Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Structure property relationships affecting the proton conductivity in imidazole loaded Al-MOFs†
T.
Homburg
a,
C.
Hartwig
a,
H.
Reinsch
a,
M.
Wark
b and
N.
Stock
*a
aInstitut für Anorganische Chemie Christian-Albrechts-Universität zu Kiel, Max-Eyth-Strasse 2, 24118 Kiel, Germany. E-mail: stock@ac.uni-kiel.de
bInstitut für Chemie, Carl von Ossietzky Universität Oldenburg, Carl-von-Ossietzky-Strasse 9–11, 26129 Oldenburg, Germany
First published on 17th August 2016
Abstract
The structures of the imidazole loaded derivatives of Al-MIL-53 [Al(OH)(1,4-BDC-(CH3)x)] (x = 0, 1, 2) and CAU-11 ([Al(OH)(SDBA)]) (1,4-H2BDC = terephthalic acid; H2SDBA = 4,4′-sulfonyldibenzoic acid) were determined from powder X-ray diffraction data. Impedance spectroscopy measurements were carried out to evaluate their proton conductivities under anhydrous conditions at temperatures up to 110 °C. In Al-MIL-53-(CH3)x_HIm (x = 0, 1, 2) the formation of hydrogen bonds between the framework and the guest molecules results in a decrease in proton conductivity (x0 = 1.7 × 10−6, x1 = 1.9 × 10−8 and x2 = 1.7 × 10−9 S cm−1 at 110 °C and Eact = 0.42, 0.41 and 0.46 eV, for 0, 1 and 2 CH3-groups, respectively). The highest conductivity has been measured for CAU-11_HIm with 3.0 × 10−4 S cm−1 at 110 °C (Eact = 0.19 eV), where no host–guest hydrogen bonding interactions are observed.
Introduction
Within the field of renewable energy and future mobility, hydrogen and methanol are common energy carriers and the most prominent candidates for utilization in fuel cells.1 Inside such fuel cells the well-known combustion reaction of H2 and O2 to H2O is split into two electrochemical half reactions (anode: H2 → 2H+ + 2e− and cathode: ½O2 + 2e− → O2−). In order to spatially separate the reaction, an external circuit for the electron transfer and an electrolyte for the flow of protons is needed. Most often these electrolytes use a water mediated system to transport the protons from the anode to the cathode.2–4 Within this context, the development of coordination polymers (CPs) as anhydrous proton conductive materials has recently attracted a lot of interest.5–12 Systematic studies have proven that membranes made of certain CPs do not show any fuel crossover and can be operated at temperatures above 100 °C.13 The latter issue is an important shortcoming of Nafion, the state of the art material in fuel cells. Not only is the mechanical stability of the Nafion membrane limited by its hydration level, but also the proton conduction itself strongly depends on the relative humidity.14,15 This implies a limitation in the operating temperature due to the removal of water from the Nafion pores above 80 °C.16–18 In recent years, several CPs and MOFs (metal–organic frameworks) have been investigated regarding their proton conduction properties, some of which show a similar value as Nafion (1 × 10−2 S cm−1) in the presence of water.19–25 Also, alternative molecules that act as proton shuttles have been studied. For example, 1,2,4-triazole was incorporated during the synthesis of Na3(THBS) (THBS3− = 2,4,6-trihydroxy-1,3,5-benzenetrisulfonate) and the final product exhibits an anhydrous proton conductivity of 5 × 10−4 S cm−1 at 150 °C.11 In addition, adsorption of histamine into [Al(OH)(1,4-NDC)] (NDC2− = 1,4 naphthalenedicarboxylate) leads to a host–guest system with a high anhydrous proton conductivity of 1.7 × 10−3 S cm−1 at 150 °C.12 Recently, imidazole (Him) was also incorporated into the pores of UiO-67, [Zr6O4(OH)4(BPDC)6] (BPDC2− = biphenyl-4,4′-dicarboxylate) and a conductivity of more than 10−3 S cm−1 at 120 °C in an anhydrous environment was observed,7 which was explained by the very weak host guest interactions. For porous coordination polymers with MIL-53-type structure it has been shown that the hydrophobisation of the framework in combination with the use of organic guest molecules can enhance the proton conductivity in such systems.26 The 3D framework of MIL-53 is built up of infinite chains of trans corner-sharing AlO4(μ-OH)2 polyhedra, which are connected by 1,4-benzenedicarboxylate (BDC2−) molecules, to form one-dimensional rhombic-shaped channels.21,27,28 Bureekaew et al. synthesised MIL-53 frameworks using 1,4-H2NDC and 1,4-H2BDC as linker molecules and subsequently impregnated the frameworks with HIm molecules.26 The different steric influences of the linker molecules leads to different pore shapes and host–guest interactions, which was demonstrated via solid-state 2H-NMR spectroscopy. Impedance spectroscopy showed that the conductivity of the compounds is correlated with the dynamic properties of imidazole adsorbed within the pores. In Al-MIL-53-1,4-NDC_HIm, the adsorbed HIm molecules can move more freely (2.2 × 10−5 S cm−1 at 120 °C) than in Al-MIL-53-BDC_HIm (1.0 × 10−7 S cm−1 at 120 °C). Furthermore, Eisbein et al. used molecular dynamics simulations to study the proton transfer in an imidazole loaded Al-MIL-53 structure.29 They observed an ordering effect of the imidazole molecules through interactions with hydrophilic groups of the framework. This supports the finding of Bureekaew et al. and suggested a general positive effect of hydrophobicity on the proton conductivity.Here, we report the results of our systematic study on the influence of the pore structure of Al-MOFs on their proton conductivity. In this context, we have synthesized various, derivatives of Al-MIL-53 [Al(OH)(1,4-BDC-(CH3)x)] (x = 0,27 1,28 2) and the recently reported MOF CAU-11 [Al(OH)(SDBA)] (4,4′-sulfonyldibenzoate = SDBA2−).30 These MOFs were loaded with imidazole molecules and their structures, as well as their proton conductivities were determined at various temperatures.
Experimental
Materials
All employed chemicals are commercially available and were used without further purification.Methods
The syntheses were carried out in custom-made steel autoclaves containing a Teflon insert with a volume of 30 mL. Powder X-ray diffraction (PXRD) data was measured on a STOE Stadi P diffractometer with Cu-Kα1-radiation, equipped with a PSD detector. Thermogravimetric experiments were carried out under a flow of air (4 K min−1, 25 mL min−1) on a Netzsch STA 409 CD analyzer. 1H-NMR spectra were measured after dissolution of the imidazole loaded MOFs in 5 wt% NaOD/D2O on a Bruker DRX 200 spectrometer. Nitrogen physisorption isotherms were recorded after activation at 150 °C under reduced pressure using a BELSorpMax. Force-field calculations for structural optimisations were performed using Materials Studio31 and all processing of PXRD data was carried out using TOPAS.32 Proton conductivity was determined by impedance spectroscopy (IS)33 using a ZahnerZennium electrochemical workstation and a custom-made cell over the frequency range 1 to 106 Hz and employing an oscillating voltage of 100 mV. The microcrystalline samples were homogenized and incorporated into a stack comprising of the sample sandwiched between two graphitic slices. The stack was placed in a PTFE sample holder, where two stainless steel electrodes (diameter 8 mm) served as working and counter electrode. The sample holder was placed in a temperature-controlled stainless steel chamber and pressed with an angular moment of 30 cNm to obtain pellets (thickness between 0.3 and 1.0 mm).34 Before each series of temperature-dependent measurements, the samples were equilibrated for 4–6 hours at 110 °C. Each sample was equilibrated for additional 2 hours at the desired temperature (110, 100, 90, and 80 °C), before measuring each data point. Every measurement was carried out three times to ensure reproducibility, and at least two pellets of each host–guest system were studied. The proton conductivity (eqn (1)) was obtained by using the Ohmic resistance of the samples determined by IS.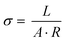 | (1) |
A Bode phase plot of the impedance was used to determine the Ohmic resistance. In a Bode plot the impedance corresponding to the phase shift closest to zero is approximately equal to the Ohmic resistance of the sample.
Synthesis
Al-MIL-53 (1) was synthesized using a mixture of 1,4 benzenedicarboxylic acid (H2BDC, 0.288 g, 1.7 mmol), Al(NO3)3·9H2O (1.30 g, 3.5 mmol) and 5 mL water, which was heated in an oven at 200 °C for 12 h (heating and cooling within 1 h, each). The resulting precipitate was filtered off, washed with DMF and water, and dried in air. Further synthesis details have been reported elsewhere.27Al-MIL-53-CH3 (2) was synthesized from a mixture of AlCl3·6H2O (1 g, 4.16 mmol), methylterephthalic acid (H2BDC-CH3, 0.75 g, 4.16 mmol) and 5 mL water using the same temperature program. Further details can be found in ref. 28. The ligand H2BDC-CH3 was synthesized according to a previously reported procedure.35
Al-MIL-53-(CH3)2 (3) was obtained from a mixture of AlCl3·6H2O (0.314 g, 1.30 mmol) and H2BDC-(CH3)2 (0.127 g, 0.65 mmol) in 5 mL water. The steel autoclave was kept at 200 °C for 24 h with heating up and cooling down for 1 h. The as-synthesized form (3-as) contains guest molecules and was activated in a two-step procedure similar to Al-MIL-53-CH3. Therefore, 3-as was heated in N,N-dimethylformamide (DMF, 60 mL) at 155 °C for 24 h. The filtered solid was washed with water and dried in air, and the narrow pore phase of 3 was obtained.
CAU-11 (4), [Al(OH)(SDBA)] (4,4′-sulfonyldibenzoate = SDBA2−), was synthesized from a mixture of AlCl3·6H2O (724 mg, 3 mmol), H2SDBA (268 mg, 1.2 mmol), 2 M aqueous solution of NaOH (1.8 mL, 3.6 mmol), and 18.2 mL H2O.30 The reaction was performed under conventional heating at 150 °C for 12 h (heating and cooling within 1 h, respectively). The resulting precipitate was filtered off and washed with DMF under microwave heating at 150 °C for 1 h, filtered again, washed with water and dried in air.
Preparation of imidazole-loaded frameworks
The water containing MOFs 1–4 (∼30 mg) were placed in a 2 mL Teflon insert, respectively (Fig. S1†). Another 30 mL Teflon insert was filled with 500 mg of imidazole (HIm). The smaller insert was placed into the larger Teflon sleeve and placed in a steel autoclave at 120 °C for 12 h, with 1 h heating up and 1 h cooling down to yield 1_HIm, 2_HIm and 3_HIm, respectively. 4_HIm was obtained after 4 h at 120 °C. PXRD patterns of the host frameworks and the intercalated compounds confirmed that the framework structures were maintained (Fig. 1). The amount of loaded imidazole was determined by thermogravimetric analysis, NMR-spectroscopy and Rietveld refinement. | ||
| Fig. 1 Sorption isotherms of the host materials Al-MIL-53, Al-MIL-53-(CH3), Al-MIL-53-(CH3)2 and CAU-11. | ||
Results and discussion
Four different microporous host materials, the three MIL-53-type compounds MIL-53, MIL-53-CH3 and MIL-53-(CH3)2 and CAU-11, were synthesized under solvothermal reaction conditions. The host materials were loaded using an excess of imidazole (HIm) as guest molecules. All samples were characterised by PXRD (Fig. 2A–D). For MIL-53-type hosts, activation and loading with imidazole molecules leads to a change of PXRD patterns due to the “breathing” behaviour (lp = large pore form, np = narrow pore form). All three MIL-53 compounds exhibit a large pore conformation upon loading with imidazole molecules. In contrast, in CAU-11 no changes of the reflection positions are observed upon activation and guest loading. The presence of guest molecules leads only to small differences in relative intensities of the reflections.Nitrogen sorption experiments were performed (Fig. 1; Table 1) prior to HIm impregnation. The three MIL-53 derivatives exhibit very different sorption isotherms which is due to the presence of the –CH3 groups. The specific surface areas and micropore volumina for Al-MIL-53 (1424 m2 g−1, 0.53 cm3 g−1) and CAU-11 (354 m2 g−1, 0.17 cm3 g−1) are in agreement with the values reported in the literature (1590 m2 g−1, 0.54 cm3 g−1 and 350 m2 g−1, 0.17 cm3 g−1).27,30 For Al-MIL-53-CH3, only the micropore volume has been reported, which also compares well to our results (0.33 cm3 g−1, literature = 0.32 cm3 g−1). As expected, Al-MIL-53-(CH3)2 has the smallest specific surface area (109 m2 g−1) and micropore volume (0.05 cm3 g−1) of the three Al-MIL-53-type compounds. Quantitative analyses yielding the number of intercalated guest molecules per formula unit were established by TG-measurements and NMR spectroscopy.
| Compound | S BET lit [m2 g−1] | S BET [m2 g−1] | V mic lit [cm3g−1] | V mic [cm3 g−1] |
|---|---|---|---|---|
| Al-MIL-53 | 1590 | 1424 | 0.54 | 0.53 |
| Al-MIL-53-(CH3) | — | 146 | 0.32 | 0.33 |
| Al-MIL-53-(CH3)2 | — | 109 | — | 0.05 |
| CAU-11 | 350 | 354 | 0.17 | 0.17 |
Quantification of guest loading
The amount of imidazole molecules in 1_, 2_, 3_ and 4_HIm was evaluated by dissolving the host–guest compounds in 5 wt% NaOD/D2O and measuring 1H-NMR spectra. Integration of the signals yields the molar ratios of imidazole![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) linker molecules (Fig. S6–S9,†Table 2). No additional signals due to DMF or its decomposition products in water were observed in the 1H-NMR spectra.
linker molecules (Fig. S6–S9,†Table 2). No additional signals due to DMF or its decomposition products in water were observed in the 1H-NMR spectra.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) linker molecules and (b) comparison of the theoretical (theo. %) and observed (obs. %) weight losses as obtained from thermogravimetric analysis (TGA)
linker molecules and (b) comparison of the theoretical (theo. %) and observed (obs. %) weight losses as obtained from thermogravimetric analysis (TGA)
| MIL-53_HIm | MIL-53-CH3_HIm | MIL-53-(CH3)2_HIm | CAU-11_HIm | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TGA theo. % | Obs. % | NMR ratio | TGA theo. % | Obs. % | NMR ratio | TGA theo. % | Obs. % | NMR ratio | TGA theo. % | Obs. % | NMR ratio | |
| Imidazole | 29.1 | 30.1 | 1.3 | 24.7 | 25.0 | 1.2 | 23.5 | 23.8 | 1.1 | 20.1 | 21.1 | 1.3 |
| Linker | 52.7 | 51.5 | 1.0 | 58.5 | 57.2 | 1.0 | 60.3 | 58.5 | 1.0 | 68.4 | 67.1 | 1.0 |
| Al2O3 | 16.2 | 16.5 | — | 16.8 | 17.8 | — | 16.0 | 17.7 | — | 11.5 | 11.8 | — |
TGA traces of the compounds 2_, 3_ and 4_HIm show two steps of weight loss, respectively (Fig. S2–S4†). The weight loss between 120 and 300 °C can be assigned to the desorption of imidazole molecules, whereas the second step is due to the combustion of the host framework. A closer look at the TGA data of 1_HIm (Fig. S1†) strikes out two additional steps. The first one from 50–100 °C (ca. 1%) was assigned to weakly bonded water molecules. The following two successive steps (between 120–190 and 195–270 °C) are attributed to the loss of imidazole molecules, suggesting varying strengths of host–guest interactions within the framework. The combustion of the framework starts around 430 °C. Similar observations were reported by S. Bureekaew et al. for HIm in Al-MIL-53 and are confirmed by the structure analysis of 1_HIm (see Crystal structures).
The results from TGA and NMR are consistent. Molar ratios of linker to imidazole between 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 and 1
1.1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 were observed in 1–4_HIm (Table 2). In the case of 1_HIm, loadings between 1.1 and 1.3 were found, however, the IS measurements did not show any change in conductivity for the samples with different degrees of loading.
1.3 were observed in 1–4_HIm (Table 2). In the case of 1_HIm, loadings between 1.1 and 1.3 were found, however, the IS measurements did not show any change in conductivity for the samples with different degrees of loading.
Proton conductivity
Subsequently, the proton conductivity of the imidazole loaded MOFs was measured under anhydrous conditions (relative humidity of 0%) and at temperatures between 80 and 110 °C by impedance spectroscopy. The proton conductivity of guest-free 1–4 is negligibly low (not shown), but varies strongly between the four title compounds 1–4_HIm (Fig. 3). | ||
| Fig. 3 Comparison of the Arrhenius plots of 1_HIm (MIL-53_HIm), 2_HIm (MIL-53-CH3_HIm), 3_HIm (MIL-53-(CH3)2_HIm) and 4_HIm (CAU-11_HIm). The resulting activation energies are displayed in Table 3. | ||
For all loaded compounds, an increase in temperature leads to an increase in conductivity and a linear correlation of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) σ vs. 1/T is observed. For 4_HIm, a proton conductivity value of 3.0 × 10−4 S cm−1 at 110 °C is found. Even though the amount of charge carriers in 1–3_HIm is almost equal to the one in 4_HIm, the proton conductivity is at least two orders of magnitude lower (1_HIm = 1.7 × 10−6, 2_HIm = 1.9 × 10−8 and 3_HIm = 1.7 × 10−9 S cm−1), suggesting that the number of charge carriers is not the most important factor. The activation energy (Eact) was calculated by fitting the proton conductivity data to the Arrhenius equation. All samples demonstrated Arrhenius behaviour between 80 and 110 °C. The highest value was found for 3_HIm (Eact = 0.46 eV), whereas no major distinction was encountered for 2_HIm (Eact = 0.41 eV) and 1_HIm (Eact = 0.42 eV). In contrast, the activation energy for 4_HIm is by far the lowest (Eact = 0.19 eV) (Table 3).
σ vs. 1/T is observed. For 4_HIm, a proton conductivity value of 3.0 × 10−4 S cm−1 at 110 °C is found. Even though the amount of charge carriers in 1–3_HIm is almost equal to the one in 4_HIm, the proton conductivity is at least two orders of magnitude lower (1_HIm = 1.7 × 10−6, 2_HIm = 1.9 × 10−8 and 3_HIm = 1.7 × 10−9 S cm−1), suggesting that the number of charge carriers is not the most important factor. The activation energy (Eact) was calculated by fitting the proton conductivity data to the Arrhenius equation. All samples demonstrated Arrhenius behaviour between 80 and 110 °C. The highest value was found for 3_HIm (Eact = 0.46 eV), whereas no major distinction was encountered for 2_HIm (Eact = 0.41 eV) and 1_HIm (Eact = 0.42 eV). In contrast, the activation energy for 4_HIm is by far the lowest (Eact = 0.19 eV) (Table 3).
| Conductivity [S cm−1] | O⋯N distance [Å] | E A [eV] | |
|---|---|---|---|
| MIL-53-(CH3)2_imi | 1.7 × 10−9 | 2.71(1) | 0.46 |
| MIL-53-(CH3)_imi | 1.9 × 10−8 | 3.06(1) | 0.41 |
| MIL-53_imi | 1.7 × 10−6 | ≥3.04(1) | 0.42 |
| CAU-11-imi | 3.0 × 10−4 | — | 0.19 |
Crystal structures
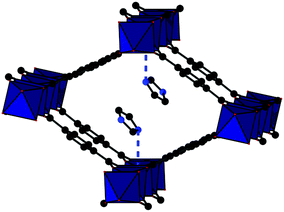
The crystal structures of Al-MIL-53-(CH3)x_HIm(x = 0, 1, 2) and CAU-11_HIm were determined from PXRD data by a combination of force-field calculations and Rietveld refinement. Details of the experimental procedures and the final Rietveld plots are given in the ESI (Table S1, Fig. S18–S21†).The structure of Al-MIL-53 is well known and has been described in great detail elsewhere.21,36,37 The frameworks of MIL-53-(CH3)x (x = 0, 1, 2) are built up by the same interconnection of infinite chains of trans corner-sharing AlO4(OH)2 polyhedra via the linker molecules. The two carboxylate groups of each BDC-X2− ion are connected to two adjacent aluminium-oxo-chains each, leading to one-dimensional rhombic channels. Depending on the presence of guest molecules, the diameter of these channels can reversibly change (“breathing”). After activation and impregnation, imidazole molecules could be successfully localized inside the pores of two of the three MIL-53 type compounds, 2_HIm and 3_HIm. The crystal structures of these compounds are shown in Fig. 4 and 5. In the channels of 2_HIm, the guest molecules adsorb in a commensurate fashion and are apparently interacting via hydrogen bonds to the μ-OH group (N⋯O distance of 3.06(1) Å). However, the reader should keep in mind that nitrogen and carbon atoms cannot be reliably distinguished and protons cannot be localized from refinements of PXRD data and thus such assignment is based on the observed interatomic distances between the host framework and the guest molecules. The host–guest interactions are also confirmed by IR spectroscopy. Comparison of the IR spectra of 2 and 3 (S15 and S16†), before and after impregnation, show a decreasing signal intensity in the range 3600–3700 cm−1 upon impregnation. Bearing in mind that this signal can be attributed to the stretching vibration of the μ-OH group of the framework,28 it hints to hydrogen bonding interactions with HIm molecules. Furthermore, only a weak signal at 2850 cm−1 is visible, indicating weakly self-associated imidazole molecules within the pores.38 In the structure of 3_HIm, the guest molecules adsorb in a similar fashion but with shorter and therefore also stronger hydrogen bonds to the host framework (N⋯O-distance of 2.71(1) Å). In addition, weaker hydrogen bonds between adjacent guest molecules in the pores are found. The strong binding in 3_HIm is in good agreement with the lowest conductivity observed among all four samples.
The weaker binding in 2_HIm also correlates with the increase in proton conductivity by one order of magnitude.
For 1_HIm, a sample loaded with approximately 1.3 imidazole molecules per formula unit was analysed by Rietveld refinement. The guest molecules assemble in two different positions, one of them shown in Fig. 6, which agrees well with the two weight loss steps observed in the TG-experiment between 120–270 °C.
However, the crystallographically independent molecules are disordered in a manner which is not chemically reasonable since this would lead to a strong overlap between the molecules. We interpret this as non-ordered adsorption of the guest molecules without full ordering of the imidazole molecules inside the channels. Nevertheless, a short distance between the host and the guest molecules with lower occupancy (accounting for ≈42% of the guest molecules) could indicate weak interactions via hydrogen bonding (N⋯O-distance of 3.04(1) Å, Fig. 6). The majority of guest molecules (≈58%) assemble in a distance from the framework that indicates no relevant interactions (host–guest distance >3.4 Å). Nevertheless, IR spectroscopy for 1_HIm (Fig. S14†) does show a sharp stretching vibration of the μ-OH group after impregnation with imidazole, indicating fewer interactions between the guest molecules and the framework itself. While this interpretation is not unambiguous, it is in very good agreement with the increase in proton conductivity of 1_HIm by two orders of magnitude compared to 2_HIm and three orders of magnitude compared to 3_HIm. Based on these results, we chose CAU-11 as a suitable host to further increase the conductivity. In the structure of CAU-11, the same inorganic building unit as in Al-MIL-53 is observed (Fig. 7). The chains are interconnected along the a-axis via the carboxylate groups of the linker molecules, resulting in layers with lozenge-shaped pores. Due to the interaction of sulfone groups with the inorganic building unit, a layered structure (ABAB-type stacking) is formed, creating a hydrophobic inner-pore surface. Thus the μ-OH groups are already interacting with the sulfone groups and should therefore not affect the conductivity of an imidazole loaded MOF.
A closer look at the IR-spectra of 4 before and after impregnation with imidazole shows a distinct signal at ∼3580 cm−1, indicating no interaction between the imidazole and the μ-OH group of the framework. Indeed, the imidazole molecules in 4_HIm assemble in the centre of the channels (host–guest distance >3.4 Å, Fig. 7). As observed for 1_HIm, the molecules overlap in a manner which is not chemically reasonable and thus we interpret this as non-ordered adsorption. Furthermore, the IR spectra for 1_HIm and 4_HIm show distinct signals at 2850 cm−1, which can be explained by the self-association of imidazole molecules to oligomers,38 suggesting hydrogen-bonding interactions between the imidazole molecules within the pores. This is substantiated by another increase in proton conductivity by two orders of magnitude, compared to 1_HIm.
In summary, we incorporated imidazole into the pores of the MOFs Al-MIL-53-(CH3)x (x = 0, 1, 2) and CAU-11. Imidazole molecules could be successfully localized inside the pores of Al-MIL-53-(CH3)x_HIm (1_HIm, 2_HIm, 3_HIm) using PXRD data by a combination of force-field calculations and Rietveld refinement. Hydrogen bonds of varying strength were found for 1–3_HIm between the μ-OH groups of the framework and the nitrogen atom of the imidazole molecules. The different values of conductivity of 1–3_HIm are consistent with the distance between the μ-OH group and the imidazole molecule (Table 3). In addition, we used CAU-11 as a host to further increase the conductivity, as the structure of CAU-11 exhibits a hydrophobic inner-pore surface (4_HIm). This results in a proton conductivity two orders of magnitude higher than the best Al-MIL-53-(CH3)x_HIm compound (3.0 × 10−4 S cm−1), and is also reflected in the activation energy which is significantly lower for 4_HIm (0.19 eV) compared to 1–3_HIm.
Conclusions
The observed ordering effect on the imidazole molecules through interactions with hydrophilic μ-OH groups within the framework leads to a direct influence on the conductivity of the host–guest compounds. Within this context, it was found that the most hydrophobic framework yields the most conductive host–guest materials. We propose that the introduction of hydrophilic groups leads to the break-down of hydrogen-bonded chains of imidazole molecules (oligomers) within the host framework. In consequence, the hydrophilic groups of the framework might slow down the rearrangement of the HIm molecules, which is causing a drop in proton conductivity. Further NMR-spectroscopic studies on the compounds are currently carried out. In addition, it would be compelling to investigate other hydrophobic frameworks towards their beneficial properties regarding the proton conductivity. Furthermore, it is necessary to address if similar effects can be found for other guest molecules possessing, for example, more acidic properties.Acknowledgements
This project was supported by the German Science Foundation (STO 643/10-1 and WA 1116/17-2). Thomas Homburg thanks the Deutsche-Bundesstiftung-Umwelt (DBU) and the Deutscher Akademischer Austauschdienst (DAAD) for financial support.Notes and references
- A. Iulianelli, P. Ribeirinha, A. Mendes and A. Basile, Renewable Sustainable Energy Rev., 2014, 29, 355–368 CrossRef CAS.
- S. Holdcroft, Chem. Mater., 2014, 26, 381–393 CrossRef CAS.
- S. Subianto, M. Pica, M. Casciola, P. Cojocaru, L. Merlo, G. Hards and D. J. Jones, J. Power Sources, 2013, 233, 216–230 CrossRef CAS.
- A. Chandan, M. Hattenberger, A. El-kharouf, S. Du, A. Dhir, V. Self, B. G. Pollet, A. Ingram and W. Bujalski, J. Power Sources, 2013, 231, 264–278 CrossRef CAS.
- Y. Ye, X. Wu, Z. Yao, L. Wu, Z. Cai, L. Wang, X. Ma, Q.-H. Chen, Z. Zhang and S. Xiang, J. Mater. Chem., 2016, 4, 4062–4070 RSC.
- Y. Ye, L. Zhang, Q. Peng, G.-E. Wang, Y. Shen, Z. Li, L. Wang, X. Ma, Q.-H. Chen, Z. Zhang and S. Xiang, J. Am. Chem. Soc., 2015, 137, 913–918 CrossRef CAS PubMed.
- S. Liu, Z. Yue and Y. Liu, Dalton Trans., 2015, 44, 12976–12980 RSC.
- D. Umeyama, S. Horike, M. Inukai, T. Itakura and S. Kitagawa, J. Am. Chem. Soc., 2012, 134, 12780–12785 CrossRef CAS PubMed.
- S. Horike, W. Chen, T. Itakura, M. Inukai, D. Umeyama, H. Asakura and S. Kitagawa, Chem. Commun., 2014, 50, 10241–10243 RSC.
- M. Inukai, S. Horike, D. Umeyama, Y. Hijikata and S. Kitagawa, Dalton Trans., 2012, 41, 13261–13263 RSC.
- J. A. Hurd, R. Vaidhyanathan, V. Thangadurai, C. I. Ratcliffe, I. L. Moudrakovski and G. K. H. Shimizu, Nat. Chem., 2009, 1, 705–710 CrossRef CAS PubMed.
- D. Umeyama, S. Horike, M. Inukai, Y. Hijikata and S. Kitagawa, Angew. Chem., Int. Ed., 2011, 50, 11706–11709 CrossRef CAS PubMed.
- P. Ramaswamy, N. E. Wong and G. K. H. Shimizu, Chem. Soc. Rev., 2014, 43, 5913–5932 RSC.
- D. Ye, Z. Tu, Y. Yu, Y. Cai, H. Zhang, Z. Zhan and M. Pan, Int. J. Energy Res., 2014, 38, 1181–1191 CrossRef CAS.
- P. Choi, N. H. Jalani, T. M. Thampan and R. Datta, J. Polym. Sci., Part B: Pol. Phys., 2006, 44, 2183–2200 CrossRef CAS.
- D. K. Paul, A. Fraser and K. Karan, Electrochem. Commun., 2011, 13, 774–777 CrossRef CAS.
- K.-D. Kreuer, Chem. Mater., 1996, 8, 610–641 CrossRef CAS.
- S. J. Hamrock and M. A. Yandrasits, Polym. Rev., 2006, 46, 219–244 CAS.
- W. Chen, S. Horike, D. Umeyama, N. Ogiwara, T. Itakura, C. Tassel, Y. Goto, H. Kageyama and S. Kitagawa, Angew. Chem., Int. Ed., 2016, 55, 5195–5200 CrossRef CAS PubMed.
- B.-L. Liu, H.-Y. Zang, H.-Q. Tan, Y.-H. Wang and Y.-G. Li, CrystEngComm, 2016, 18, 3300–3305 RSC.
- A. S. Munn, R. S. Pillai, S. Biswas, N. Stock, G. Maurin and R. I. Walton, Dalton Trans., 2016, 45, 4162–4168 RSC.
- N. Reimer, B. Bueken, S. Leubner, C. Seidler, M. Wark, D. De Vos and N. Stock, Chem. – Eur. J., 2015, 21, 12517–12524 CrossRef CAS PubMed.
- T. N. Tu, N. Q. Phan, T. T. Vu, H. L. Nguyen, K. E. Cordova and H. Furukawa, J. Mater. Chem. A, 2016, 4, 3638–3641 CAS.
- T. Yamada, K. Otsubo, R. Makiura and H. Kitagawa, Chem. Soc. Rev., 2013, 42, 6655–6669 RSC.
- M. Yoon, K. Suh, S. Natarajan and K. Kim, Angew. Chem., Int. Ed., 2013, 52, 2688–2700 CrossRef CAS PubMed.
- S. Bureekaew, S. Horike, M. Higuchi, M. Mizuno, T. Kawamura, D. Tanaka, N. Yanai and S. Kitagawa, Nat. Mater., 2009, 8, 831–836 CrossRef CAS PubMed.
- T. Loiseau, C. Serre, C. Huguenard, G. Fink, F. Taulelle, M. Henry, T. Bataille and G. Férey, Chem. – Eur. J., 2004, 10, 1373–1382 CrossRef CAS PubMed.
- S. Biswas, T. Ahnfeldt and N. Stock, Inorg. Chem., 2011, 50, 9518–9526 CrossRef CAS PubMed.
- E. Eisbein, J.-O. Joswig and G. Seifert, Microporous Mesoporous Mater., 2015, 216, 36–41 CrossRef CAS.
- N. Reimer, H. Reinsch, A. K. Inge and N. Stock, Inorg. Chem., 2015, 54, 492–501 CrossRef CAS PubMed.
- A. I. Materials, Studio Version 5.0, San Diego, CA, 2009 Search PubMed.
- C. S. Topas, Academics 4.2, 2007 Search PubMed.
- J. R. Macdonald and W. B. Johnson, in Impedance Spectroscopy, John Wiley & Sons, Inc., 2nd Edition edn, 2005, DOI:10.1002/0471716243.ch1.
- G. Alberti, M. Casciola, L. Massinelli and B. Bauer, J. Membr. Sci., 2001, 185, 73–81 CrossRef CAS.
- T. Devic, P. Horcajada, C. Serre, F. Salles, G. Maurin, B. Moulin, D. Heurtaux, G. Clet, A. Vimont, J.-M. Grenèche, B. L. Ouay, F. Moreau, E. Magnier, Y. Filinchuk, J. Marrot, J.-C. Lavalley, M. Daturi and G. Férey, J. Am. Chem. Soc., 2010, 132, 1127–1136 CrossRef CAS PubMed.
- H. Reinsch, R. S. Pillai, R. Siegel, J. Senker, A. Lieb, G. Maurin and N. Stock, Dalton Trans., 2016, 45, 4179–4186 RSC.
- P. G. Yot, Z. Boudene, J. Macia, D. Granier, L. Vanduyfhuys, T. Verstraelen, V. Van Speybroeck, T. Devic, C. Serre, G. Ferey, N. Stock and G. Maurin, Chem. Commun., 2014, 50, 9462–9464 RSC.
- H. Wolff, H. Müller and H. Müller, Ber. Bunsenges. Phys. Chem., 1974, 78, 1241–1244 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis details, IR-, NMR- and impedance spectroscopy, thermal analyses, Rietveld refinements. See DOI: 10.1039/c6dt03048c |
| This journal is © The Royal Society of Chemistry 2016 |