Open Access Article
Open Access ArticleExpanding the family of bis-cyclometalated chiral-at-metal rhodium(III) catalysts with a benzothiazole derivative†
Jiajia
Ma
a,
Xiaodong
Shen
a,
Klaus
Harms
a and
Eric
Meggers
*ab
aPhilipps-Universität Marburg, Hans-Meerwein-Strasse 4, 35043 Marburg, Germany. E-mail: meggers@chemie.uni-marburg.de
bCollege of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, People's Republic of China
First published on 22nd April 2016
Abstract
Synthetic access to previously elusive single enantiomers of an octahedral chiral-at-metal rhodium(III) complex containing two cyclometalated 2-phenylbenzothiazoles and two acetonitrile ligands is reported. The complex is a superior chiral Lewis acid catalyst compared to its benzoxazole congener which can be rationalized with a higher steric congestion around the coordination sites.
Chiral Lewis acids play an important role in asymmetric catalysis because many reactions are amenable to Lewis acid activation.1 Recently, we introduced a new class of chiral Lewis acids based on octahedral iridium(III)2–7 and rhodium(III)8–11 complexes which draw their chirality exclusively from the metal-centered chirality (metal centrochirality).12,13 These chiral-only-at-metal complexes are cyclometalated by two 5-tert-butyl-2-phenylbenzoxazoles or the analogous benzothiazole ligands in addition to two exchange-labile acetonitriles, which generates a C2-symmetric, propeller-type geometry.
Whereas the iridium complexes Λ/Δ-IrO and Λ/Δ-IrS have been demonstrated to be excellent catalysts for visible light induced photoredox reactions,3,4,6,7 the rhodium congener Λ/Δ-RhO features advantages for regular Lewis acid catalysis,8–10 apparently due to a more rapid ligand exchange kinetics (Table 1). We expected that the related complex Λ/Δ-RhS, in which the coordinating benzoxazole moieties are replaced by benzothiazoles, should provide a higher asymmetric induction due to an increased bond length of C–S over C–O which will position the two tert-butyl groups somewhat closer to the substrate coordination site. Here we disclose the previously elusive access to the enantiomerically pure benzothiazole complexes Λ- and Δ-RhS, characterize their structures and configurational stability, and demonstrate their excellent performance as asymmetric catalysts.
| Entry | Complex | M | X | Remarks |
|---|---|---|---|---|
| a Synthesis by an auxiliary-mediated strategy. | ||||
| 1 | Λ- and Δ-IrO | Ir | O | Ref. 2 and 4 |
| 2 | Λ- and Δ-IrS | Ir | S | Ref. 3 and 5–7 |
| 3 | Λ- and Δ-RhO | Rh | O | Ref. 8–11 |
| 4 | Λ- and Δ-RhS | Rh | S | This study |
The auxiliary-mediated synthesis14–18 starts with rhodium trichloride hydrate which is first converted into rac-RhS in a yield of 73% by reaction with 2 equiv. of 5-tert-butyl-2-phenylbenzothiazole (1), followed by a treatment with 1.2 equiv. of AgPF6 in MeCN (Fig. 1). The complex rac-RhS is then reacted with the monofluorinated salicyloxazoline (S)-219 to provide a diastereomeric mixture of Λ-(S)-3 and Δ-(S)-3 which can be resolved into pure diastereomers (46% each) based on their different solubilities in EtOH or by silica gel chromatography, or a combination thereof depending on the reaction scale. Configurations were assigned based on the crystal structure of Λ-(S)-3 as shown in Fig. 2. Finally, starting with Λ-(S)-3 and Δ-(S)-3, an acid induced replacement of the coordinated auxiliary ligand with two acetonitriles under retention of the configuration affords the individual enantiomers Λ-RhS (85%) and Δ-RhS (80%). The key aspect of this auxiliary-mediated synthesis is the fluorinated auxiliary (S)-2 which was first introduced by Ceroni and co-workers.19 All other tested auxiliaries did not provide intermediate rhodium auxiliary complexes with distinct solubilities and were not stable enough for a resolution via silica gel chromatography.
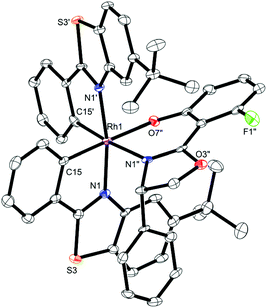 | ||
| Fig. 2 Crystal structure of the auxiliary complex Λ-(S)-3. ORTEP drawing with 50% thermal ellipsoids. CCDC number 1455732. | ||
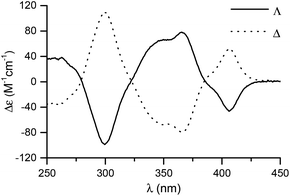
The CD spectra of the complexes Λ- and Δ-RhS are shown in Fig. 3, and confirm their mirror-imaged structures. HPLC performed on a chiral stationary phase validates the high enantiomeric purity of the individual enantiomers (Fig. 4).20 For the Δ-enantiomer an er of 99.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 was determined, while peak tailing prevents an accurate validation of the Λ-enantiomer and an er of >99
0.1 was determined, while peak tailing prevents an accurate validation of the Λ-enantiomer and an er of >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was estimated.
1 was estimated.
Fig. 5 shows the superimposed crystal structures of Λ-RhS and mirror-imaged Δ-RhO, not only confirming the assigned metal-centered configuration of Λ-RhS, but also revealing the differences in how the two tert-butyl groups flank the coordination site around the two exchange-labile acetonitrile ligands. In comparison with RhO, the tert-butyl groups of RhS are in closer proximity to the labile acetonitriles as quantified by a 0.9 Å shorter intramolecular distance between the quaternary carbons of the two tert-butyl groups in RhS (10.5 Å) over RhO (11.4 Å). This is consistent and analogous with a comparison of the related benzoxazole and benzothiazole iridium complexes.3,5
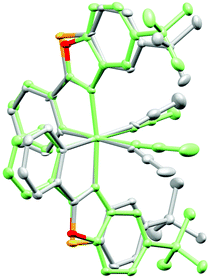 | ||
| Fig. 5 Superimposed crystal structure of Λ-RhS (grey) with inverted Δ-RhO (green). Fitted is the central metal together with the metal-bound atoms. Atoms are displayed as 50% thermal ellipsoids. CCDC number 1455731 (Λ-RhS). | ||
The increased steric hindrance provided by the two tert-butyl groups should make RhS an improved asymmetric catalyst for many applications. This is confirmed by the preliminary results shown in Fig. 6. In both enantioselective Michael addition8 and a photoinduced enantioselective radical reaction,11 the determined enantioselectivities are appreciably higher for the benzothiazole (RhS) over the benzoxazole (RhO) catalyst.21
In conclusion, here we reported a new chiral-at-metal benzothiazole complex Λ/Δ-RhS which expands the family of bis-cyclometalated rhodium(III) complexes for applications in asymmetric catalysis. Compared to the previously reported benzoxazole complex Λ/Δ-RhO, the benzothiazole ligands in Λ/Δ-RhS provide a higher steric congestion around the labile acetonitrile ligands, thereby making Λ/Δ-RhS a superior asymmetric catalyst. Applications of the new chiral Lewis acid catalyst to challenging asymmetric transformations are underway in our laboratory.
We thank the German Research Foundation (DFG) for financial support of this research (ME 1805/13-1).
Notes and references
- Reviews and accounts on different aspects of chiral Lewis acid catalysis: (a) K. Narasaka, Synthesis, 1991, 1–11 CrossRef CAS; (b) S. Saito and H. Yamamoto, Chem. Commun., 1997, 1585–1592 RSC; (c) K. A. Jørgensen, M. Johannsen, S. Yao, H. Audrain and J. Thorhauge, Acc. Chem. Res., 1999, 32, 605–613 CrossRef; (d) J. S. Johnson and D. A. Evans, Acc. Chem. Res., 2000, 33, 325–335 CrossRef CAS PubMed; (e) G. Desimoni, G. Faita and K. A. Jørgensen, Chem. Rev., 2006, 106, 3561–3651 CrossRef CAS PubMed; (f) S. Kobayashi and C. Ogawa, Chem. – Eur. J., 2006, 12, 5954–5960 CrossRef CAS PubMed; (g) S. Kanemasa, M. Hasegawa and F. Ono, Chem. Rec., 2007, 7, 137–149 CrossRef CAS PubMed; (h) J. Christoffers, G. Koripelly, A. Rosiak and M. Rössle, Synthesis, 2007, 1279–1300 CrossRef CAS; (i) M. North, D. L. Usanov and C. Young, Chem. Rev., 2008, 108, 5146–5226 CrossRef CAS PubMed; (j) P. Li and H. Yamamoto, Top. Organomet. Chem., 2011, 37, 161–183 CrossRef CAS; (k) J. Zhou and Y. Tang, Top. Organomet. Chem., 2011, 36, 287–312 CrossRef CAS; (l) L. C. Dias, E. C. de Lucca Jr., M. A. B. Ferreira and E. C. Polo, J. Braz. Chem. Soc., 2012, 23, 2137–2158 CrossRef CAS; (m) H. Yamamoto, Top. Organomet. Chem., 2013, 44, 315–334 CrossRef CAS.
- H. Huo, C. Fu, K. Harms and E. Meggers, J. Am. Chem. Soc., 2014, 136, 2990–2993 CrossRef CAS PubMed.
- H. Huo, X. Shen, C. Wang, L. Zhang, P. Röse, L.-A. Chen, K. Harms, M. Marsch, G. Hilt and E. Meggers, Nature, 2014, 515, 100–103 CrossRef CAS PubMed.
- C. Wang, Y. Zheng, H. Huo, P. Röse, L. Zhang, K. Harms, G. Hilt and E. Meggers, Chem. – Eur. J., 2015, 21, 7355–7359 CrossRef CAS PubMed.
- X. Shen, H. Huo, C. Wang, B. Zhang, K. Harms and E. Meggers, Chem. – Eur. J., 2015, 21, 9720–9726 CrossRef CAS PubMed.
- H. Huo, C. Wang, K. Harms and E. Meggers, J. Am. Chem. Soc., 2015, 137, 9551–9554 CrossRef CAS PubMed.
- C. Wang, J. Qin, X. Shen, R. Riedel, K. Harms and E. Meggers, Angew. Chem., Int. Ed., 2016, 55, 685–688 CrossRef CAS PubMed.
- C. Wang, L.-A. Chen, H. Huo, X. Shen, K. Harms, L. Gong and E. Meggers, Chem. Sci., 2015, 6, 1094–1100 RSC.
- Y. Huang, L. Song, L. Gong and E. Meggers, Chem. – Asian J., 2015, 10, 2738–2743 CrossRef CAS PubMed.
- Y. Tan, W. Yuan, L. Gong and E. Meggers, Angew. Chem., Int. Ed., 2015, 54, 13045–13048 CrossRef CAS PubMed.
- X. Shen, K. Harms, M. Marsch and E. Meggers, submitted for publication.
- For reviews on different aspects of metal-centered chirality, see: (a) J.-L. Pierre, Coord. Chem. Rev., 1998, 178–180, 1183–1192 CrossRef CAS; (b) U. Knof and A. von Zelewsky, Angew. Chem., Int. Ed., 1999, 38, 302–322 CrossRef; (c) P. D. Knight and P. Scott, Coord. Chem. Rev., 2003, 242, 125–143 CrossRef CAS; (d) H. Amouri and M. Gruselle, Chirality in Transition Metal Chemistry, Wiley, Chichester, UK, 2008 Search PubMed; (e) E. Meggers, Eur. J. Inorg. Chem., 2011, 2911–2926 CrossRef CAS; (f) J. Crassous, Chem. Commun., 2012, 48, 9684–9692 RSC; (g) E. C. Constable, Chem. Soc. Rev., 2013, 42, 1637–1651 RSC; (h) A. von Zelewsky, Chimia, 2014, 68, 297–298 CrossRef CAS PubMed.
- For reviews covering chiral-at-metal complexes in catalysis, see: (a) H. Brunner, Angew. Chem., Int. Ed., 1999, 38, 1194–1208 CrossRef CAS; (b) M. Fontecave, O. Hamelin and S. Ménage, Top. Organomet. Chem., 2005, 15, 271–288 CrossRef CAS; (c) E. B. Bauer, Chem. Soc. Rev., 2012, 41, 3153–3167 RSC; (d) L. Gong, L.-A. Chen and E. Meggers, Angew. Chem., Int. Ed., 2014, 53, 10868–10874 CrossRef CAS PubMed; (e) Z.-Y. Cao, W. D. G. Brittain, J. S. Fossey and F. Zhou, Catal. Sci. Technol., 2015, 5, 3441–3451 RSC.
- E. Meggers, Chem. – Eur. J., 2010, 16, 752–758 CrossRef CAS PubMed.
- L. Gong, M. Wenzel and E. Meggers, Acc. Chem. Res., 2013, 46, 2635–2644 CrossRef CAS PubMed.
- M. Helms, Z. Lin, L. Gong, K. Harms and E. Meggers, Eur. J. Inorg. Chem., 2013, 4164–4172 CrossRef CAS.
- L.-A. Chen, W. Xu, B. Huang, J. Ma, L. Wang, J. Xi, K. Harms, L. Gong and E. Meggers, J. Am. Chem. Soc., 2013, 135, 10598–10601 CrossRef CAS PubMed.
- For related studies from other groups on auxiliary mediated synthesis of chiral, non-racemic metal complexes, see: (a) C. F. Liu, N. C. Liu and J. C. Bailar, Inorg. Chem., 1964, 3, 1085–1087 CrossRef CAS; (b) U. Koelle, K. Bücken and U. Englert, Organometallics, 1996, 15, 1376–1383 CrossRef CAS; (c) D. Hesek, Y. Inoue, S. R. L. Everitt, H. Ishida, M. Kunieda and M. G. B. Drew, Chem. Commun., 1999, 403–404 RSC; (d) D. Hesek, Y. Inoue, H. Ishida, S. R. L. Everitt and M. G. B. Drew, Tetrahedron Lett., 2000, 41, 2617–2620 CrossRef CAS; (e) D. Hesek, Y. Inoue, S. R. L. Everitt, H. Ishida, M. Kunieda and M. G. B. Drew, Inorg. Chem., 2000, 39, 317–324 CrossRef CAS PubMed; (f) F. Pezet, J.-C. Daran, I. Sasaki, H. Aït-Haddou and G. G. A. Balavoine, Organometallics, 2000, 19, 4008–4015 CrossRef CAS; (g) O. Chepelin, J. Ujma, X. Wu, A. M. Z. Slawin, M. B. Pitak, S. J. Coles, J. Michel, A. C. Jones, P. E. Barran and P. J. Lusby, J. Am. Chem. Soc., 2012, 134, 19334–19337 CrossRef CAS PubMed; (h) D. L. Davies, K. Singh, S. Singh and B. Villa-Marcos, Chem. Commun., 2013, 49, 6546–6548 RSC.
- E. Marchi, R. Sinisi, G. Bergamini, M. Tragni, M. Monari, M. Bandini and P. Ceroni, Chem. – Eur. J., 2012, 18, 8765–8773 CrossRef CAS PubMed.
- For other reports on non-racemic, chiral-at-metal octahedral rhodium(III) complexes, see: (a) A. H. Krotz, L. Y. Kuo, T. P. Shields and J. K. Barton, J. Am. Chem. Soc., 1993, 115, 3877–3882 CrossRef CAS; (b) A. Sitlani, C. M. Dupureur and J. K. Barton, J. Am. Chem. Soc., 1993, 115, 12589–12590 CrossRef CAS; (c) L. Ghizdavu, B. Kolp, A. von Zelewsky and H. Stoeckli-Evans, Eur. J. Inorg. Chem., 1999, 1271–1279 CrossRef CAS; (d) L. Ghizdavu, A. von Zelewsky and H. Stoeckli-Evans, Eur. J. Inorg. Chem., 2001, 993–1003 CrossRef CAS; (e) L. Ghizdavu, O. Lentzen, S. Schumm, A. Brodkorb, C. Moucheron and A. Kirsch-De Mesmaeker, Inorg. Chem., 2003, 42, 1935–1944 CrossRef CAS PubMed; (f) N. Yoshinari and T. Konno, Inorg. Chem., 2008, 47, 7450–7452 CrossRef CAS PubMed; (g) A. Damas, J. Moussa, M. N. Rager and H. Amouri, Chirality, 2010, 22, 889–895 CrossRef CAS PubMed; (h) S. Mollin, S. Blanck, K. Harms and E. Meggers, Inorg. Chim. Acta, 2012, 393, 261–268 CrossRef CAS; (i) S. Mollin, R. Riedel, K. Harms and E. Meggers, J. Inorg. Biochem., 2015, 148, 11–21 CrossRef CAS PubMed; (j) R. Rajaratnam, E. K. Martin, M. Dörr, K. Harms, A. Casini and E. Meggers, Inorg. Chem., 2015, 54, 8111–8120 CrossRef CAS PubMed.
- For asymmetric catalysis with octahedral rhodium(III) complexes, see also: (a) H. Nishiyama, H. Sakaguchi, T. Nakamura, M. Horihata, M. Kondo and K. Itoh, Organometallics, 1989, 8, 846–848 CrossRef CAS; (b) Y. Motoyama, H. Narusawa and H. Nishiyama, Chem. Commun., 1999, 131–132 RSC; (c) D. Cuervo, M. P. Gamasa and J. Gimeno, J. Mol. Catal. A: Chem., 2006, 249, 60–64 CrossRef CAS; (d) J.-i. Ito and H. Nishiyama, Synlett, 2012, 509–523 CAS; (e) T. Wang, J.-L. Niu, S.-L. Liu, J.-J. Huang, J.-F. Gong and M.-P. Song, Adv. Synth. Catal., 2013, 355, 927–937 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and analytical data. CCDC 1455731 and 1455732. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6dt01063f |
| This journal is © The Royal Society of Chemistry 2016 |