpotential and larger current density. However they suffer from the lack of inherent tolerance to catalyst poisons (like CO) and stability in the electrochemical environment. Furthermore, their high cost and limited availability are also drawbacks. Clearly there is a need to replace Pt or PGM catalysts by less costly and easily synthesizable catalysts. In this context, non-precious metal catalysts especially carbon based materials have been under the limelight of research. We have found for the first time borocarbonitrides (BCNs) to be efficient HER electrocatalysts, with a carbon-rich sample exhibiting an onset potential of −56 mV (vs. RHE) and a current density of 10 mA cm−2 at an overpotential of 70 mV (vs. RHE). The performance of the carbon-rich BCN found by us is superior to that reported for other non-metal electrocatalysts for HER. BCNs are low cost materials and have the potential to replace Pt based electrocatalysts. |
Generation of hydrogen from water is potentially an important means of using solar energy for the benefit of mankind. The hydrogen evolution reaction (HER) is not only a vital part of electrochemical water splitting but also provides a way to understand the underlying mechanism of electron transfer processes in electrocatalysis. It is well known that platinum supported on carbon exhibits very good electrocatalytic HER activity.1–6 Other transition metal catalysts have also been tried for HER,7–17 but it would be ideal to have a metal-free catalyst for the purpose, partly because of the scarcity and high cost of Pt.18 Most of the photocatalysts and electrocatalysts reported so far suffer from low quantum efficiency as well as stability. A composite of carbon nitride (C3N4) and nitrogen-doped graphene (NG) has recently been reported to possess unique properties for successful electrocatalytic H2 production.19 Films of porous C3N4 layers with NG have also been shown to display excellent electrochemical HER performance with a highly positive onset potential20 and high exchange current density and stability comparable to platinum. The use of bimetallic core–shell electrocatalysts with carbonitrides for fuel cell applications has also been reported.21–23 Other materials which have shown promising electrocatalytic HER activity are MoS2 nanoparticles grown on graphene24 and Mo2C-carbon nanocomposites.25 We considered it most appropriate to investigate the electrochemical HER activity of borocarbonitrides, BxCyNz, which have been shown to have impressive surface and catalytic properties26,27 and are also low cost materials. These materials are nanoplatelets containing graphene and BN domains, possibly along with BCN rings.28–30 They contain B–C, B–N, C–N, C–C bonds but no B–B and N–N bonds. They would have defect sites (e.g. sp3–C, Stone–Wales defects) in the carbon network which can act as active sites (as nucleophiles for H+ ions) for electron transfer reactions. BxCyNz is different from B, N-codoped graphene in some ways such as thermal stability and the presence of covalent BN domains in the carbon matrix. Although excess of BN domains impedes the electrochemical activity, their presence in trace amounts gives rise to the (BN)x/Cy interface which is shown to have interesting adsorption properties. In this communication, we report the electrocatalytic activity of borocarbonitrides (designated as BCNs in the text for simplicity) for HER. The high surface area and the low charge transfer resistance for electron transfer due to the presence of B and N atoms in the carbon network would be expected to reduce the overpotential for hydrogen production. To the best of our knowledge, there has been no report in the literature on the use of BCNs as electrocatalysts for hydrogen production although they have been used for the oxygen reduction reaction (ORR).31 The present study demonstrates that the carbon-rich BCN (BC7N2) exhibits outstanding electrocatalytic activity for HER. In order to understand the experimental results, we have carried out first-principles calculations which reveal the unique features of the highly active carbon-rich BCN.
We have prepared five compositions (BCN-1 to BCN-5) of borocarbonitrides, BxCyNz, by the reaction of boric acid, urea and activated charcoal.28,29 Of these, BCN-1 is the most carbon rich sample with the composition of BC7N2 and BCN-5 has the least carbon content with the composition of BC1.1N (see Table S1, ESI†). Fig. 1 shows the TEM images of the BCN sheets as well as a schematic depicting their structure and morphology. The surface areas are obtained from N2 adsorption isotherms and BCN-1 was shown to have the highest surface area (∼1950 m2 g−1) (Fig. S1, ESI†) and BCN-4 the lowest surface area of 1241 m2 g−1. The presence of mesopores and micropores is indicated by the isotherms and pore size distribution. The pore diameter calculated by the DFT method is in the range of 3–19 nm and the pore volume in the range of 0.7–1.1 cm3 g−1 for all the samples (see the inset of Fig. S1, ESI†). The powder X-ray diffraction pattern of BCN-1 shows broad peaks centered at 24.9° and 43.2° (2θ with FWHM values of 6.8 and 3.5 respectively) (Fig. S2a, ESI†), due to the (002) and (100) reflections, the broadening of peaks arising from the nanosized domains. Raman spectra show a prominent D-band (1335 cm−1) due to defects along with the G-band (1590 cm−1) and a weak 2D-band (2775 cm−1) (see Fig. S2b, ESI†). The BCN samples were free from metal impurities except for iron which is shown to be catalytically inactive (see ESI†).
 | ||
| Fig. 1 (a) TEM images of BCN-1 showing the sheet morphology. (b) Schematic of carbon rich BxCyNz sheets depicting the incorporation of B and N into the carbon network. | ||
X-ray photoelectron spectroscopy (XPS) was employed to establish the nature of the chemical species in the BCN samples (see Fig. 2 for the spectra of BCN-1). Table S1 (ESI†) gives a summary of the compositions of the BCN samples. The core level spectra of the individual elements were deconvulated to obtain a detailed understanding of the bonding characteristics (see Fig. 2 and Fig. S2c, ESI†). The B 1s signal consists of two peaks centered at 190.8 eV and 192.3 eV, which corresponds to the presence of B–C and B–N bonds respectively. The C-1s peak can be deconvoluted to four peaks at 284.4, 285.1, 285.9, and 298.8 eV attributed to sp2 carbon, C–B, C–N and C–O respectively. The deconvolution of the N-1s peak suggests the presence of different kinds of N–C bonds in BCN-1 (mainly pyridinic and pyrollic) as shown in Table S2 (ESI†). Thus the XPS analysis revealed the presence of B–C, B–N, C–N, and C–C bonds in BCN samples suggesting the presence of graphene and BN (as depicted in Fig. 1b). Table S3 (ESI†) gives the percentages of B–C and B–N bonds on the surface of the BCN samples. We have also analyzed the O-1s signal (see ESI†). EDAX and CHN elemental analysis gave results which corresponded well with the compositions obtained from XPS.
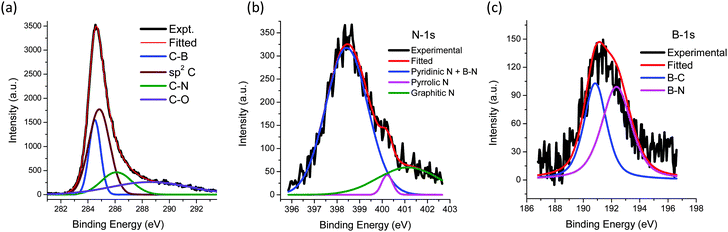 | ||
| Fig. 2 (a) to (c) X-ray photoelectron spectrum of BCN-1 showing the core level spectrum of C, N and B respectively. | ||
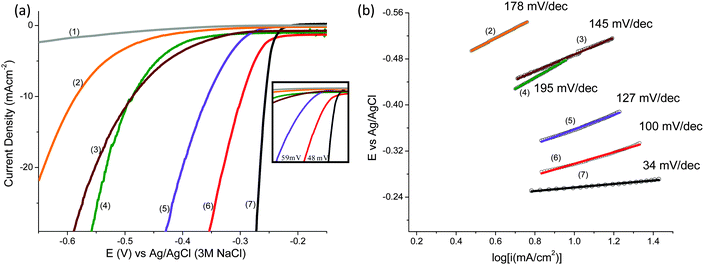
Electrochemical performance of the BCN samples for HER was investigated by Linear Sweep Voltammetry (LSV) at 1600 rpm and 5 mV s−1 scan rate with deaerated 0.5 M H2SO4 as the electrolyte. The cathodic polarization curves of all the samples are shown in Fig. 3a.
For the purpose of comparison, data for 40% Pt/C and the bare GCE are also included. The GCE exhibits almost null activity in comparison with BCN and Pt/C polarization curves. The onset potential for HER was found to be −0.28 V (Ag/AgCl) whereas that for Pt/C was −0.23 V (Ag/AgCl). The value of the overpotential (η) gives an idea of the extent of polarization upon passage of faradaic current (in this case due to H2 evolution), a lower η signifying better catalytic activity generating more H2 upon application of smaller cathodic potential. The values of η at 10 and 20 mA cm−2 are −298 mV and −330 mV signifying the rapid rate of electron transfer on BCN-1 (see Table 1). The inset in Fig. 3a shows the difference in the overpotential required to produce a current density of 10 mA cm−2 for the BCN samples and the Pt/C. In comparison with the recently reported non-precious metal catalysts, BCN-1 shows markedly better performance for HER (Table 2). For the ease of comparison, the potentials reported in Table 1 are against the Reversible Hydrogen Electrode (RHE) obtained through RHE calibration using the equation,36 .
.
| Sample | BET (m2 g−1) | Onseta (mV) |
η@10 mA cm−2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a, (mV) a, (mV) |
η@20 mA cm−2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a, (mV) a, (mV) |
Tafel slope (mV dec−1) | I o (A cm−2) | R ct (ohm) | C dl (mF cm−2) |
|---|---|---|---|---|---|---|---|---|
a Against RHE the values can be converted by adding 227.7 mV, following the equation, 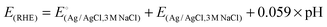 . .
|
||||||||
| BCN-1 | 1950 | −284 | −298 | −330 | 100 | 5.1 × 10−5 | 13.6 | 0.108 |
| BCN-2 | 1635 | −314 | −357 | −401 | 127 | 3.2 × 10−5 | 60.1 | 0.103 |
| BCN-3 | 1470 | −451 | −487 | −642 | 195 | 1.09 × 10−5 | 64.3 | 0.033 |
| BCN-4 | 1241 | −444 | −487 | −533 | 145 | 9.1 × 10−6 | 268.9 | 0.022 |
| BCN-5 | 1580 | −428 | −586 | −542 | 178 | 4.4 × 10−6 | 384.6 | 0.020 |
| Pt/C | — | −230 | −250 | −266 | 34 | — | — | — |
| Catalyst | Onset (mV) vs. RHE | η@20 mA cm−2 (mV) vs. RHE | Tafel slope (mV dec−1) | Ref. |
|---|---|---|---|---|
a @5 mA cm−2.
b
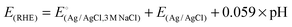 . .
|
||||
| 3D-MoS2/N-GAs | −236 | −261 | 230 | 32 |
| N-MPG | −220 | −239 | 109 | 33 |
| C3N4@NG | −180 | −240 | 51 | 19 |
| N-Graphene on Si | −200 | −260 | 74 | 34 |
| g-C3N4nanoribbons | −80 | −200 | 54 | 35 |
| MoS2/RGO | −100 | −150 | 41 | 24 |
| Defect rich MoS2 nanosheets | −120 | −100 | 57 | 24 |
| MoC2/C nanocomposite | −100 | −270a | 110 | 25 |
| 40% Pt/C | 2.3 | 22 | 34 | This work |
| BC7N2 | −56b | −70b | 100 | This work |
The high HER activity of BCN nanosheets is further manifested by the Tafel plots (Fig. 3b) and the corresponding mechanism for H2 generation (See ESI†). The Tafel equation can be deduced from the Butler–Volmer equation and the final form of the equation is given as eqn (1),
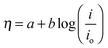 | (1) |
 | ||
| Fig. 4 Electrocatalytic performance testing of BxCyNz. (a) Nyquist plot of BxCyNz at the onset potential. (b) Magnified image of (a) to show the same plot for low Rct value. (c) Activity retention test by amperometric i–t plot taken at −0.32 V for BCN-1 in comparison to Pt/C. (d) Chronopotentiometry plot to produce a current density of 20 mA cm−2. The inset shows the comparative plot for BCN-1 and Pt/C. The letters in (a) represent the same as in Fig. 3. | ||
To confirm the high activity and lower charge transfer resistance we have compared the electrochemical active surface areas of the samples by calculating the double layer capacitance (see ESI,† for detailed calculations). The value of Cdl is 0.108 mF cm−2 for BCN-1, which is the highest value amongst all the BCN samples (Fig. S3, ESI,† and Table 1). Since double layer capacitance is directly proportional to the electrochemical active surface area for carbon based materials, the values obtained for BCN-1 correspond to an enhancement factor of one order of magnitude relative to the other samples (Table 1). The higher activity with lower Rct for BCN-1 is due to the high electrical conductivity owing to the higher proportion of carbon in the network29 and the larger active surface area. It is noteworthy that the electronic structure and properties which enhance electron transfer reactions are related to the composition and the relative ordering of graphene and BN domains. BCN-1 has a high B–C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B–N ratio of 6.7 (Table S3, ESI†) leading to a lower proportion of BN domains. Furthermore graphitic N–C interaction would favour defect sites which play a key role in electron transfer reactions. The samples obtained from the controlled experiments without urea and boric acid respectively show that singly doped carbon (with N and B) does not show enhanced HER activity.
B–N ratio of 6.7 (Table S3, ESI†) leading to a lower proportion of BN domains. Furthermore graphitic N–C interaction would favour defect sites which play a key role in electron transfer reactions. The samples obtained from the controlled experiments without urea and boric acid respectively show that singly doped carbon (with N and B) does not show enhanced HER activity.
Stability is an important parameter of catalyst activity. We have employed chronoamperometry (CA), chronopotentiometry (CP) (Fig. 4c and d) and cyclic voltammetry (CV) to test the %retention of the electrochemical activity as a function of time or cycle. CA was performed at −0.32 V (Ag/AgCl, 3 M NaCl) and CP was done at a current density of 20 mA cm−2. Samples were also potentially swiped between 0.1 V and −0.35 V and after the cycling, LSV was again taken for comparison. BCN-1 shows exceptional stability in comparison to the Pt/C catalyst where ∼80% of the activity is retained in comparison to Pt/C (only ∼10% activity retention) in 1000 seconds (Fig. 4c and Fig. S4, ESI†) to obtain current at −0.32 V. From the CP study, (see the inset of Fig. 4d), BCN-1 shows activity at par with Pt/C to produce a current density of 20 mA cm−2. We have performed CP for 24 hours (Fig. 4d) and found BCN-1 to be a highly stable catalyst for HER. To reinforce these conclusions, we have performed CV in the potential window of −0.35 V to 0.1 V for 1000 cycles, recording the polarization curves before and after the cycling (Fig. 5a). We observe ∼83% retention in activity as also seen from the chronoamperometric i–t (CA) test (Fig. S3, ESI†). The degradation of graphitic carbon in acidic medium is negligible and the possible loss of active surface area is less than that for Pt/C (catalyst detachment, agglomeration, carbon corrosion, etc.).
 | ||
| Fig. 5 (a) LSV plot before and after 1000 cycles (between −0.35 V and 0.1 V) for both BCN-1 and Pt/C. (b) Schematic showing the HER activity of BxCyNz sheets. | ||
In our first principles calculations, we have examined the electronic density of states (DOS) and the partial density of states (PDOS) of the three compositions, BC7N2 (I), B2C2N2 (II) and BC8N (III), by shifting the energy suitably to have a vacuum level at 0 eV. For all the configurations of BC7N2 (Fig. S5, ESI†), Fermi energy (EF) is around −3.2 eV, which gives an overpotential of about 1.2 eV relative to the redox potential of HER. Secondly, it is in a conduction band separated by a gap of about 0.5 eV from the valence band giving n-type carriers at relatively high density, highest for the configuration Ia (Fig. 6). The bottom of the conduction band is just above the HER potential. From the PDOS, it is clear that these states are contributed largely by p orbitals of C and N atoms, and reflect that the two substituted N atoms have different chemical characters. Visualization of the frontier occupied state of the lowest energy configurations reveals that C–C and C–N bonds are relevant to the electrocatalytic activity. The electronic structures of both BC8N and B2C2N2 (Fig. 6c and d) exhibit a gap of about 0.5 eV, and the top of the valence band (with fairly low density of states) is just above or barely touches the redox potential of the HER, consistent with low electrocatalytic activity. Their frontier occupied states involve mainly C–C bonds. While the valence and conduction band edges of the configurations of BC7N2 (Ia, Ib and Ic) straddle the HER redox potential (−4.44 eV), those of BC8N and B2C2N2 do not (Fig. 7 and Table S4, ESI†). A comparative study of the electronic structures of C8N2 and BC7N2 reveals that the electrostatic balance provided by the B atom is essential to get stability and optimal alignment of the electronic structure with respect to the HER potential (see Fig. S9, ESI†). We have examined around 60 configurations for the interaction between H2O and BC7N2 (including van der Waals interactions, see Fig. S10, ESI†). We find that the energy of adsorption of H2O with the 1a configuration spans the widest range from 11 to 26 kJ mol−1 among all the configurations of BC7N2 (see Fig. S11, ESI†). Moreover, the electric dipoles of H2O and the N–B–N structural motifs of 1a configuration couple with each other and with the external electric field, an aspect of relevance to the electrocatalytic properties. While all the four chemically ordered states of BC7N2 are comparable in their electronic properties, our results indicate that 1a may be more optimal for electrocatalysis in HER. Thus, of the four configurations, Ia, where both N atoms are bonded to B atoms, is the best candidate as an electrocatalyst for hydrogen generation. We also include a comparative analysis of the electronic structure of B2C5N3 containing carbon nitride rings, and BC7N2 with assessment of their activity towards HER (see Fig. S12, ESI†). Electronic DOS of B2C5N3 and BC7N2 are qualitatively similar, but with a lower overpotential of the former for HER, with the conduction band edge above the HER by ∼0.25 eV. B2C5N3 with CN-rings is indeed suitable for the catalysis of HER and water splitting (see Fig. S12, ESI,† bottom), However, a comparison of the energies of B2C5N3 and B2C6N2 relative to graphene, boronitride and BC7N2 shows that the two structures are energetically not favorable, with B2C5N3 less stable than B2C6N2. The BCN containing carbon-nitride rings is energetically less favorable than BC7N2.
 | ||
| Fig. 6 (a) DOS for four configurations of BC7N2 (I), (b) PDOS of its first configuration (Ia), (c) DOS for two configurations of BC8N (III) and (d) PDOS of B2C2N2 (II). See Fig. S5 (ESI†) for different configurations. | ||
 | ||
| Fig. 7 Bandgaps and valence (black) and conduction (red) band edge positions of the different configurations. The energy level for hydrogen evolution reaction (HER) is denoted by a dotted line and their Fermi energies by green circles. Here, IV and V refer to B2C6N2 and B2C5N3 (see ESI,† for configurations). | ||
Conclusions
We have successfully demonstrated the efficacy of borocarbonitride sheets as excellent low-cost, metal-free (see ESI,† for the analysis) catalysts for hydrogen generation (Fig. 5b). This is the first report on the use of BCNs as HER catalysts. Amongst all the BCN samples, carbon-rich BCN-1 shows the best activity with a performance superior to that of other non-precious metal electrocatalysts (Table 2). The onset potential of −0.28 V (vs. Ag/AgCl) is close to that of Pt (−0.23 V). BCN-1 also exhibits stability up to 24 hours requiring an overpotential of −0.32 V (vs. Ag/AgCl) to produce a current density of 20 mA cm−2. It is noteworthy that BCN-1 contains a larger proportion of pyridinic than pyrollic nitrogens and a high percentage of B–C bonds, the low proportion of BN bonds also contributing to higher conductivity. Our theoretical studies show that substitution of B and N at equal concentrations opens up a gap, with the valence band unfavourably located relative to the HER potential. Substitution of excess N results in the population of the conduction bands with electrons, shifts the valence and conduction bands to lower energy and favours the alignment of bands to facilitate electrocatalysis. We believe that with compositional and morphological modifications, the electrochemical activity of BCNs can be further improved.Acknowledgements
MC thanks UGC, India, for JRF and JNCASR for the advanced facilities. U.V.W. and H.C. acknowledge discussions and interactions with M. L. Klein and are grateful to the support through the Centre for the Computational Design of Functional Layered Materials, an Energy Frontier Research Centre funded by the U.S. Department of Energy, Office of Science, Basic Energy Sciences, under Award No. DE-SC0012575. We also acknowledge S. Sampath, Indian Institute of Science, for the help with Faradaic efficiency measurements.Notes and references
- J. K. Norskov, T. Bligaard, J. Rossmeisl and C. H. Christensen, Nat. Chem., 2009, 1, 37–46 CrossRef CAS PubMed.
- K. Neyerlin, W. Gu, J. Jorne and H. A. Gasteiger, J. Electrochem. Soc., 2007, 154, B631–B635 CrossRef CAS.
- S. A. Grigoriev, P. Millet and V. N. Fateev, J. Power Sources, 2008, 177, 281–285 CrossRef CAS.
- P. Millet, R. Ngameni, S. A. Grigoriev, N. Mbemba, F. Brisset, A. Ranjbari and C. Etiévant, Int. J. Hydrogen Energy, 2010, 35, 5043–5052 CrossRef CAS.
- D. V. Esposito, S. T. Hunt, A. L. Stottlemyer, K. D. Dobson, B. E. McCandless, R. W. Birkmire and J. G. Chen, Angew. Chem., Int. Ed., 2010, 122, 10055–10058 CrossRef.
- N. M. Marković, B. N. Grgur and P. N. Ross, J. Phys. Chem. B, 1997, 101, 5405–5413 CrossRef.
- S. Bai, C. Wang, M. Deng, M. Gong, Y. Bai, J. Jiang and Y. Xiong, Angew. Chem., Int. Ed., 2014, 53, 12120–12124 CrossRef CAS PubMed.
- E. J. Popczun, J. R. McKone, C. G. Read, A. J. Biacchi, A. M. Wiltrout, N. S. Lewis and R. E. Schaak, J. Am. Chem. Soc., 2013, 135, 9267–9270 CrossRef CAS PubMed.
- D. Brown, M. Mahmood, A. Turner, S. Hall and P. Fogarty, Int. J. Hydrogen Energy, 1982, 7, 405–410 CrossRef CAS.
- D. Brown, M. Mahmood, M. Man and A. Turner, Electrochim. Acta, 1984, 29, 1551–1556 CrossRef CAS.
- C. Fan, D. Piron, A. Sleb and P. Paradis, J. Electrochem. Soc., 1994, 141, 382–387 CrossRef CAS.
- I. A. Raj and K. Vasu, J. Appl. Electrochem., 1990, 20, 32–38 CrossRef CAS.
- M. Wang, L. Chen and L. Sun, Energy Environ. Sci., 2012, 5, 6763–6778 CAS.
- J.-F. Capon, F. Gloaguen, F. Y. Pétillon, P. Schollhammer and J. Talarmin, Coord. Chem. Rev., 2009, 253, 1476–1494 CrossRef CAS.
- V. Artero, M. Chavarot-Kerlidou and M. Fontecave, Angew. Chem., Int. Ed., 2011, 50, 7238–7266 CrossRef CAS PubMed.
- D. L. DuBois and R. M. Bullock, Eur. J. Inorg. Chem., 2011, 1017–1027 CrossRef CAS.
- X. Fan, Z. Peng, R. Ye, H. Zhou and X. Guo, ACS Nano, 2015, 9, 7407–7418 CrossRef CAS PubMed.
- H. Vrubel and X. Hu, Angew. Chem., 2012, 124, 12875–12878 CrossRef.
- Y. Zheng, Y. Jiao, Y. Zhu, L. H. Li, Y. Han, Y. Chen, A. Du, M. Jaroniec and S. Z. Qiao, Nat. Commun., 2014, 5 Search PubMed.
- J. Duan, S. Chen, M. Jaroniec and S. Z. Qiao, ACS Nano, 2015, 9, 931–940 CrossRef CAS PubMed.
- V. Di Noto, E. Negro, S. Polizzi, F. Agresti and G. A. Giffin, ChemSusChem, 2012, 5, 2451–2459 CrossRef CAS PubMed.
- V. Di Noto, E. Negro, S. Polizzi, K. Vezzù, L. Toniolo and G. Cavinato, Int. J. Hydrogen Energy, 2014, 39, 2812–2827 CrossRef CAS.
- E. Negro, S. Polizzi, K. Vezzù, L. Toniolo, G. Cavinato and V. Di Noto, Int. J. Hydrogen Energy, 2014, 39, 2828–2841 CrossRef CAS.
- Y. Li, H. Wang, L. Xie, Y. Liang, G. Hong and H. Dai, J. Am. Chem. Soc., 2011, 133, 7296–7299 CrossRef CAS PubMed.
- N. S. Alhajri, D. H. Anjum and K. Takanabe, J. Mater. Chem. A, 2014, 2, 10548–10556 CAS.
- S. Sen, K. Moses, A. J. Bhattacharyya and C. N. R. Rao, Chem. – Asian J., 2014, 9, 100–103 CrossRef CAS PubMed.
- K. Raidongia, A. Nag, K. P. S. S. Hembram, U. V. Waghmare, R. Datta and C. N. R. Rao, Chem. – Eur. J., 2010, 16, 149–157 CrossRef CAS PubMed.
- N. Kumar, K. Moses, K. Pramoda, S. N. Shirodkar, A. K. Mishra, U. V. Waghmare, A. Sundaresan and C. N. R. Rao, J. Mater. Chem. A, 2013, 1, 5806–5821 CAS.
- M. Kota, N. S. Sharmila, U. V. Waghmare and C. N. R. Rao, Mater. Res. Express, 2014, 1, 025603 CrossRef.
- C. N. R. Rao, K. Gopalakrishnan and U. Maitra, ACS Appl. Mater. Interfaces, 2015, 7, 7809–7832 CAS.
- K. Moses, V. Kiran, S. Sampath and C. N. R. Rao, Chem. – Asian J., 2014, 9, 838–843 CrossRef CAS PubMed.
- Y. Hou, B. Zhang, Z. Wen, S. Cui, X. Guo, Z. He and J. Chen, J. Mater. Chem. A, 2014, 2, 13795–13800 CAS.
- X. Huang, Y. Zhao, Z. Ao and G. Wang, Sci. Rep., 2014, 4 CAS.
- U. Sim, T.-Y. Yang, J. Moon, J. An, J. Hwang, J.-H. Seo, J. Lee, K. Y. Kim, J. Lee, S. Han, B. H. Hong and K. T. Nam, Energy Environ. Sci., 2013, 6, 3658–3664 CAS.
- Y. Zhao, F. Zhao, X. Wang, C. Xu, Z. Zhang, G. Shi and L. Qu, Angew. Chem., Int. Ed., 2014, 53, 13934–13939 CrossRef CAS PubMed.
- J. Guo, F. Li, Y. Sun, X. Zhang and L. Tang, J. Power Sources, 2015, 291, 195–200 CrossRef CAS.
- C. G. Morales-Guio, L.-A. Stern and X. Hu, Chem. Soc. Rev., 2014, 43, 6555–6569 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Faradaic efficiency and methods of first principles calculations. See DOI: 10.1039/c5ee02521d |
| This journal is © The Royal Society of Chemistry 2016 |