Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Palladium nanoparticles supported on a nickel pyrazolate metal organic framework as a catalyst for Suzuki and carbonylative Suzuki couplings†
A. W.
Augustyniak
a,
W.
Zawartka
a,
J. A. R.
Navarro
*b and
A. M.
Trzeciak
*a
aFaculty of Chemistry, University of Wrocław, 14 F. Joliot-Curie, 50-383 Wrocław, Poland. E-mail: anna.trzeciak@chem.uni.wroc.pl
bDepartamento de Química Inorgánica, Universidad de Granada, Av. Fuentenueva S/N, 18071 Granada, Spain. E-mail: jarn@ugr.es
First published on 27th July 2016
Abstract
Methanolic reduction of [PdCl2(CH3CN)2] on a [Ni(2,5-di(1H-pyrazol-4-yl)benzenesulfonate)2] metal organic framework gives rise to Pd2+/Pd0 nanocomposites with Suzuki and carbonylative Suzuki heterogeneous catalytic activities.
One of the major challenges in modern catalysis is the efficient separation of a metal catalyst from organic products.1–7 This is particularly important in palladium catalysed cross-coupling reactions that lead to products which can be utilized in the pharmaceutical industry. For example, the Suzuki–Miyaura reaction presents an attractive pathway to substituted biaryls, precursors of anti-inflammatory drugs.8–12 The carbonylative Suzuki–Miyaura coupling offers easy access to diarylated ketones used in UV-screens, dyes, agrochemicals and pharmaceuticals.13–15
Application of heterogeneous catalysts to these reactions enables improvement of the separation step and minimizes the palladium content in the reaction products. In this regard, metal organic frameworks, MOFs, have attracted an increasing attention as potential supports for palladium.16–22 Advantageously, MOFs possess large surface areas with well-defined pores of shapes that can be modulated. These highly porous materials can be easily modified by several functional groups facilitating the bonding of a variety of metal ions and metal nanoparticles (NPs).16–22
One of the first reports dealing with the application of the MOF in the Suzuki–Miyaura reaction was the palladium(II) based system [Pd(2-pymo)2]n (2-pymo = 2-hydroxypyrimidinolate)23,24 which remained unaltered under the reaction conditions, at 150 °C, as proven by X-ray powder diffraction.24 Other MOFs, mainly carboxylate systems of the MIL family, were used as supports for palladium catalysts of the Suzuki–Miyaura coupling.25–36 Thus, Pd NPs immobilized on MIL-101(Cr) formed efficient heterogeneous catalysts of the water-mediated coupling of aryl chlorides at 80 °C with a TBAB additive.25 Palladium supported on amino-functionalized MIL-53(Al)NH2 catalyzed the Suzuki–Miyaura coupling with high activity and recyclability.26 The effect of palladium loading on the catalytic performance was studied for Pd NPs immobilized on MIL-101(Cr)NH2 and the best activity and recyclability were obtained for 8 wt% of Pd.27 Further studies with this catalyst indicated that the type of the base used in the catalytic reaction has a decisive influence on the stability of the MOF structure.28 Noteworthily, the MOF catalyst was degraded by the presence of carbonates, while it remained crystalline with fluorides.28 Decomposition of Pd-MIL-101(Cr) and leaching of the active phase were observed.29 A similar catalyst, Pd-MIL-101, containing well dispersed Pd NPs obtained by a “double solvent” method acted as the heterogeneous and recyclable catalyst of bromo- and iodobenzene coupling.30
Pd-NH2-MIL-125 catalyzed the Suzuki–Miyaura coupling of aryl chlorides at 100 °C in methanol with a yield of ca. 80%.31 It was also successfully recycled and used in four subsequent runs. The catalyst Pd-UiO-66, obtained by the microwave-assisted method, exhibited high catalytic activity in the Suzuki–Miyaura coupling of aryl bromides and chlorides at 30 °C.32 Pd NPs dispersed on the surface of DUT-67 micro-crystals were used for coupling of iodobenzene at 70 °C in water–ethanol.33 Interestingly, the Pd(II) complex with a 2,2,-bypyridine-5,5,-dicarboxylate linker incorporated into the MOF (UiO-67) showed a remarkably higher activity than the homogeneous Pd counterparts.34
Noteworthily, while the Pd–MOF catalysts found application in the Suzuki–Miyaura coupling, this work represents the first example of their use under a CO atmosphere. To the best of our knowledge, the only example until now was reported for the Sonogashira carbonylative coupling with a Pd(II)–Zr–MOF–BIPY system.37
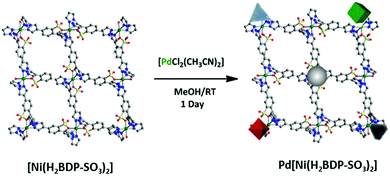
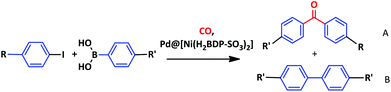
Most of the studies on the Pd–MOF systems in the Suzuki–Miyaura reaction are based on carboxylate MOFs of the MIL/UiO families. In this regard, testing of other MOF materials of high chemical stability as supports for palladium could be beneficial. To this aim, MOFs containing strong donor azolate ligands like imidazolates or pyrazolates could be considered.38,39 Following this idea, we decided to choose pyrazolato based MOF containing sulfonate functionalities, [Ni(H2BDP-SO3)2], for palladium immobilization.40 We expected that the system, built from the assembly of Ni(II) cations and 2,5-di(1H-pyrazol-4-yl)benzenesulfonate linkers might be a suitable MOF support for Pd2+ and Pd NPs since both palladium forms are present in the Suzuki–Miyaura catalytic cycle (Scheme 1).4,6,7
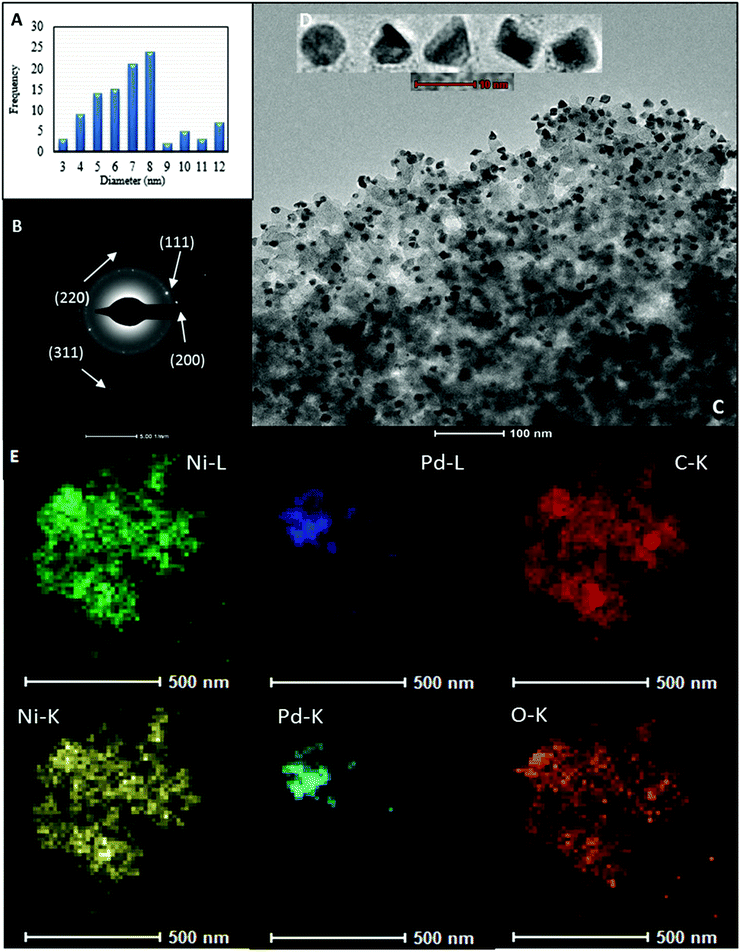
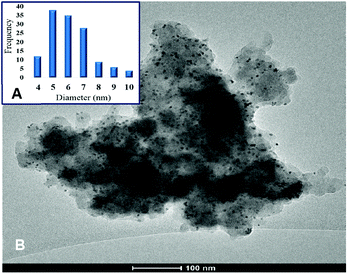
Noteworthily, the impregnation of the [Ni(H2BDP-SO3)2] system with a methanolic solution of [PdCI2(CH3CN)2] at room temperature gave rise to the straightforward formation of Pd@[Ni(H2BDP-SO3)2] containing both Pd2+ ions and Pd NPs embedded in the MOF material. The uploaded amount of Pd, as determined by induced coupling plasma (ICP) analysis, is up to 5.6 wt%. TEM images indicated that the Pd NPs are homogeneously distributed on the MOF microparticles (Fig. 1) (for details see the ESI†). Remarkably, MeOH acted as both a solvent and a reductant.41 Indeed, we have observed a quick change of the colour of the [PdCI2(CH3CN)2] solution from yellow to dark brown, while the MOF micropowder changed the colour from yellow to dark red, indicating that the palladium precursor was reduced to Pd NPs. The as-prepared Pd NPs were characterized by transmission electron microscopy (TEM) (see Fig. 1C and D) and elemental mapping images (Fig. 1E). The corresponding electron diffraction pattern (SAED) for the selected area is shown in Fig. 1B demonstrating that Pd NPs are crystalline. In the SAED pattern we observe the diffraction rings matched with (111), (200), (220), and (311) crystallographic planes of Pd nanocrystals, respectively. The TEM images also established that the palladium nanocrystals are in the 4–8 nm size distribution (Fig. 1A). It should also be noted that Pd NPs exhibit very-well defined geometrical shapes even though the synthesis conditions were very mild. This is unusual since the production of Pd NPs typically involves the addition of a reducing agent (i.e. N2H4, NaBH4, H2) leading to less defined shapes in most cases.42,43 In this regard, the use of methanol as a reducing agent, under the reported mild conditions, seems to be advantageous since it leads to a higher control over the shape and size of palladium nanocrystals compared to traditional reducing agents (Fig. 1D). Moreover, analysis of X-ray photoelectron spectroscopy (XPS) spectra confirmed the presence of both Pd(0) (334.0, 339.3 eV) and Pd2+ (336.9, 342.6 eV) in a 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85 ratio. On the other hand, XPS lines of Pd2+ are shifted towards lower binding energies, as compared to pristine [PdCl2(CH3CN)2] indicating a change of the palladium environment. In fact, FT-IR analysis exhibited that the characteristic absorbance band for a C
85 ratio. On the other hand, XPS lines of Pd2+ are shifted towards lower binding energies, as compared to pristine [PdCl2(CH3CN)2] indicating a change of the palladium environment. In fact, FT-IR analysis exhibited that the characteristic absorbance band for a C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond at ca. 2340 cm−1 was absent (Fig. S4†), suggesting that CH3CN is removed from the coordination sphere of palladium during immobilization. The FT-IR region 600–100 cm−1 was also studied. There is a strong absorption band centred at 352 cm−1 which can unequivocally be assigned to ν(Pd–Cl).44 Additionally, EDX analysis shows a ca. 1
N bond at ca. 2340 cm−1 was absent (Fig. S4†), suggesting that CH3CN is removed from the coordination sphere of palladium during immobilization. The FT-IR region 600–100 cm−1 was also studied. There is a strong absorption band centred at 352 cm−1 which can unequivocally be assigned to ν(Pd–Cl).44 Additionally, EDX analysis shows a ca. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 atomic ratio of Pd
1 atomic ratio of Pd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cl. These results suggest that PdCl+ cations might be anchored to the outer surface of the Pd NPs and further stabilized in the [Ni(H2BDP-SO3)2] structure by interactions with sulfonate oxygen atoms of the organic linkers. Indeed, two additional bands centred at 420 and 455 cm−1 might be assigned to ν(Pd–O), supporting the plausible interaction of the core shell NPs with the sulfonate groups of the organic spacers. Alternatively, additional stabilizing interactions between Pd–Cl cations and NH groups of pyrazole could also be considered.
Cl. These results suggest that PdCl+ cations might be anchored to the outer surface of the Pd NPs and further stabilized in the [Ni(H2BDP-SO3)2] structure by interactions with sulfonate oxygen atoms of the organic linkers. Indeed, two additional bands centred at 420 and 455 cm−1 might be assigned to ν(Pd–O), supporting the plausible interaction of the core shell NPs with the sulfonate groups of the organic spacers. Alternatively, additional stabilizing interactions between Pd–Cl cations and NH groups of pyrazole could also be considered.
The low-angle XPRD pattern of the as-prepared [Ni(H2BDP-SO3)2] (Fig. 2A) is in agreement with the simulated pattern reported by Colombo et al.40 In addition, the structure of [Ni(H2BDP-SO3)2] remains intact after palladium uploading and no changes in the XPRD patterns were detected, which supports that [Ni(H2BDP-SO3)2] was chemically stable during the palladium reduction process and ulterior catalytic reaction. We have also evaluated the effect of palladium incorporation into the [Ni(H2BDP-SO3)2] system by means of N2 adsorption isotherms at 77 K (see Fig. 2B and Table 1). Noteworthily, the porous structure in Pd@[Ni(H2BDP-SO3)2] is still highly accessible, although a diminution of approx. 45% in the specific surface area (SA) and pore volume is noticed. On the other hand, the TGA analysis reveals that the [Ni(H2BDP-SO3)2] material possesses a notable thermal stability of up to 350 °C (Fig. S1†).
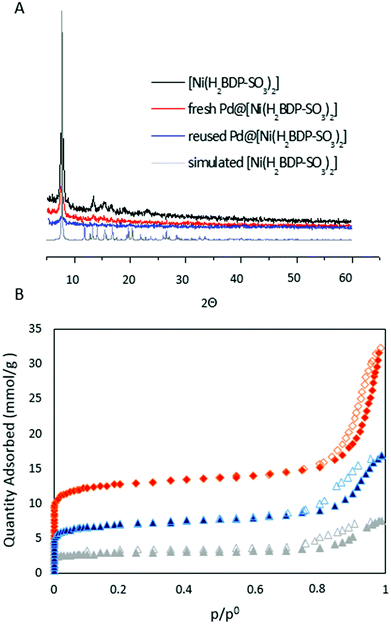 | ||
| Fig. 2 (A) XPRD patterns of [Ni(H2BDP-SO3)2] as synthesized (black), Pd@[Ni(H2BDP-SO3)2] (red), 4-use catalytic cycle (blue), simulated (grey);40 (B) N2 adsorption isotherms measured at 77 K for [Ni(H2BDP-SO3)2] (red), Pd@[Ni(H2BDP-SO3)2] (blue) and reused Pd@[Ni(H2BDP-SO3)2] (grey). Filled and empty symbols represent adsorption and desorption, respectively. | ||
Once the physicochemical features of the Pd@[Ni(H2BDP-SO3)2] composites were established we proceed to evaluate their plausible catalytic properties.
The C–C coupling reactions between aryl halides (–Br, –Cl) and phenylboronic acid were selected as model reactions for testing the catalytic activity of Pd@[Ni(H2BDP-SO3)2]. With this aim, the reaction parameters were optimized using 4-bromoanisole as a screening substrate, and the results are summarized in Tables 2 and S1 (see the ESI†).
| Entry | Aryl halide | Yielda (%)* | Yielda (%)** |
|---|---|---|---|
| a Reaction conditions: aryl halide (1 mmol), phenylboronic acid (1.5 mmol), K2CO3 (1.5 mmol), iPrOH (2.5 mL), H2O (2.5 mL), Pd@[Ni(H2BDP-SO3)2] (0.5 mol% of Pd), *8 h at 25 °C, **4 h at 60 °C, ***20 h at 100 °C. | |||
| 1 |
|
85 | 99 |
| 2 |
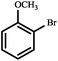
|
58 | 75 |
| 3 |
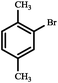
|
88 | 94 |
| 4 |
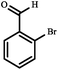
|
69 | 76 |
| 5 |
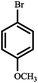
|
91 | 98 |
| 6 |
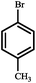
|
91 | 97 |
| 7 |
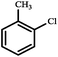
|
— | 24*** |
The reaction did not proceed either in the absence of a catalyst (Table S1, entry 1†) or in the presence of pristine [Ni(H2BDP-SO3)2] (Table S1, entry 2†), which confirms the catalytic activity of Pd@[Ni(H2BDP-SO3)2]. Indeed, Pd@[Ni(H2BDP-SO3)2] was active, in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
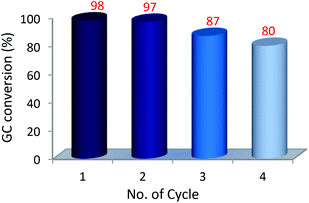
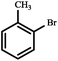
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 2-propanol and water solvent mixtures at 25 °C forming up to 91% of the coupling product. Under these conditions (25 °C) reactions of different aryl halides with phenylboronic acid showed conversions from 30% for 2-chlorotoluene to 91% for 4-bromoanisole and 4-bromotoluene. The highest conversions, up to 97–99%, were obtained for 2-bromotoluene, 4-bromotoluene and 4-bromoanisole at 60 °C after a 4 h reaction (Table 2). Kinetic profiles of Suzuki–Miyaura reactions of 4-bromoanisole, illustrating a positive effect of a higher temperature on the reaction course, are shown in Fig. 3. By contrast, in the reaction of 2-chlorotoluene at 100 °C only 24% of the corresponding coupling product was formed as a result of higher strength of the C–Cl bond compared to C–Br (Table 2). The recyclability of the Pd@[Ni(H2BDP-SO3)2] catalyst was tested over the Suzuki–Miyaura reaction (Fig. 4). After each run, the solid catalyst was easily collected by centrifugation, washed with MeOH, and used for a further run up to four consecutive runs under the same conditions.
1 2-propanol and water solvent mixtures at 25 °C forming up to 91% of the coupling product. Under these conditions (25 °C) reactions of different aryl halides with phenylboronic acid showed conversions from 30% for 2-chlorotoluene to 91% for 4-bromoanisole and 4-bromotoluene. The highest conversions, up to 97–99%, were obtained for 2-bromotoluene, 4-bromotoluene and 4-bromoanisole at 60 °C after a 4 h reaction (Table 2). Kinetic profiles of Suzuki–Miyaura reactions of 4-bromoanisole, illustrating a positive effect of a higher temperature on the reaction course, are shown in Fig. 3. By contrast, in the reaction of 2-chlorotoluene at 100 °C only 24% of the corresponding coupling product was formed as a result of higher strength of the C–Cl bond compared to C–Br (Table 2). The recyclability of the Pd@[Ni(H2BDP-SO3)2] catalyst was tested over the Suzuki–Miyaura reaction (Fig. 4). After each run, the solid catalyst was easily collected by centrifugation, washed with MeOH, and used for a further run up to four consecutive runs under the same conditions.
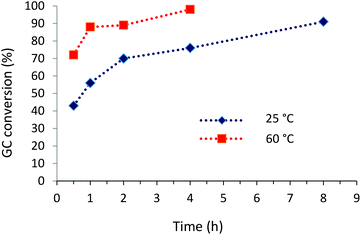 | ||
| Fig. 3 Time conversion plot for the Suzuki–Miyaura coupling of 4-bromoanisole with phenylboronic acid. | ||
The results show a relatively small conversion decrease, from 98 to 80%, highlighting the good stability of the Pd@[Ni(H2BDP-SO3)2] catalyst. After the 4th use, the catalyst was examined by XPRD (Fig. 2A) and TEM (Fig. S3†) and showed a certain degree of MOF crystallinity loss. Noteworthily, the amount of Pd(0) increased from the original 15% to 39% similarly to other heterogenized palladium systems in Suzuki–Miyaura reactions45–47 (Fig. S2†). To estimate the role of soluble palladium forms leached from the solid support, in the catalytic process, the activity of the liquid phase was investigated. However, the filtrate obtained after separation of the Pd@[Ni(H2BDP-SO3)2] catalyst showed no activity (Table S1, entry 7†). According to ICP analysis, the palladium content in the liquid phase was 0.035 ppm evidencing insignificant palladium leaching. Consequently, the heterogeneous reaction pathway can be proposed as the main one, with catalytic activity taking place on the Pd NP surface.
Furthermore, TEM images of the recovered catalyst indicate that the Pd NPs are homogeneously dispersed even after the catalytic reactions (Fig. 5A and B).
Moreover, agglomeration of Pd NPs was not appreciable but some decrease of an average size was noted. The morphology was also changed and Pd NPs became more round. These observations support the suggestion that Pd NPs do actively participate in the catalytic reaction taking place on their surface. The Pd amount found in the reused Pd@[Ni(H2BDP-SO3)2], as determined by ICP analysis, was 5.3 wt%. The specific area determined by N2 adsorption decreased to 246 m2 g−1, probably as a result of incorporation of organic products into pores (Table 1). As the leaching of palladium was not significant, it can be assumed that the decrease of the catalytic activity during recycling is caused by lower availability of active palladium centres blocked by organic products.
Once we proved that the Pd@[Ni(H2BDP-SO3)2] system was catalytically active in the Suzuki–Miyaura coupling, we decided to test it in a more demanding reaction, like carbonylative Suzuki–Miyaura coupling. It was interesting to check whether the catalytic activity can be switched from standard coupling to the carbonylative coupling by a simple change in the reaction conditions. The carbonylative coupling of iodoanisole with phenylboronic acid was selected as the model reaction, and the results are summarized in Tables 3 and S2 (see the ESI†). Anisole, toluene and DMF were chosen as solvents, while K2CO3, KHCO3 and KOH were selected as bases. At 1 bar of CO only 21% of diarylketone was formed, however, its yield increased to 51% after pressure was increased to 5 bar. At 10 bar of CO diarylketone was obtained as the only product. Consequently, this pressure was used in further experiments performed in anisole as a solvent with 1 mol% of Pd@[Ni(H2BDP-SO3)2]. Reactions of different aryliodides with phenylboronic acid show conversions from 33% to 71% (Table 3). The highest conversion, up to 71%, was obtained for 3-iodoanisole. Typically, a correlation between the conversion and position of the substituent in the aryl ring is observed.
| Entry | Aryl halides | Boronic acid | Conv. (%) | A (%) | B (%) |
|---|---|---|---|---|---|
| a Reaction conditions: aryl halide (1 mmol), phenylboronic acid (1.2 mmol), Pd@[Ni(H2BDP-SO3)2] (1.0 mol% of Pd), K2CO3 (3 mmol), solvent (anisole) 5 mL, CO (10 bar) at 105 °C. *CO balloon, **CO (5 bar), ***CO (10 bar). b 0.5 mol% of Pd at 105 °C. | |||||
| 1 |
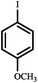
|
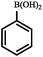
|
80*,b | 21 | 59 |
| 2 |
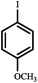
|
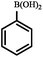
|
66**,b | 51 | 15 |
| 3 |
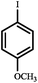
|
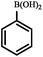
|
50***,b | 50 | 0 |
| 4 |
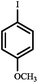
|
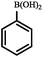
|
58 | 58 | 0 |
| 5 |
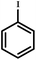
|
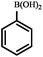
|
33 | 33 | 0 |
| 6 |
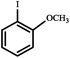
|
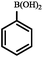
|
54 | 54 | 0 |
| 7 |
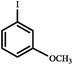
|
|
71 | 71 | 0 |
| 8 |
|
|
59 | 59 | 0 |
| 9 |
|
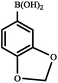
|
53 | 53 | 0 |
| 10 |
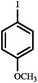
|
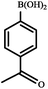
|
35 | 35 | 0 |
The much higher reactivity of 3-iodoanisole, as compared to 4-iodoanisole and 2-iodoanisole, may be attributed to the reduced pore availability of Pd@[Ni(H2BDP-SO3)2] for a 3-iodoanisole molecule facilitating the reaction on the surface of Pd@[Ni(H2BDP-SO3)2]. Reactions with substituted boronic acids showed conversions ranging from 59% for 3,5-dimethoxyboronic acid to 35% for 4-acetylphenylboronic acid. Interestingly the best yield was observed for bulky 3,5-dimethoxyboronic acid. In all reactions at 10 bar of CO the Pd@[Ni(H2BDP-SO3)2] catalyst gave exceptionally 100% selectivity.
Conclusions
A new green synthetic method leading to Pd@[Ni(H2BDP-SO3)2] containing Pd2+ and Pd NPs in a Ni(II) pyrazolate MOF has been presented. Noteworthily, the obtained crystalline Pd NPs supported on the [Ni(H2BDP-SO3)2] MOF material can be regarded as a highly active heterogeneous catalyst for the Suzuki–Miyaura coupling in an environmentally friendly mixture of solvents (2-propanol/water). The catalyst can be recovered and reused for at least four runs without a significant loss in its activity. At 25 °C our catalyst converted 91% of 4-bromotoluene and 4-bromoanisole to the coupling products. As far as we know, only for a Zn-free MOF-5-NPC-Pd catalyst very good results were obtained at 25 °C.36 In other MOF systems a higher temperature (40–200 °C) was applied. The catalytic activity reported by us at 60 °C with 0.5 mol% of Pd is similar to that found for Pd/MIL-53-NH2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 and Pd-MCM-41.51 On the other hand, a higher activity in the Suzuki–Miyaura reaction was shown by Pd/MOF which produced 92% of the coupling product at 40 °C in 0.5 h.35 Under the same conditions this catalyst converted other bromoarenes to appropriate products in 87–95%.35
30 and Pd-MCM-41.51 On the other hand, a higher activity in the Suzuki–Miyaura reaction was shown by Pd/MOF which produced 92% of the coupling product at 40 °C in 0.5 h.35 Under the same conditions this catalyst converted other bromoarenes to appropriate products in 87–95%.35
The activity of the Pd@[Ni(H2BDP-SO3)2] catalyst can be easily switched to carbonylative coupling by introduction of CO and the excellent selectivity to diarylketone was noted at 10 bar of CO. According to the best of our knowledge we have reported for the first time the results of carbonylative coupling with a MOF supported Pd catalyst. At 1 bar of CO our catalyst produced 21% of ketone, showing lower activity than Pd nanoparticles supported on dopamine-functionalized magnetite.49 Palladium nanoparticles immobilized onto SILLP formed diarylketones with the yield of 60–87% in 12 h at 30 atm of CO at 120 °C.50 These conditions are significantly harsher than used by us. The Pd catalyst supported on amino-modified silica microspheres used at 5 atm of CO in an amount of 0.05 mol% gave better results, up to 90% conversion, in 5 h.48
Further work on exploring new catalytic reactions with such a Pd@MOF system is currently underway.
Acknowledgements
We are grateful for financial support from National Science Centre (NCN, Poland) with grant 2014/15/B/ST5/02101 (A. W. A., W. Z., A. M. T.), Spanish MINECO (CTQ2014-53486-R) and EU FEDER Funding (JARN).Notes and references
- B. C. Gates, Chem. Rev., 1995, 95, 511 CrossRef CAS.
- D. Astruc, F. Lu and J. R. Aranzaes, Angew. Chem., Int. Ed., 2005, 44, 7852 CrossRef CAS PubMed.
- A. Molnar, in Palladium catalyzed coupling reactions, ed. A. Molnar, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2013 Search PubMed.
- N. T. S. Phan, M. van der Sluys and C. W. Jones, Adv. Synth. Catal., 2006, 348, 609 CrossRef CAS.
- L. Yin and J. Liebscher, Chem. Rev., 2007, 107, 133 CrossRef CAS PubMed.
- A. M. Trzeciak and J. J. Ziółkowski, Coord. Chem. Rev., 2005, 249, 2308 CrossRef CAS.
- A. M. Trzeciak and J. J. Ziółkowski, Coord. Chem. Rev., 2007, 251, 1281 CrossRef CAS.
- N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457 CrossRef CAS.
- A. Suzuki, J. Organomet. Chem., 1999, 576, 147 CrossRef CAS.
- N. Miyaura, Top. Curr. Chem., 2002, 219, 11 CrossRef CAS.
- C. Torborg and M. Beller, Adv. Synth. Catal., 2009, 351, 3027 CrossRef CAS.
- J. Magano and J. R. Dunetz, Chem. Rev., 2011, 111, 2177 CrossRef CAS PubMed.
- X.-F. Wu, H. Neumann and M. Beller, Chem. Soc. Rev., 2011, 40, 4986 RSC.
- S. T. Gadge and B. M. Bhanage, RSC Adv., 2014, 4, 10367 RSC.
- R. Skoda-Földes and L. Kollár, Curr. Org. Chem., 2002, 6, 1097 CrossRef.
- J. Gascon, A. Corma, F. Kapteijn and F. X. Llabres i Xamena, ACS Catal., 2014, 4, 361 CrossRef CAS.
- Y. Wu, D. Wang and Y. Li, Chem. Soc. Rev., 2014, 43, 2112 RSC.
- J. Liu, L. Chen, H. Cui, J. Zhang, L. Zhang and Ch. Su, Chem. Soc. Rev., 2014, 43, 6011 RSC.
- Y. Yoon, R. Srirambalaji and K. Kim, Chem. Rev., 2011, 112, 1196 CrossRef PubMed.
- A. H. Chughtai, N. Ahmad, H. A. Younus, A. Laypkov and F. Verpoort, Chem. Soc. Rev., 2015, 44, 6804 RSC.
- Y. Zhang, Y. Zou, Y. Zhao and C.-J. Liu, Catal. Today, 2016, 263, 61 CrossRef CAS.
- Q.-L. Zhu and Q. Xu, Chem. Soc. Rev., 2014, 43, 5468 RSC.
- J. A. R. Navarro, E. Barea, J. M. Salas, N. Masciocchi, S. Galli, A. Sironi, C. O. Ania and J. B. Parra, Inorg. Chem., 2006, 45, 2397 CrossRef CAS PubMed.
- F. X. Llabrés i Xamena, A. Abad, A. Corma and H. Garcia, J. Catal., 2007, 250, 294 CrossRef.
- B. Yuan, Y. Pan, Y. Li, B. Yin and H. Jiang, Angew. Chem., Int. Ed., 2010, 49, 4054 CrossRef CAS PubMed.
- Y. Huang, Z. Zheng, T. Liu, J. Lű, Z. Lin, H. Li and R. Cao, Catal. Commun., 2011, 14, 27 CrossRef CAS.
- V. Pascanu, Q. Yao, A. B. Gomez, M. Gustafsson, Y. Yun, W. Wan, L. Samain, X. Zou and B. Martin-Matute, Chem. – Eur. J., 2013, 19, 17483 CrossRef CAS PubMed.
- F. Carson, V. Pascanu, A. B. Gómez, Y. Zhang, A. E. Platero-Prats, X. Zou and B. Martin-Matute, Chem. – Eur. J., 2015, 21, 10896 CrossRef CAS PubMed.
- J. Juan-Alcañiz, J. Ferrando-Soria, I. Luz, P. Serra-Crespo, E. Skupien, V. P. Santos, E. Pardo, F. X. Llabrés i Xamena, F. Kapteijn and J. Gascon, J. Catal., 2013, 307, 295 CrossRef.
- N. Shang, S. Gao, X. Zhou, Ch. Feng, Z. Wang and Ch. Wang, RSC Adv., 2014, 4, 54487 RSC.
- P. Puthiaraj and W.-S. Ahn, Catal. Commun., 2015, 65, 91 CrossRef CAS.
- W. Dong, Ch. Feng, L. Zhang, N. Shang, S. Gao, Ch. Wang and Z. Wang, Catal. Lett., 2016, 146, 117 CrossRef CAS.
- G.-L. Zhuang, J.-Q. Bai, L. Tan, H.-L. Huang, Y.-F. Gao, X. Zhong, C.-L. Zhong and J.-G. Wang, RSC Adv., 2015, 5, 32714 RSC.
- L. Chen, S. Rangan, J. Li, H. Jiang and Y. Li, Green Chem., 2014, 16, 3978 RSC.
- L. Zhang, Z. Su, F. Jiang, Y. Zhou and W. Xu, Tetrahedron, 2013, 69, 9237 CrossRef CAS.
- W. Dong, L. Zhang, Ch. Wang, Ch. Feng, N. Shang, S. Gao and Ch. Wang, RSC Adv., 2016, 6, 37118 RSC.
- C. Bai, S. Jian, X. Yao and Y. Li, Catal. Sci. Technol., 2014, 4, 3261 CAS.
- V. Colombo, S. Galli, H. J. Choi, G. D. Han, A. Maspero, G. Palmisano, N. Masciocchi and J. R. Long, Chem. Sci., 2011, 2, 1311 RSC.
- J. J. Low, A. I. Benin, P. Jakubczak, J. F. Abrahamian, S. A. Faheem and R. R. Willis, J. Am. Chem. Soc., 2009, 131, 15834 CrossRef CAS PubMed.
- V. Colombo, C. Montoro, A. Maspero, N. Masciocchi, S. Galli, E. Barea and J. A. R. Navarro, J. Am. Chem. Soc., 2012, 134, 12830 CrossRef CAS PubMed.
- M. Pourkhosravani, S. Dehghanpour and F. Farzaneh, Catal. Lett., 2016, 146, 499 CrossRef CAS.
- H. Li, Z. Zhu, F. Zhang, S. Xie, H. Li, P. Li and X. Zhou, ACS Catal., 2011, 1, 1604 CrossRef CAS.
- B. Gole, U. Sanyal, R. Banerjee and P. S. Mukherjee, Inorg. Chem., 2016, 55, 2345 CrossRef CAS PubMed.
- A. de Leon, M. Guerrero, J. Garcia-Antón, J. Ros, M. Font-Bardia and J. Pons, CrystEngComm, 2013, 15, 1762 RSC.
- A. Gniewek, J. J. Ziółkowski, A. M. Trzeciak, M. Zawadzki, H. Grabowska and J. Wrzyszcz, J. Catal., 2008, 254, 121 CrossRef CAS.
- W. Zawartka, P. Pośpiech, M. Cypryk and A. M. Trzeciak, J. Mol. Catal. A: Chem., 2015, 407, 230 CrossRef CAS.
- E. Mieczyńska, T. Borkowski, M. Cypryk, P. Pośpiech and A. M. Trzeciak, Appl. Catal., A, 2014, 470, 24 CrossRef.
- W. Zawartka, P. Pośpiech, M. Cypryk and A. M. Trzeciak, J. Mol. Catal. A: Chem., 2016, 417, 76 CrossRef CAS.
- Y. Long, K. Liang, J. Niu, X. Tong, B. Youan and J. Ma, New J. Chem., 2015, 39, 2988 RSC.
- N. Jiao, Z. Li, Y. Wang, J. Liu and Ch. Xia, RSC Adv., 2015, 5, 26913 RSC.
- S. Jana, S. Haldar and S. Koner, Tetrahedron Lett., 2009, 50, 4820 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, optimization of the reaction conditions, FT-IR spectra, XPS spectra, TGA curve, TEM image of Pd@[Ni(H2BDP-SO3)2] after 4 uses. See DOI: 10.1039/c6dt02242a |
| This journal is © The Royal Society of Chemistry 2016 |