Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Antimony oxofluorides – a synthesis concept that yields phase pure samples and single crystals†
Sk Imran
Ali
and
Mats
Johnsson
*
Department of Materials and Environmental Chemistry, Stockholm University, SE-106 91 Stockholm, Sweden. E-mail: mats.johnsson@mmk.su.se; Fax: +46-8-152187; Tel: +46-8-162169
First published on 30th June 2016
Abstract
The single crystals of the new isostructural compounds Sb3O4F and Y0.5Sb2.5O4F and the two previously known compounds M-SbOF and α-Sb3O2F5 were successfully grown by a hydrothermal technique at 230 °C. The new compound Sb3O4F crystallizes in the monoclinic space group P21/c; a = 5.6107(5) Å, b = 4.6847(5) Å, c = 20.2256(18) Å, β = 94.145(8)°, z = 4. The replacing part of Sb with Y means a slight increase in the unit cell dimensions. The compounds M-SbOF and α-Sb3O2F5 have not been grown as single crystals before and it can be concluded that hydrothermal synthesis has proved to be a suitable technique for growing single crystals of antimony oxofluorides because of the relatively low solubility of such compounds compared to other antimony oxohalides that most often have been synthesised at high temperatures by solid state reactions or gas–solid reactions.
Introduction
There are several compounds described in the Sb3+–O–X (X = F, Cl, Br, I) system e.g. SbOCl, Sb3O4Cl, Sb8O11Cl2 and Sb8O11Br2, α- and β-Sb3O4I.1–4 Five different oxofluorides have previously been reported, three of them are different forms of SbOF denoted as L-SbOF, M-SbOF and H-SbOF and the remaining two are α-Sb3O2F5 and β-Sb3O2F5.5,6 The hardness/softness properties of the halide ions are reflected in how they are bonded in the different crystal structures. Fluoride ions form covalent bonds like oxygen to antimony and are integrated in the Sb–O–F framework while the other halide ions act more as counter ions and the structures become separated into oxide parts and halide parts.The one-sided coordination around p-block cations having stereochemically active lone-pairs increases the chances to find compounds crystallizing in non-centrosymmetric space groups that thus can show non-linear optical properties, e.g. Te2SeO7, Na2TeW2O9 and Bi2TeO5.7–9 Oxohalide glasses can also have non-linear optical properties.10 Further the lone-pairs open up crystal structures and when combining with transition metals it is very often so that the latter arrange in low dimensional arrangements especially in oxohalides like Cu2Te2O5Cl2 and CuNi5(TeO3)4Cl2 where the metal cations tend to bond to both oxygen and halide ions while the lone-pair element tends to bond only to oxygen.11,12 Several such compounds show e.g. magnetic frustration.
We have utilized hydrothermal reaction techniques to grow single crystals of oxofluoride compounds and to form monophasic synthesis products. The new compound Sb3O4F has been synthesized starting with Sb2O3 and SbF3. The compound Y0.5Sb2.5O4F was obtained by introducing YF3 in the reaction mixture. Two previously known compounds M-SbOF and α-Sb3O2F5 were synthesized for the first time as single crystals; the synthesis products were found to be phase pure. The synthesis technique has proved to be suitable for synthesizing oxofluorides, however less suitable for oxohalides comprising Cl, Br, or I due to the higher solubility product of such compounds.
Experimental
Single crystals of Sb3O4F, Y0.5Sb2.5O4F, M-SbOF and α-Sb3O2F5 were synthesized by a hydrothermal technique. The compounds were found during investigations of the Sb–O–F and Y–Sb–O–F systems. All compounds were found from experiments using autoclaves equipped with 18 mL Teflon liners heated to 230 °C at a rate of 1.6 °C min−1. The plateau temperature was maintained for four days and thereafter the temperature was lowered to 30 °C with the same rate as the heating. As starting materials the following chemicals were used: Sb2O3 (99.97%, Sigma-Aldrich), SbF3 (99.8%, Sigma-Aldrich) and YF3 (99.97%, Sigma-Aldrich).Sb3O4F crystals were obtained from a stoichiometric mixture of Sb2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SbF3 = 4
SbF3 = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in 1 mL deionized water. The starting amount was 1.00 mmol Sb2O3. The experiment yielded phase pure colourless Sb3O4F crystals. Crystals of Y0.5Sb2.5O4F and M-SbOF were found from a mixture of Sb2O3
1 in 1 mL deionized water. The starting amount was 1.00 mmol Sb2O3. The experiment yielded phase pure colourless Sb3O4F crystals. Crystals of Y0.5Sb2.5O4F and M-SbOF were found from a mixture of Sb2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) YF3 = 3
YF3 = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in 1 mL deionized water and some few droplets of HF. The synthesis product was a mixture of yellowish crystals (Y0.5Sb2.5O4F) and transparent colourless crystals (M-SbOF). Phase pure M-SbOF was synthesized from a stoichiometric mixture of Sb2O3
1 in 1 mL deionized water and some few droplets of HF. The synthesis product was a mixture of yellowish crystals (Y0.5Sb2.5O4F) and transparent colourless crystals (M-SbOF). Phase pure M-SbOF was synthesized from a stoichiometric mixture of Sb2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SbF3 = 1
SbF3 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in 1 mL deionized water, and α-Sb3O2F5 was obtained from an experiment starting with the same molar ratio Sb2O3
1 in 1 mL deionized water, and α-Sb3O2F5 was obtained from an experiment starting with the same molar ratio Sb2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SbF3 = 1
SbF3 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, however in 1 mL deionized water plus some few droplets of HF. The weight in amounts is given in the ESI.†
1, however in 1 mL deionized water plus some few droplets of HF. The weight in amounts is given in the ESI.†
Chemical compositions were obtained by EDS using a Hitachi M3000 Table top scanning electron microscope and a JEOL JSB-7000F. The EDS results for Y0.5Sb2.5O4F are shown in the ESI.†
Single crystal X-ray data were collected using a Bruker D8 Venture diffractometer equipped with a PHOTON 100 detector. Data integration, including the application of a correction for oblique incidence, was performed with the software package SAINT.13 Absorption correction was applied by the computer program SADABS.14 The crystal structures were solved using the program Superflip and refined by using the program JANA2006.15,16 All atoms are refined with anisotropic temperature displacement parameters. Crystallographic data for all compounds are shown in Table 1.
| Sb3O4F | Y0.5Sb2.5O4F | M-SbOF | α-Sb3O2F5 | |
|---|---|---|---|---|
| Chemical formula | Sb3O4F | Y0.49Sb2.51O4F | M-SbOF | α-Sb3O2F5 |
| Formula weight/g mol−1 | 448.24 | 432.12 | 156.75 | 492.24 |
| Temperature/K | 293 | 293 | 293 | 293 |
| Crystal system | Monoclinic | Monoclinic | Orthorhombic | Monoclinic |
| Space group | P21/c | P21/c | Pbca | P2/c |
| a/Å | 5.6107 (5) | 5.6329 (3) | 11.6718 (3) | 13.2103 (8) |
| b/Å | 4.6847 (5) | 4.7150 (3) | 5.5855 (7) | 5.9519 (3) |
| C/Å | 20.2256 (18) | 20.3675 (12) | 12.2688 (8) | 9.0530 (5) |
| β/° | 94.145 (8) | 94.304 (13) | 90 | 107.865 (18) |
| V/Å3 | 530.2 | 539.4 | 799.8 | 677.5 |
| ρ/g cm−3 | 5.615 | 5.32 | 5.21 | 4.82 |
| Z | 4 | 4 | 16 | 4 |
| Crystal size/mm3 | 0.45 × 0.3 × 0.15 | 0.5 × 0.2 × 0.15 | 0.4 × 0.4 × 0.2 | 0.25 × 0.2 × 0.1 |
| Radiation type | Mo-Kα | Mo-Kα | Mo-Kα | Mo-Kα |
| Wavelength/Å | 0.71069 | 0.71069 | 0.71069 | 0.71069 |
| Indices range | −10 ≤ h ≤ 10 | −9 ≤ h ≤ 9 | −21 ≤ h ≤ 8 | −20 ≤ h ≤ 21 |
| −8 ≤ k ≤ 8 | −7 ≤ k ≤ 7 | −10 ≤ k ≤ 6 | −9 ≤ k ≤ 9 | |
| −36 ≤ l ≤ 35 | −33 ≤ l ≤ 33 | −22 ≤ l ≤ 21 | −14 ≤ l ≤ 14 | |
| No. of reflections | ||||
| Measured/unique | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 860/3296 860/3296 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 095/2615 095/2615 |
9232/2499 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 126/2928 126/2928 |
| Observed [I > 3 σ(I)] | 2785 | 2533 | 1894 | 1831 |
| R int | 0.029 | 0.043 | 0.034 | 0.056 |
(sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ/λ)max/Å−1 θ/λ)max/Å−1 |
0.90 | 0.84 | 0.90 | 0.80 |
| R F/wRF [F > 3σ(F)] | ||||
| All reflections (%) | 2.47/3.05 | 2.93/5.14 | 2.12/2.52 | 2.85/2.78 |
| Goodness of fit (all) | 3.81 | 2.65 | 1.12 | 1.06 |
Further details on the crystal structural investigations may be obtained from the Fachinformationszentrum Karlsruhe, 76344 Eggenstein-Leopoldshafen, Germany (Fax: +49-7247-808-666; e-mail: crysdata@fiz-karlsruhe.de), on quoting the deposit numbers CSD-431207 for Sb3O4F, CSD-431208 for Y0.5Sb2.5O4F, CSD-431209 for M-SbOF, and CSD-431210 for α-Sb3O2F5.
Powder X-ray data were collected on a Panalytical X'Pert PRO powder X-ray diffractometer in Bragg–Brentano geometry with Cu-Kα radiation. Rietveld refinement and the comparison of powder patterns against single crystal data were made by using the program Jana2006.
Thermogravimetric analyses (TG) were performed using a TA instruments Discovery equipment. The measurements were carried out in air with a heating rate of 5 °C min−1 up to 700 °C, starting with an ∼0.5 mg sample.
Results and discussion
It was found to be possible to synthesize the compounds Sb3O4F, Y0.5Sb2.5O4F, α-Sb3O2F5, and M-SbOF by hydrothermal synthesis. The two isostructural compounds Sb3O4F and Y0.5Sb2.5O4F are new while α-Sb3O2F5 and M-SbOF are known for a long time. α-Sb3O2F5 in the form of powder has previously been synthesised by reacting SbF3 and NH4F in water at room temperature and M-SbOF has previously been synthesized as powder by solid state reactions in gold tubes at 220–260 °C.6,17The crystal structure of Sb3O4F and Y0.5Sb2.5O4F
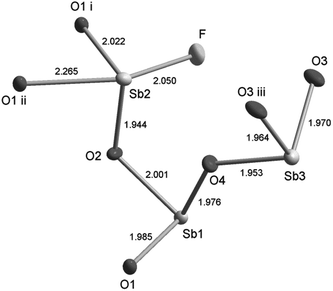
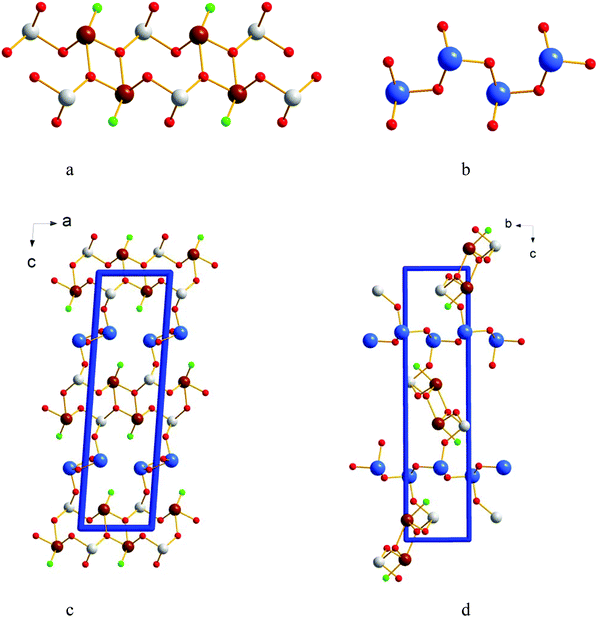
The Sb3O4F crystal structure consists of three crystallographically independent Sb atoms having [Sb(1)O3], [Sb(2)O3F] and [Sb(3)O3] trigonal pyramidal and see-saw co-ordinations, see Fig. 1. If bond distances on the wedge to be included in the primary coordination sphere are also included we end up with [Sb(1)O3+1F], [Sb(2)O3+1F] and [Sb(3)O3] co-ordinations. The long Sb(1)–O(2) and Sb(2)–O(4) bond distances are 2.586(2) Å and 2.679(2) Å respectively and the long Sb(1)–F distance is 2.630(2) Å. The operative definition of the outer primary coordination sphere according to Brown suggests this to be 2.76 Å for Sb–O and 2.67 Å for Sb–F.18 The Sb–O bond distances of ∼2.0 Å in the [SbO3] building blocks show very close proximity to the Sb–O distances in cubic Sb2O3, however the Sb–O–Sb angles differ slightly.19The pairs of edge sharing [Sb(2)O3F] polyhedra are further bridged by two corner sharing [Sb(1)O3] units to make [Sb2O3F]n chains extending along [100], see Fig. 2a. The [Sb(3)O3] trigonal pyramids are corner sharing and make up [Sb(3)O2]n chains extending along [010], see Fig. 2b. The two chain systems connect via Sb(1)–O(4)–Sb(3)–O(3)–Sb(3)–O(4)–Sb(1) to make up the three dimensional framework of Sb3O4F where the F atoms protrude into cavities in the structure and are thus not participating in building the framework, see Fig. 2c and d. The allocation of O and F was based on BVS calculations that show slight hyper-valence for Sb and O and hypo-valence for F indicating its more ionic nature (ESI†). It is not uncommon in oxohalides that halide ions show hypo-valence.20,21 The lone electron pairs, E, on the three crystallographically different Sb3+ ions are stereochemically active and occupy space in the crystal structure and thus become responsible for the open framework that can be seen in Fig. 2c and d. For Sb(1) and Sb(3) the lone-pairs take apex positions in [SbO3E] tetrahedra, and for Sb(2) it forms a [SbO3FE] trigonal bipyramid where E sits at one of the corners of the base plane of the pyramid. The lone-pairs on Sb3+ point into the voids (channels) of the crystal structure as the F-atoms.
A phase pure sample of Sb3O4F could be obtained from a stoichiometric ratio of the starting materials, see Fig. 3.
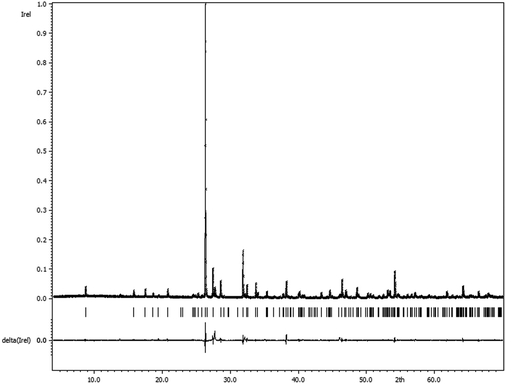 | ||
| Fig. 3 Comparison of the measured powder X-ray diffractogram and the calculated pattern for Sb3O4F based on the single crystal X-ray determination of the crystal structure (Rp = 9.43). | ||
Y0.5Sb2.5O4F is isostructural to Sb3O4F and the presence of Y is responsible for the yellow colour as Sb3O4F is colourless. Y partly occupies all three Sb positions in the crystal structure. The insertion of Y is responsible for the 0.14 Å elongation of the c-axis and while the a- and b-axes are not significantly influenced. All Sb–O and Sb–F distances are slightly longer in Y0.5Sb2.5O4F compared to those in Sb3O4F, see the ESI.† When synthesizing Y0.5Sb2.5O4F the addition level of HF turned out to be very important in order to incorporate Y into the structure. Synthesis attempts were made with increased water content but it did not yield Y incorporated in Sb3+–O–F compounds.
M-SbOF and α-Sb3O2F5
The compounds α-Sb3O2F5 and M-SbOF were found while attempting to synthesize Y0.5Sb2.5O4F. It was also possible to synthesize both phase pure α-Sb3O2F5 and M-SbOF from a mixture of SbF3 and Sb2O3, cf. the Experimental section. The structure determination resulted in the same model as has previously been reported.6,17 However, the present data also allowed refining the ADPs, see Fig. 4a. The present model has a slightly smaller unit cell and small changes in bond distances compared to the older model. Bond-valence sum (BVS) calculations support that the valences are Sb3+, O2− and F− with slightly over-bonded antimony cations and oxygen anions and under-bonded fluorine atoms (see the ESI†).22 The compound M-SbOF has been described by Åström.17 The present structure determination also allows for anisotropic ADPs, see Fig. 4b.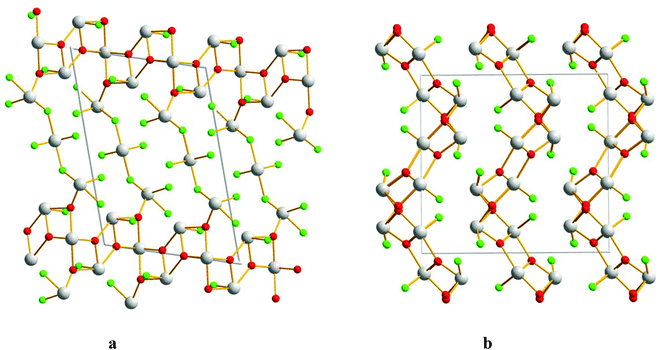 | ||
| Fig. 4 Crystal structures of (a) α-Sb3O2F5 and (b) M-SbOF, projected along [010]. Grey atoms indicate Sb, red stands for O, and green stands for F. | ||
Thermal gravimetry
The thermal decomposition of the compounds Sb3O4F, M-SbOF and α-Sb3O2F5 is shown in Fig. 5. Based on the weight changes the new compound Sb3O4F decomposes in two steps to first give off SbF3 (340–380 °C) and subsequently SbOF in the second step (450–550 °C), see reactions (1) and (2) below. Finally at 550–600 °C there is a slight weight increase when Sb2O3 partly oxidizes to Sb2O5, the latter step is in accordance with previous observations.23| 4Sb3O4F(s) → 5Sb2O3(s) + L-SbOF(s) + SbF3(g) | (1) |
| 5Sb2O3(s) + L-SbOF(s) → 5Sb2O3(s) + L-SbOF(g) | (2) |
| Sb2O3(s) + O2(g) → Sb2O5(s) | (3) |
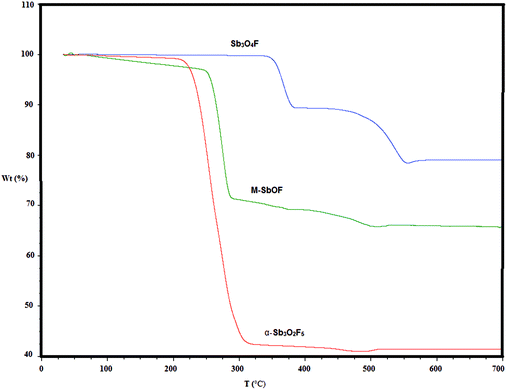 | ||
| Fig. 5 Thermal decomposition of the compounds Sb3O4F, M-SbOF and α-Sb3O2F5, see the text for interpretation of the decomposition steps. | ||
The compound M-SbOF decomposes (250–500 °C) in one step (4) with subsequent oxidation according to step (3).
| 3M-SbOF(s) → Sb2O3(s) + SbF3(g) | (4) |
The compound α-Sb3O2F5 also decomposes (225–325 °C) in one step (5) with subsequent oxidation according to step (3).
| 3α-Sb3O2F5(s) → 2Sb2O3(s) + 5SbF3(g) | (5) |
It can be concluded that Sb3O4F is thermally more stable than M-SbOF and α-Sb3O2F5.
Comparison with other Sb–O–X compounds
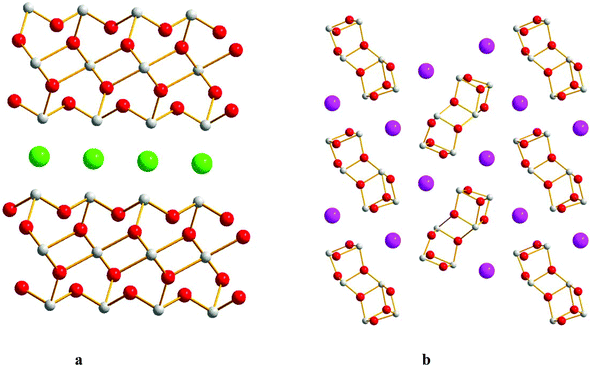
Most compounds in the system Sb3+–O–X (X = F, Cl, Br, I) are layered e.g. Sb4O5(Cl, Br2),24,25 Sb5O7I,26 Sb8O11(Cl, Br, I)2,3,20 Sb3O4I,2 SbOCl,1 and M-SbOF.17 Exceptions are Sb3O4I4 that show ladders of [Sb3O4]n with I-atoms in between, the compounds α-Sb3O2F5, L-SbOF5 and the present compound Sb3O4F that are 3D-frameworks. The compounds in the system Sb3+–O–F have direct covalent bonds in between Sb3+ and F while when the halide ion is one of Cl, Br or I there is a separation into an oxide part consisting of a Sb–O framework made up of [SbO3] and [SbO4] building blocks and a halide part where those ions take the role of counter ions in the crystal structures. However, there is one exception, SbOCl, where there is covalent Sb3+–Cl bonds and trigonal pyramidal [SbO2Cl] building blocks.The oxohalides with the common formula Sb3O4X (X = Cl, I) have completely different crystal structures compared to the present compound Sb3O4F. Sb3O4Cl has a monoclinic structure that crystallizes in the space group P2/c.27 It consists of [Sb3O4]n layers parallel to (100) with Cl atoms situated in between the layers, see Fig. 6a. BVS calculations show the ionic character of the Cl atoms (Vi = 0.64). Sb3O4I exists in two similar forms; orthorhombic α-phase (space group Pbn21) and monoclinic β-phase (space group P21/c). The structures are composed of [Sb3O4] infinite Sb–O tubes along [100] that are separated by I atoms.4 The α-phase is shown in Fig. 6b. BVS calculation on I (Vi = 0.80) reveals a more covalent character for I than for Cl. The main difference between Sb3O4F and the Cl- and I analogues is that F is incorporated in the Sb–O–F network while for the other two the oxide part and the halide part of the crystal structures are separated.
Conclusions
The single crystals of Sb3O4F, Y0.5Sb2.5O4F and the two previously known Sb–O–F compounds, 3M-SbOF and α-Sb3O2F5, were successfully synthesized by hydrothermal methods at 230 °C. Depending on subtle differences in the synthesis method the different compounds were obtained. An interesting outcome of the work is the incorporation of yttrium in Sb3O4F to form Y0.5Sb2.5O4F at such a low temperature used.The new compound Sb3O4F is built from SbO3 and SbO3F polyhedra to form a three dimensional network. Incorporation of yttrium to form Y0.5Sb2.5O4F causes an elongation of the unit cell parameters. The three crystallographically different Sb sites in the crystal structure are all partially occupied by Y. The compounds M-SbOF and α-Sb3O2F5 were previously synthesized by solid state reactions and in this work it was shown that they can form also by hydrothermal reactions. Accurate crystal structures of both compounds were determined from the single-crystal X-ray diffraction data. With respect to the previous structure refinement against powder X-ray diffraction the principal difference is the present refinement of anisotropic ADPs in the present study.
Acknowledgements
Financial support from Stiftelsen Olle Engkvist Byggmästare and the Swedish Research Council is acknowledged.References
- M. Edstrand, Ark. Kemi, 1953, 6, 89–112 CAS.
- H. Katze, Y. Oka, Y. Kanke and T. Z. Yao, Z. Kristallogr., 1999, 214, 284–289 Search PubMed.
- Z. Mayerova, M. Johnsson and S. Lidin, Solid State Sci., 2006, 8, 849–854 CrossRef CAS.
- Z. Hugonin, M. Johnsson and S. Lidin, Solid State Sci., 2009, 11, 24–28 CrossRef CAS.
- A. Åström and S. Andersson, Acta Chem. Scand., 1971, 25, 1519–1520 CrossRef.
- A. A. Udovenko, L. A. Zemnukhova, E. V. Kovaleva and G. A. Fedorishcheva, Russ. J. Coord. Chem., 2004, 30, 618–624 CrossRef CAS.
- Y. Porter, K. M. Ok, N. S. P. Bhuvanesh and P. S. Halasyamani, Chem. Mater., 2001, 13, 1910–1915 CrossRef CAS.
- J. Goodey, J. Broussard and P. S. Halasyamani, Chem. Mater., 2002, 14, 3174–3180 CrossRef CAS.
- K. M. Ok, N. S. P. Bhuvanesh and P. S. Halasyamani, Inorg. Chem., 2001, 40, 1978–1980 CrossRef CAS PubMed.
- R. E. de Araujo, C. B. de Araújo, G. Poirier, M. Poulain and Y. Messaddeq, Appl. Phys. Lett., 2002, 25, 4694–4696 CrossRef.
- M. Johnsson, K. W. Törnroos, F. Mila and P. Millet, Chem. Mater., 2000, 12, 2853–2857 CrossRef CAS.
- M. Johnsson, K. W. Törnroos, P. Lemmmens and P. Millet, Chem. Mater., 2003, 15, 68–73 CrossRef CAS.
- Bruker AXS Inc., Madison, Wisconsin, USA, 2012.
- G. M. Sheldrick, SADABS, Version 2008/1 Search PubMed.
- L. Palatinus and G. Chapuis, J. Appl. Crystallogr., 2007, 40, 785–790 Search PubMed.
- V. Petricek, M. Dusek and L. Palatinus, Z. Kristallogr., 2014, 229, 345–352 CAS.
- A. Åström, Acta Chem. Scand., 1972, 26, 3849–3854 CrossRef.
- I. D. Brown, Chemical bond in inorganic chemistry, Oxford University Press, New York, 2002 Search PubMed.
- C. Svensson, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1975, 31, 2016–2018 CrossRef.
- S. Lidin, M. Johnsson and Z. Hugonin, Solid State Sci., 2009, 11, 1198–1205 CrossRef CAS.
- S. Hu, M. Johnsson, P. Lemmens, D. Schmid, D. Menzel, J. Tapp and A. Möller, Chem. Mater., 2014, 26, 3631–3636 CrossRef CAS.
- N. E. Brese and M. O'Keeffe, Acta Crystallogr., Sect. B: Struct. Sci., 1991, 47, 192–197 CrossRef.
- P. Čerič and B. Bukovec, Thermochim. Acta, 1992, 195, 73–84 CrossRef.
- M. Edstrand, Acta Chem. Scand., 1947, 1, 178–203 CrossRef CAS.
- C. Saernstrand, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1978, 34, 2402–2407 CrossRef.
- V. Kraemer, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1975, 31, 234–237 CrossRef.
- T. Katzke, H. Oka, Y. Kanke, Y. Kato and K. Yao, Z. Kristallogr., 1999, 284, 284–289 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6dt01897a |
| This journal is © The Royal Society of Chemistry 2016 |