The influence of gold(i) on the mechanism of thiolate, disulfide exchange†
Abstract
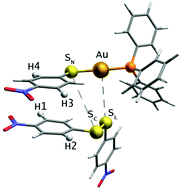
The mechanism of gold(I)-thiolate, disulfide exchange was investigated by using initial-rate kinetic studies, 2D (1H–1H) ROESY NMR spectroscopy, and electrochemical/chemical techniques. The rate law for exchange is overall second order, first order in gold(I)-thiolate and disulfide. 2D NMR experiments show evidence of association between gold(I)-thiolate and disulfide. Electrochemical/chemical investigations do not show evidence of free thiolate and are consistent with a mechanism involving formation of a [Au–S, S–S], four-centered metallacycle intermediate during gold(I)-thiolate, disulfide exchange.

 Please wait while we load your content...
Please wait while we load your content...